
- Group Menu ->
- Data Access
- Vision, Mission & Values
- Investor Relations
- Leadership Charter
- Agroscience Services
- BioPharma Services
- Clinical Diagnostics
- Consumer Product Testing
- Environment Testing
- Food Testing
- Food Auditing and Compliance Services
- Food Label Review
- Genomic Services
- Sensory and Consumer Research
- Materials and Engineering Sciences

Eurofins CRL Piscataway

Get Directions
Eurofins CRL, Inc. was first incorporated in 1992 as Clinical Research Laboratories, Inc. Today, Eurofins CRL, Inc. operates as a contract laboratory dedicated to providing a wide range of in vitro and in vivo clinical safety and efficacy testing to the cosmetic, beauty, personal care and pharmaceutical industries.
Eurofins CRL, Inc. combines a highly skilled technical staff with state-of-the-art equipment and facilities located in New Jersey, North Carolina, and Texas.
We are committed to providing cost-effective testing of the highest quality, combined with timely scheduling, comprehensive final reports, and a commitment to customer service. Areas of expertise include: dermatology, photobiology, ophthalmology, bioinstrumentation, microbiology, cosmetology, clinical trials, and associated services.
Our Leadership Team
Board of Directors
Board Committees
Our Commitment to Diversity, Equity & Inclusion
The Feed by C-P
Our History
Our Policies
Awards & Recognitions
View Our Brands
Innovation at Colgate-Palmolive
Clinical Trials
Our Innovation Stories
Innovation History
Sustainability
Colgate-Palmolive Sustainability Overview
Our 2025 Sustainability & Social Impact Strategy
Sustainability & Social Impact Report
Our Sustainability Stories
Sustainability Policies
Reporting Data & Downloads
Our Community Impact
Bright Smiles, Bright Futures ® (BSBF)
Colgate Women’s Games
Hill’s Food, Shelter & Love Program
Estrellas Colgate
We are Colgate, a caring, innovative growth company that is reimagining a healthier future for all people, their pets and our planet.

Colgate-Palmolive Opens One-of-a-Kind Volpe Clinical Research Center to Reimagine a Healthier Future

New Clinical Facility in Piscataway Accelerates Research and Innovation
As an innovative growth company, Colgate-Palmolive is opening the Volpe Clinical Research Center in Piscataway, NJ, which will accelerate development of the Company’s cutting-edge technology and product formulas, fulfilling our purpose to reimagine a healthier future for all.
Colgate hosted a grand opening ceremony on June 1 to officially open the new, state-of-the-art, in-house clinical research facility, named in honor of the Company’s formative leader for more than 50 years, the late Tony Volpe, Colgate’s Vice President of Worldwide Clinical Research.

Accelerating Innovation
The Volpe Clinical Research Center, an in-house clinical research center based within our largest global technology center, empowers Colgate to more easily test and learn about the benefits of new formulations and devices, gain immediate feedback on prototypes, and receive direct consumer insights from study participants.
By leveraging a unique cross-team structure that brings together Colgate’s R&D, innovation team, clinical and consumer insights specialists, the Volpe Center is designed to accelerate R&D and speed the innovation timeline significantly.
This facility enables dentists, hygienists, dermatologists, aestheticians and other members of the clinical research team to co-create health care products with our early research, product development, marketing, design and packaging teams, and with the consumers who will ultimately use the products. And with input from all stakeholders, from Colgate clinicians to everyday consumers, we can design and develop the best possible products.
In addition to advancing oral care, Colgate researchers will also use the new facility to support innovation for the company’s skin health business.
Reimagining a Healthier Future
With the new Volpe Center, Colgate furthers its commitment to bringing consumers the most efficacious products based on clinical research.
Our powerhouse clinical research team and vast global clinical research network is a differentiating factor for our company, and enables us to achieve our purpose – to reimagine a healthier future for all – with scientific excellence. Colgate conducts over 200 clinical studies each year with the help of academic partners, clinical research organizations and practice-based networks in addition to our own in-house clinical operations in Piscataway and at our Dental Health Units throughout the world.
The research conducted in the Volpe Center will also support Colgate’s comprehensive education program that is transforming how the world thinks about and approaches oral health. Despite the alarming fact that nearly half of all people globally suffer from oral disease, many are unaware that oral health is linked to overall health. With our Know Your OQ campaign, Colgate is on a mission to increase oral health literacy and empower people to understand that a healthier future starts with a healthy mouth.

Honoring Tony Volpe’s Legacy
The new center pays tribute to the late Tony Volpe, Colgate’s Vice President of Worldwide Clinical Research. Tony was an extraordinary leader for Colgate for more than 50 years and personified our company’s commitment to improving oral health, to developing products with scientific excellence, and to establishing our leadership among dental professionals.
Recognized as one of the world’s leading experts on clinical dental research and preventive dentistry, Tony published more than 200 papers in major international scientific journals. He was also an accomplished practitioner, dental educator, industry leader and philanthropic volunteer.
Tony was a member of the first graduating class of the Rutgers School of Dental Medicine, marking the beginning of an important and growing partnership between Rutgers and Colgate. Today, to continue our longstanding relationship with Rutgers, Colgate supports an endowed scholarship for historically underrepresented populations in the field of dentistry and powers the New Horizons Program to expose dental students to alternate careers in dentistry. In addition, Colgate supports the American Dental Association's New Volpe Research Scholar to advance oral health research and their prestigious Gold Medal Award to recognize individuals, who through basic or clinical research, have contributed to the advancement of the profession of dentistry or to major improvement of the oral health of the public.
Celebrating the Grand Opening
The new Volpe Center strengthens our commitment to our purpose – to reimagine a healthier future for all – through innovation and scientific excellence.
To celebrate the Center’s opening, and to honor Tony, Colgate hosted a ceremony on June 1 at Colgate’s Global Technology Center in Piscataway. The festivities opened with remarks from Chairman, President and Chief Executive Officer, Noel Wallace; Chief Clinical Officer Maria Ryan; and Chief Technology Officer Pat Verduin.
Colgate hosted esteemed guests from Rutgers, the American Dental Association, and the New Jersey Dental Association to recognize our strong partnerships and honor the longstanding relationships Tony fostered. The celebration included a presentation of a replica of Tony’s sculpture and tour groups for guests to explore the new clinic
For more information about how Colgate is reimagining a healthier future and driving growth through innovation, visit: innovation.colgatepalmolive.com and follow the Company on LinkedIn .

You might also be interested in...
The story behind suavitel shed shield, a liquid fabric softener that repels pet hair and fights pet odors.

How Colgate-Palmolive’s Digital Upskilling Is Leading to E-commerce Growth

The Story Behind Hill’s Prescription Diet ONC Care — Nutrition Designed Specifically For Pets With Cancer

Community Impact
Colgate.com
Where to Buy
United States (US English)
ColgateProfessional.com
Shop.Colgate.com
Submit an Idea
© YYYY Colgate-Palmolive Company. All rights reserved.
Terms Of Use
Privacy Policy
Children's Privacy Policy
Terms Of Sale
Cookie Consent Tool
Do Not Sell My Personal Information
Piscataway, New Jersey
- Actively Recruiting
About Eurofins CRL

Over the years, Eurofins CRL Inc (CRL) has earned a leadership position in providing independent, human clinical testing services. Products are tested to determine safety and efficacy, and for claims support substantiation. The extensive professional experience of Eurofins CRL encompasses testing for a wide range of products including cosmetics, over-the-counter drug products, pharmaceuticals and household products.
Eurofins CRL is at the forefront of method development and acts as a technical consultant to many leading global cosmetic and pharmaceutical companies. To ensure panelists’ safety, every facet of our operation is monitored by our quality assurance program. As Eurofins CRL continues to evolve, new services will be developed to accommodate a broader base of applications to satisfy the requirements of a larger cross-section of the cosmetic, personal care, medical device and pharmaceutical industries.

- Group Menu ->

- Field Studies
- Ecotoxicology
- Regulatory Affairs
- Agro Testing
- Bioanalytical Services
- BioPharma Product Testing
- Contract Development & Manufacturing Organisation (CDMO)
- Pharma Discovery Services
- Pharma Early Development
- Pharma Central Laboratory
- Medical Device Testing
- Human Safety Testing
- Search Clinical Diagnostics
- Information
- Cosmetics & Personal Care
- Electrical & Electronics
- Water Testing
- Air Testing
- Soil Testing
- Building Materials
- Pollution Testing
- Waste Testing
- Fuel / Oil Testing
- Environment Laboratories
- Environmental Testing News
- Food Testing Services
- Food Testing Industries
- Food Testing Flyers
- Food Testing Laboratories
- Forensic DNA Analysis
- Forensic toxicology
- Quality and Safety
- Next Generation Sequencing
- Gene Synthesis & Molecular Biology
- Genotyping & Gene Expression
- DNA & RNA Oligonucleotides
- Custom DNA Sequencing
- News & Events
- In Vitro Diagnostics Solutions
- Maritime Services
- Materials and Engineering
- PSS Insourcing Solutions ®
- Testing Services
- News and useful links
- Sensory and Consumer Research
- Sustainability Services
- Eurofins Fact Sheet
- Eurofins Group overview
- Eurofins Strategy and Objectives
- Eurofins Entrepreneurship Model
- 30th Anniversary (2017)
- Our Vision, Mission and Values
- Leadership Charter
- Board of Directors
- Group Operating Council
- Eurofins Foundation
- CO2 Emissions
- ISO 14001 (Environmental Management)
- Spotlight: Examples of Local Environmental Initiatives
- Recruitment
- Training and Academy
- FT Diversity Leader by Financial Times
- OHSAS 18001 / ISO 45001 (Occupational Health and Safety)
- Total Recordable Incidence Rate (TRIR)
- Spotlight: Examples of Local Social Initiatives
- Code of Ethics and Values
- Eurofins Core Compliance Documents
- Whistleblowing
- Giving Back
- Carbon Neutrality - 2025 Objective
- Our Commitment to the UN Sustainable Development Goals
- Scientific Collaborations
- Your Career at Eurofins
- Our Global Locations
- COVID-19-Response
- Testing for Life
- A New Era of Oncological Data
- Fighting an Animal Health Endemic
- 20 Million Tests a Month to Help Protect the World Against COVID-19
- Protecting Crops and Human Health at the Same Time
- Ensuring Liquid Gold is the Real Deal
- Detecting a Silent Threat – Transplant Rejection
- What Do Pizza Boxes, Raincoats and Frying Pans Have in Common?
- Seeking Out a Silent Killer
- Protecting Cancer Patients
- Safeguarding the Health of the World’s Most Vulnerable Consumers – Infants
- Tackling the Big Problem of Tiny Particles
- Protecting Transplant Patients and Saving Lives
- Busting the Identical Twin Myth
- New Generation of Non-Invasive Prenatal Testing Methods
- SNIF-NMR - How it All Began
- The Future of Bees, the Future of Life
- Helping to stop Zika in its tracks
- A Pioneer in Dioxins Detection
- Acrylamide on the Tip of Everyone’s Tongue
- Fuelling the First Astronauts
- Testing for Traceability of Meat From Farm to Fork Down to Individual Animals
- The Forefathers of Pesticide Testing
- 30 Years of Data for Life
- Finding Adulteration Even When Not Looking for it
- Modern Farming is Precision Farming
- Beating Drug-Assisted Rape Forensically
- High-Flying Innovation
- Testing for Tailored Cancer Treatment
- Nurturing Cells to Grow
- Out of the Box Product Testing
- Inventing The Most Sensitive Heavy Metals Detection Method
- A Passive Revolution In Water Sampling
- Algae, Algae, Everywhere. Toxic and What a Stink
- Solving Old Crimes with New Technology
- Leading the World in GM Fish Detection
- Not Wanted Dead or Alive Mould and Bacteria
- Scientific Publications
- Shareholder Information
- Our Success in Numbers
- Audit and Risk Committee
- Sustainability and Corporate Governance Committee
- Nomination and Remuneration Committee
- Executives’ Dealings Disclosures
- Analyst Coverage
- Shares in Issue
- Corporate Timetable
- Bond Instruments
- Hybrid Capital Instruments
- Bond & Hybrid Capital Maturity Profile
- Commercial Paper Program
- Investor Relations Events Timetable
- Share buy-back programmes
- Press Releases
- Eurofins BioPharma Services Newsletter 34 - February 2023
- Eurofins BioPharma Services Newsletter 33 - October 2022
- Eurofins BioPharma Services Newsletter 32 - June 2022
- Eurofins BioPharma Services Newsletter 31 - February 2022
- Eurofins BioPharma Services Newsletter 30 - October 2021
- Eurofins BioPharma Services Newsletter 29 - June 2021
- Eurofins BioPharma Services Newsletter 28 - February 2021
- Eurofins BioPharma Services Newsletter 27 - October 2020
- Eurofins BioPharma Services Newsletter 26 - July 2020
- Eurofins BioPharma Services Newsletter 25 - February 2020
- Eurofins BioPharma Services Newsletter 24 - October 2019
- Eurofins BioPharma Services Newsletter 23 - June 2019
- Eurofins BioPharma Services Newsletter 22 - February 2019
- Group Directory PDF
- Czech Republic
- French Guiana
- Hong Kong (China)
- The Netherlands
- New Zealand
- Philippines
- Saudi Arabia
- South Africa
- Switzerland
- United Arab Emirates
- United Kingdom
Eurofins CRL

Get Directions
Eurofins CRL, Inc. was first incorporated in 1992 as Clinical Research Laboratories, Inc. Today, Eurofins CRL, Inc. operates as a contract laboratory dedicated to providing a wide range of in vitro and in vivo clinical safety and efficacy testing to the cosmetic, beauty, personal care and pharmaceutical industries. Eurofins CRL, Inc. combines a highly skilled technical staff with state-of-the-art equipment and facilities located in New Jersey, North Carolina, and Texas. We are committed to providing cost-effective testing of the highest quality, combined with timely scheduling, comprehensive final reports, and a commitment to customer service. Areas of expertise include: dermatology, photobiology, ophthalmology, bioinstrumentation, microbiology, cosmetology, clinical trials, and associated services.
*Safety/Clinical Trials
Follicular Biopsy
Facial Sting
Safety-in-Use (Derm, Comedo, Op, Ped, Gyn, Dental)
Acnegenicity/Comedogenicity
*Claims/Bioinstrumentation
Anti-Aging Claims Support
Moisturization &Skin Hydration
Cell Turnover/Cell Renewal
Skin Elasticity, Extensibility, & Firmness
TEWL
VISIA-CR
Clarity System
Skin Luminosity, Skin Color/Skin Tone, Evenness of Color
Video Microscopy of the Skin & Skin Structures
*Photobiology
In Vivo Testing:
Static SPF Testing (FDA/ISO 24444)
Water Resistant, Sweat Resistant, and Sand Resistant Testing
Wet Skin Application
In Vitro Testing:
Determination of Sunscreen UVA Factor (UVAPF/SPF Ratio and CWL)
Critical Wavelength (FDA Broad Spectrum)
Blue Light Testing
BOOTS Star Rating
Fabric Ultraviolet Protection Factor (UPF) Testing
*Microbiology
Healthcare Professional Hand Wash (ASTM 1174)
European Hand Wash & Rub (EN 1499 & EN 1500)
Surgical Scrub Evaluation (ASTM 1115)
Persistent & Residual Efficacy
Food Service Hand Wash
Finger Pad Method: Bacterial (ASTM 2276) Viral (ASTM 1838)
Preoperative & Preinjection Skin Prep (ASTM 1173)
Hand Sanitizers (ASTM 2755)
Virucidal Hand Wash (ASTM 2011)
*Cosmetology
*Pediatrics
*Consumer Research
*Product Procurement, Blinding, and Distribution
Accreditations:
*ISO 9001:2015
*GCP Compliant Facilities
*GLP Compliant Facilities
Tel: +1 515 280 8378
Email: [email protected]
Web: www.eurofinsus.com

- Patient Login
- Partner Login
- Become A Member
- Search Clinical Trials
- About Clinical Trials
- Virtual Clinical Trials
Latest Blog Posts
Services for research sites.
- Study Listings
- Clinical Ad Network™
- Patient Direct Mail Alerts
- Create Partner Account
National Recruitment Services
- Multicenter Study Listings
- Digital Marketing Strategies
- Site Identification Services
- Direct to Patient Advertising
News And Information
- Case Studies
- Patient Recruitment Insider Blog
- For Research Sites
- Study Screening Websites
Clinical Trials in Piscataway, New Jersey
- Search Results
Enter your email to receive custom alerts for new clinical trials in Piscataway, New Jersey
Top searches.
- Alzheimer's Disease
- Ulcerative Colitis
- Lupus (SLE)
- Scleroderma
- Endometriosis
- Retinitis Pigmentosa
Search Results from C linical C onnection

© 1998-2024 | All trademarks are property of their legal owners. | All Rights Reserved
ClinicalConnection.com is a resource that provides information on clinical trials and other research opportunities worldwide.
ClinicalConnection.com does not conduct or endorse this research. Please consult your physician before participating.
- Exploring the Microbiome: Gut Health, Probiotics and Clinical Trials
- Nutrition and Clinical Trials: Latest Clinical Research on Diet's Role in Disease
- Personalized Medicine: The Power of Genetics in Clinical Trials
- Depression Clinical Trials Near Me: Current Research and How to Participate
Useful Links
1998-2024 © All Rights Reserved. Privacy Policy | Terms of Service
Please read our guidelines before visiting Rutgers Cancer Institute. | COVID-19: What Cancer Patients Should Know
Clinical Research
The Rutgers Cancer Institute of New Jersey Clinical Research Program is organized based on disease teams, mainly related to specific diagnoses. Some of the Disease Specific Groups are more thematic: Early Drug Development (Phase 1), Precision Medicine, and Cellular Therapies. Each disease team reviews available clinical trials or develops their own trials to meet the clinical needs of patients within their area of expertise (for example, breast cancer) at Rutgers Cancer Institute and RWJBarnabas Health sites for all patients across the system. The DSG trial portfolio covers the majority of situations for our patients and meets the needs of cancer patients in our catchment area, which is the entire state of New Jersey. DSGs work closely with our Community Outreach and Engagement team to address the trial needs of underrepresented patient groups across the state. Learn more about the Clinical Research Program of each Disease Specific Group below.
Disease Specific Groups
Gastrointestinal oncology disease specific group.
The Gastrointestinal Cancers Disease Specific Group (GI DSG) is dedicated to clinical and translational research in all tumors of the GI tract. We conduct clinical trial on cancers of the entire GI tract including esophageal cancer, gastric and G-E junction cancers, biliary tract, hepatocellular carcinoma, pancreas, small bowel, colon, rectal and anal cancer. In 2022 the GI DSG ran approximately 20 trials and accrued 100 patients to therapeutic trials. We also work with scientists at Rutgers Cancer Institute of New Jersey, Princeton University, other Cancer Centers and industry on treatment trials and on non-treatment trials understanding the biology of GI Cancers.
The group is composed of Medical, Surgical and Radiation Oncologists who work closely in a multi-disciplinary fashion. We have special interest in pancreatic cancer, colon cancer and peritoneal carcinomatosis, and the use of Precision Medicine to direct therapies for novel molecular subsets. We have active trials in these areas at all times and surgical trials involving surgery and HIPEC and liver directed therapies. We always have late-line colon cancer studies focused on patients who have previously received the standard drugs. We are working closely with the Cellular Therapies DSG to develop cell therapy for solid tumors of the GI tract. Additionally, we have a strong interest in developing blood-based DNA testing for tumor prognosis, treatment and for microbiome research. We work closely with investigators at Rutgers Center for Advance Biotechnology and Medicine, and Rutgers Cancer Institute to understand the microbiome and how it influences cancer growth and control. We are conducting microbiome directed treatment trials.
- Learn more about our Gastrointestinal Oncology Program.
- Find GI Cancer Clinical Trials.
- To request an appointment, call 844-CANCERNJ.
Genitourinary Oncology Disease Specific Group

The Genitourinary Cancers Disease Specific Group (GU DSG) is dedicated to advancing prevention, screening and treatment of all GU cancers. We work across the cancer continuum and across multiple disciplines to improve outcomes for our patients. We conduct clinical trials on cancers of the entire GU tract including prostate, bladder, kidney, penile and testicular cancers. We offer trials of first-in-human (phase 1) through randomized phase 3 studies. We offer trials on improving screening and improving cancer treatments by offering advanced surgical and radiation techniques and medical therapies. We also work with our colleagues from the School of Public Health, School of Nursing and School of Communication at Rutgers University to improve care delivery of cancer patients with communication interventions to improve palliative care outcomes. We work with our colleagues and investigators at Rutgers Center for Advanced Biotechnology and Medicine to understand the microbiome and its impact on outcomes for bladder cancer surgery.
In 2022, the GU DSG ran approximately 20 trials and accrued 60 patients to therapeutic trials. Our team of medical, surgical and radiation oncologists are actively collaborating with each other and across the United States as part of cooperative groups, the Hoosier Cancer Research Network, Big Ten Cancer Research Consortium and pharmaceutical companies. We train future investigators in medical, urological and radiation oncology. We welcome anyone interested in further collaboration in advancing patient care reach one of our physicians in the link below. We also welcome scientific inquiries to any one of us directing at the contact information below.
- Learn more about our Urologic Oncology Program , Prostate Oncology Program
- Find GU Cancer Clinical Trials.
Gynecologic Oncology Disease Specific Group
The Gynecologic Disease Specific Group is comprised of a multidisciplinary group of gynecologic oncologists, pathologists, radiologists, radiation oncologists, nurses, and scientists. We are committed to offering a diverse array of clinical protocols that encompass the spectrum of gynecologic malignancies. These include ovarian cancers, uterine cancers, cervical cancers, as well as rarer histologies. We participate in trials through groups such as the NRG Oncology Group and Gynecologic Oncology Group (GOG) Foundation as well as industry sponsored trials. A unique feature of our DSG is that all gynecologic oncologists are members of the group and enroll patients on clinical trials. This means that the patient is able to continue care with their surgeon who can lead them through their participation in a clinical trial. We have monthly DSG meetings across all RWJBarnabas Health partner sites where clinical trial portfolios, patient accruals and safety data are reviewed. Our team members hold key roles in regional and national organizations, such as Society of Gynecologic Oncology, Gynecologic Oncology Group, and Big Ten Cancer Research Consortium. We also actively participate in the Precision Medicine Oncology group at Rutgers Cancer Institute of New Jersey and offer personalized treatment options based on molecular tumor profile.
The past few years have been a very exciting time for exploring new treatments for gynecologic malignancies. We have seen this lead to an exponential increase in the number of trials and enrolled patients at Rutgers Cancer Institute. One example is the incorporation of checkpoint inhibitors. Trials for both endometrial and ovarian cancers are testing the use of this treatment as an addition to or alternative to standard of care chemotherapy. We have participated in a national clinical trial using a checkpoint inhibitor for endometrial cancer, where interim analysis showed a possible survival benefit. Other ongoing research includes the testing of antibody drug conjugates for platinum sensitive recurrent ovarian cancer. Cell cycle inhibitors are an encouraging modality for gynecologic tumors that have a high degree of replication stress. These include high-grade uterine and ovarian cancers, and ongoing research is examining the effectiveness of these inhibitors for these tumor types. We are strong believers that clinical trials should be considered throughout the care of a gynecologic cancer patient. This is true whether it is a newly diagnosed patient, someone seeking maintenance therapy or a patient with recurrent disease. We are dedicated to ensuring that all our patients have equitable access to clinical trials and to outstanding care.
- Learn more about our Gynecologic Oncology Program.
- Find Gynecologic Cancer Clinical Trials.
Hematologic Malignancies Oncology Disease Specific Group
The Hematologic Malignancies Disease Specific Group (HEME DSG) is focused on the care of patients facing a diagnosis of various blood disorders. Blood disorders include illnesses of the bone marrow (the ‘factory’ for our blood) and cancers of the immune system. This includes cancers such as leukemia, lymphoma, multiple myeloma and other plasma cell cancers, myelodysplasia and myeloproliferative disorders. Caring for patients with such a diverse range of illnesses requires both breadth and depth of expertise, and the care teams within our group offer tremendous expertise in the diagnosis, treatment, and research in these fields. This expertise is at its heart multi-disciplinary, and we fully embrace the spirit of teamwork required to support patients and their loved ones through this journey. Excellent physicians, nurse clinicians and advanced practice providers, nurse navigators, social workers, dieticians, and genetic counselors work together both at Rutgers Cancer Institute of New Jersey and across RWJBarnabas Health to provide patients the holistic care that is so critical to achieving the best outcomes.
Increasingly, cutting-edge care of blood disorders requires consideration of the newest treatments such as cellular therapy, including stem cell transplantation as well as chimeric antigen receptor-modified T-cell (CAR-T) therapy. Our Blood and Marrow Transplant Program, led by Dr. Ira Braunschweig, offers tremendous expertise in the use of both FDA-approved and next-generation cellular therapies. Indeed, the Division is leading the way not only in treating patients with the best treatments currently available, but also in leading research to redefine the standards of care. Clinical research into more effective and less toxic treatments for patients with a diagnosis of a blood disorder is at the core of the Division’s mission, and we are leading pivotal clinical trials across the spectrum of illnesses faced by our patients to work towards achieving our mission of ending blood cancers and blood disorders, period. Please reach out if interested in a consultation with one of our experts, either in person or via telemedicine.
- Learn more about our Blood and Marrow Transplant Program, Leukemia/Lymphoma/Hematologic Malignancies Program.
- Find Hematologic Cancer Clinical Trials.
Cell Therapy Disease Specific Group

The Cell Therapy DSG is a vital component of Rutgers Cancer Institute of New Jersey's mission to develop effective treatments for solid tumors. Through clinical trials, the team translates discoveries made in the Cell Therapy Research laboratory into promising therapies for patients. The Cell Therapy DSG utilizes the Rutgers Cancer Institute Process Development Laboratory and Good Manufacturing Practice Facility to develop cutting-edge treatments, while investigators also collaborate with industry partners to ensure patients have access to the latest therapies.
The team is at the forefront of developing several types of cell therapy, including tumor-infiltrating lymphocyte therapy and engineered T cells therapies like CAR T-cells and TCR T-cells. The Cell Therapy DSG is conducting clinical trials for a variety of solid tumor types, including HPV-associated cancers, gastric cancer, melanoma, and ovarian cancer. Active clinical trials include both investigator-initiated interventional and non-interventional studies, as well as clinical trials of investigator-sponsored IND agents.
- Learn more about the Cancer Metabolism & Immunology Research Program.
- Find Immunotherapy Cancer Clinical Trials
Lung and Head & Neck Cancer Disease Specific Group
The Lung and Head & Neck Cancer Disease Specific Group (Lung/H&N DSG) at Rutgers Cancer Institute of New Jersey is charged with maintaining a portfolio of clinical trials of highest clinical and scientific value that covers the diversity of disease manifestations within our catchment area, advancing translational science and enhancing access to investigations of the most promising therapies for patients throughout New Jersey. Recent scientific advances in lung and head and neck cancers have led to transformational therapies for patients, resulting in some of the largest declines in national cancer death rates, with participants in current clinical trials contributing toward a brighter future.
Our philosophy of access relies on our partnership with the RWJBarnabas Health System, with a focus on bringing trials to patients in their local communities. Collectively, our efforts have led to a year over year improvement in trials enrollment since 2019, with a doubling of enrolled participants to therapeutic interventional trials in calendar year 2022. Our trials portfolio includes high priority trials sponsored by the National Cancer Institute, pharmaceutical companies and an increasing number are led by our own Rutgers Cancer Institute investigators.
- Learn more about the Lung/Thoracic Oncology Program.
- Find Lung/Thoracic Cancer Clinical Trials.
Melanoma/Sarcoma Disease Specific Group
The Melanoma/Sarcoma Disease Specific Group at Rutgers Cancer Institute of New Jersey provides comprehensive multidisciplinary care to patients with melanoma and other non-melanoma skin cancers such as Merkel cell carcinoma, basal cell carcinoma and squamous cell carcinoma, as well as sarcomas. Our team consists of experts in the fields of surgical oncology, medical oncology, radiation oncology, dermatology, and pathology. Our investigators meet weekly to discuss patient management and to formulate individualized treatment plans and approaches for each patient. We have clinical expertise in managing early through advanced stage melanoma, non-melanoma skin cancer, and sarcomas.
Rutgers Cancer Institute is the state’s only National Cancer Institute-designated Comprehensive Cancer Center. As such, our group is heavily focused on conducting clinical and translational research to improve patient outcomes and to develop better and newer therapeutic strategies for advanced skin cancers and sarcomas. Our goal is to offer the most cutting-edge therapies to our patients and to offer clinical trials whenever possible. Many of our clinical trials focus on immune-based approaches for patients with skin cancers who have not responded to standard immunotherapies and/or targeted therapies.
- Learn more about the Melanoma Oncology Program and Sarcoma & Soft Tissue Oncology Program.
- Find Melanoma Clinical Trials and Sarcoma Clinical Trials.
Neuro-Tumor Disease Specific Group
The Neuro-Tumor Disease Specific Group (DSG) is comprised of members from Rutgers Cancer Institute of New Jersey and multiple institutions within the RWJBarnabas Health system. We are focused on offering clinical trials for patients with primary central nervous system (CNS) tumors and metastatic tumors to the CNS at every stage of their journey. Currently we have several trials open for patients with glioblastoma (GBM), including NRG-BN011 for patients with newly diagnosed, methylated GBM; NRG-BN007 for patients with newly diagnosed, unmethylated GBM; and Berubicin for patients at first recurrence of GBM. We are also working to open TVI-Brain-1, a novel adoptive cell transfer approach in patients with newly diagnosed unmethylated GBM. We also have a number of trials for pediatric patients with primary CNS tumors, including diffuse intrinsic pontine gliomas (DIPG), medulloblastoma, papillary craniopharyngioma and low-grade glioma in neurofibromatosis type 1.
There are also active research trial efforts in the setting of metastatic CNS disease including studies in patients with Her2/neu positive breast cancer, small cell lung cancer and resected metastatic disease. We are also preparing to offer a trial utilizing targeted therapy tailored to the molecular profile of resected metastatic disease. Finally, in coordination with the early phase group, we have a trial of a novel compound for patients with several targetable mutations. We look forward to the future and honored to participate in this team effort to develop increasingly effective therapies with the goal of defeating cancer.
- Learn more about the Neurologic Oncology Program.
- Find Neurologic Cancer Clinical Trials.
Pediatric Hematology Oncology Disease Specific Group

The Pediatric Hematology and Oncology Disease Specific Group is committed to improving outcomes for children with cancer or blood disorders through clinical research. We offer novel therapeutic approaches for children with a wide variety of common childhood cancers, including acute lymphoblastic leukemia, acute myelogenous leukemia, chronic myelogenous leukemia, Hodgkin lymphoma, non-Hodgkin lymphomas, brain tumors, bone tumors, and soft tissue sarcomas. We offer clinical trials for children with blood disorders such as sickle cell anemia and hemophilia. In addition, we offer clinical trials focused on innovative approaches to reduce symptoms and improve quality of life during treatment or in the survivorship period. Our clinical trial portfolio includes investigator-initiated trials, industry-sponsored trials, and trials offered by cooperative groups, such as the Children's Oncology Group and the Dana Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium.
The multidisciplinary group includes pediatric hematologists and oncologists, radiation oncologists, pediatric surgeons, and psychologists. We work closely with laboratory scientists and population scientists at Rutgers Cancer Institute of New Jersey to bring the latest science to the development of novel clinical trials.
- Learn more about the Pediatric Hematology/Oncology Program.
- Find Pediatric Hematology Cancer Clinical Trials.
Phase I/Investigational Therapeutics Disease Specific Group

The Phase I/Developmental Therapeutics Disease Specific Group at Rutgers Cancer Institute of New Jersey is a multidisciplinary scientific group designed to develop new therapies for the treatment of cancer. The program includes a team of medical oncologists, surgical oncologists, advanced practice providers, research nurses, research scientists, clinical trial specialists, data coordinators, and social workers who have extensive experience in providing novel therapeutic strategies. The program conducts clinical trials of novel drugs, including cytotoxic chemotherapeutic agents, targeted therapeutic approaches (VEGF, claudin 18.2, among others), molecularly driven agents (targeting MTAP null tumors, FGFR fusions, her-2, PIK3CA mutations), and novel immuno-oncology approaches (including novel checkpoints, CAR-T, bi-specifics and TRINKETs). The clinical trials include first in human trials of novel drugs as well as novel combination approaches. These may also include combinations of drugs with radiation or surgery. Clinical trial offerings are diverse and include partnerships with academia, industry, and government.
The objectives of the program are twofold. At a systemic level, we strive to contribute to the effort of rational development of the next generation of breakthrough drugs that will help improve the lives of patients with cancer. At a more localized and personal level, we strive to make available therapeutic options within the context of a clinical trial for every patient with cancer who has progressive cancer despite the best efforts of their health care team.
- Learn more about the Phase I/Investigational Therapeutics Oncology Program.
- Find Phase I Cancer Clinical Trials.
Population Science Disease Specific Group

The Population Science Disease Specific Group (DSG) promotes high quality interventional and observational trials related to cancer prevention and control research. The DSG reviews and monitors investigator-initiated studies across a range of behavioral, epidemiological, and health services research that spans the cancer control continuum from primary prevention to survivorship. This DSG also monitors accrual and works with principal investigators to overcome any challenges to successfully enrolling and achieving the aims of the study.
The Population Science DSG portfolio contains a range of peer-reviewed externally funded trials as well as internally funded studies. Areas of strength include studies identifying and addressing disparities in cancer risk, screening, treatment, and survivorship, tobacco cessation and control strategies, cancer risk reduction, digital health interventions for cancer survivors, and improving care coordination during healthcare transitions.
- Learn more about our Section of Pediatric Population Science, Outcomes, and Disparities Research Program.
- Find Pediatric Cancer Clinical Trials.
Radiation Oncology Disease Specific Group
The Radiation Oncology Disease Specific Group (DSG) was newly created in 2022. The Radiation Oncology DSG fosters multiple innovative interventional radiotherapeutic studies across tumor types and across RWJBarnabas Health. The DSG reviews new studies for feasibility and interest and monitors the accrual of the clinical trials within the portfolio. The DSG meets monthly to discuss enrollment, addresses barriers to trial accrual, and reviews the status of trials through the pre-activation period. The DSG assesses areas of potential need and growth within the trial portfolio.
The Radiation Oncology DSG has several original investigator-initiated studies, both internally and externally funded. Some of these studies evaluate: the timing of radiation in breast cancer, an intensified regimen of preoperative chemoradiation and chemotherapy in rectal cancer, immunotherapy combinations with radiation in GI and lung cancers, radiation dose questions in bony and non-bone sites of painful metastases, and the use of early radiation oncologist involvement in the setting of palliation. The Radiation Oncology DSG also runs many National Cancer Institute funded trials through the National Clinical Trials Network (NCTN) and Experimental Therapeutics Clinical Trials Network (ETCTN) as well as industry funded clinical trials. These important clinical trials advance care for our Radiation Oncology patients.
- Learn more about our Radiation Oncology Program.
- Find Radiation Cancer Clinical Trials.
Eurofins CRL
Photos & videos.
See all 10 photos

You Might Also Consider

Kofinas Fertility Group
Vanessa P. said "I had no idea what to expect when coming here and I had read some great reviews and this place is amazing. From the receptionist, to the nurses that work there, and then the doctor himself, everyone was great and made me feel at…" read more
in Fertility

Mazhar Elamir, MD
24.0 miles away from Eurofins CRL
Ekaterina F. said "If you don't mind a 1-1.5 hour wait then you will find a wonderful and kin, very knowledgeable and friendly doctor who has been treating me for over 20 year. I have used his pulmonary skills before but he is my internis. The staff…" read more
in Internal Medicine, Pulmonologist, Sleep Specialists
About the Business
Join us at Clinical Research Laboratories! Here at CRL, Panelists receive compensation to participate in a variety of different clinical studies that involve testing new products from numerous clients. …
Location & Hours
Suggest an edit
Amenities and More
Ask the community.
Ask a question
Yelp users haven’t asked any questions yet about Eurofins CRL .
Recommended Reviews
- 1 star rating Not good
- 2 star rating Could’ve been better
- 3 star rating OK
- 4 star rating Good
- 5 star rating Great
Select your rating
Overall rating
Horrible company does not respect there clients!! Stay away... the use you and then do not respect you,.

Honestly this is the best place to make fast, easy money. I do this with my friends and everyone here is so nice!!!! They do a lot of makeup & skincare related studies - which is a win-win for me since I love makeup and skincare. All the ladies are so cute, funny, and friendly. It's easy to sign up. The paper work isn't that hectic and isn't long, but you do get a lot of calls for recruitment of new studies. Which I don't mind because my phone is always on do not disturb anyways. They leave voicemails so if you do want to sign up for a new study in the future, you already have the information in your phone.

Sophia M. said "When I was diagnosed with fibroids and recommended to have surgery, I wanted to make sure that I found a doctor who was concerned with preserving my fertility. I went to see a doctor a Columbia and a doctor at NYU for opinions, both…" read more

Benjamin C H Peng, MD
Oscar A. said "This review is long overdue, but had to be shared. Dr. Peng came highly recommended by my PCP when I was searching for a Urologist to perform my vasectomy. I don't think I need to elaborate on how much concern and apprehension went…" read more
in Urologists
People Also Viewed

Carrier Clinic
Priyanka Sanjay, MD

RWJUH Lab Services at Edison
Klots Larisa MD DO PC

Bridgeway Care and Rehabilitation Center at Hillsborough

Brunswick Urgent Care

MedFirst Urgent Care - Bridgewater
Associated Radiologists
Central Jersey Internal Medicine

Avenel Primary Care
Browse Nearby
Urgent Care
Things to Do
Restaurants
Emergency Rooms
Drug Screening Near Me
Laboratory Testing Near Me
Other Laboratory Testing Nearby
Find more Laboratory Testing near Eurofins CRL

We bridge the talent gap and kick-start careers simultaneously.
Forget about keeping up. It’s time to get ahead.
Get the sollers advantage.
We partner with corporations, design curricula around their business needs and train our students to become ready-on-day-one job candidates.


With Great Experience Comes Great Opportunity
The world is evolving fast. Here is your chance to get ahead. Information Technology and Life Sciences, two fields that improve life, are in greater need than ever before. Now, you can make a difference, at any industry-leading organization.
Information Technology
Cloud Computing and Data Science are the future of Information Technology. And you can play an important role in shaping it.
Life Sciences
Sollers offers future-relevant, career-defining courses in Clinical Data Science, Drug Safety, Project Management and more.
Upskilling America for the jobs of the future
Level up. Emerge head and shoulders above the rest. Contribute meaningfully at any top organization.

Sollers supports you with student-friendly financial options that clear a path for your future.
Real-time work environments, the latest technologies and continuous upskilling for two years.

Sollers builds curricula around the needs of corporations . This means you get trained for the job.
Financial Options

Employer Sponsorships
A clear win-win for both parties. You will be able to get your tuition reimbursed by your employer.

Women’s Scholarship
Sollers offers a special scholarship for specific courses to women who are eligible for it.

Payment Plans
Students who cannot pay the entire tuition sum upfront can pay it off in installments over the duration of the course.
Partnerships that Grow Your Business
Sollers creates impactful partnerships with industry-leading employers in the fields of Information Technology and Life Sciences. We make it our responsibility to do a detailed analysis of your needs and prepare a comprehensive plan from initiation to delivery and provide “ready on day one” candidates with both the background and applied skills needed to succeed at low cost.
Testimonials
“Sollers has great certificate and internship programs in Clinical Trials domain. I have recently completed Clinical Data Science program with Sollers. Their faculty, facilitators, career services all are knowledgeable and experienced in the field of Clinical Trial. I was very happy with the internship program too. They provided me with valuable guidance throughout my program, internship and job application process. I definitely would recommend Sollers.edu to anyone who wants to pursue education in Clinical Trial industry ??”

“Sollers College is a great institution of learning, I had a memorable time attending advance drug safety and pharmacovigilance program. They helped me financially, the lecturers were so friendly and knowledgeable. The internship program was so flexible and on point. They have one of the best career services department with a very dedicated director. Keep it up.”
“My experience with Sollers is nothing short if a instant family. They are always right there to guide and mentor you along the way. Whenever I was stuck I always reached out, I am pretty sure they are happy that I passed my course, because now some of the staff can get some sleep. Sollers was there for me, and they helped me land my dream job. Every stage was amazing. Thank you Sollers, thanks for everything.”
"We are pleased to welcome Sollers College as an SEI Partner". "Our Partners are a valuable extension of the SEI, helping to meet the demand for SEI technologies and services worldwide with a level of quality that matches the SEI's own."
SEI Partners are trained, evaluated, and selected by the SEI and are the only source of official SEI technologies services from outside the SEI. SEI Partners provide courses, consulting, and/or coaching services in one or more of the following areas: information security, software architecture, software engineering measurement and analysis, and the Team Software Process.

"Sollers College provides strategic sourcing and subject matter expertise to tackle a variety of complex functions for the pharmaceutical industry. I chose Sollers for a number of reasons. The school provides a hybrid learning format where students can attend classes in person or online via internet."
Their staff is well trained with broad skill sets that range from Drug Safety/Pharmacovigilance, Clinical Trial Management, Clinical Research, Oracle Argus safety database, and Statistical Analysis System (SAS). Staff and faculty members are accessible and willing to give students advice about their professional questions and concerns. I plan to work at a pharmaceutical company or contract research organization as a pharmacovigilance scientist. I am looking forward to my future career thanks to the skills and professional network that Sollers College has helped me build.

Syune arrived at Sollers, having completed her PhD in Biology at a Swedish University. Despite having worked as a research scientist in Armenia, Poland, and Sweden, she had no US-based experience, which was limiting her ability to pursue a satisfying science focused career.
After finishing the Clinical Trial Management program at Sollers in Jan. 2019, Syune was hired by Catalyst Pharmaceuticals as a Clinical Research Associate.
“A well-designed curriculum, Project-based, mentor-guided program combined with their helpful and friendly career services and support staff made for a wonderful experience as a student. I was able to get a job by the end of the program in Cognizant and I am grateful for all the support from Sollers. Definitely worth investing your time and going through the bootcamp.”
“I would like to thank Sollers College for providing this wonderful opportunity to learn and gain knowledge on the AWS & Database Concepts. The labs were a good starter for obtaining practical knowledge. The tutors go above and beyond: they explained each topic with examples and provided hands-on practice.”
Eurofins CRL
[email protected] | (512) 243-6426
- About the Lab
With ECRL, innovative companies Collaborate. Test. Succeed.
Eurofins CRL, Inc. is a highly specialized clinical testing lab focused on the evaluation of topical antiseptics such as hand sanitizers, surgical site preps, and surgical scrubs. Previously the ECRL Texas location operated as Vivo Clinical Testing, a joint venture between Microchem Laboratory based in Round Rock, TX and Clinical Research Laboratories based in Piscataway, NJ which combined Microchem's decades of expertise in antimicrobial testing with Clinical Research Laboratories' decades of experience working with volunteers in clinical studies. Following its acquisition by Eurofins Scientific Group, ECRL now offers a broader range of services while maintaining the same dedication to quality. Clients can count on ECRL's skillful and experienced scientific staff to deliver fast, reliable results, study after study.
ECRL offers testing in compliance with current Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) regulations. Clients are welcome to tour the lab, observe studies, and audit the lab's quality system.
The lab is staffed by a capable, friendly group of scientists, mostly microbiologists. We understand what our clients need: fairly priced testing services, done accurately and reported quickly.
Main Testing Services:
- Consumer hand sanitizer and hand wash testing
- Health care personnel hand sanitizer and hand wash testing
- Pre-operative skin preparation testing
- Surgical hand scrub testing
- CHG compatibility testing
- Custom residual and persistent efficacy testing of topical antiseptics
- Custom topical antiseptic testing
Essential Information
- ECRL is FDA GCP and GLP-compliant.
- ECRL's expert staff is responsive.
- ECRL is well-regarded in industry.
- ECRL generates accurate reports.
- Phone the Laboratory: 512-243-6426.
- Email: [email protected].

Select Region or Brand
- Charleston, SC
- Columbia, SC
- Greenville, SC
- Pennsylvania
- Lehigh Valley, PA
- Long Island, NY
- Mecklenburg, NC
- New Orleans, LA
- Oklahoma City, OK
- Rochester, NY
- South Carolina
- Color Magazine
- Massachusetts
- North Carolina
- Rhode Island
- Milwaukee, WI
- Designers Today
- Furniture Today
- Gifts & Decorative Accessories
- Home Accents Today
- Home Furnishings News
- Home Textiles Today
- Manage Your Print or Online Subscription
- Manage Your Email Subscriptions
Upcoming Event
NJBIZ Leaders in Finance 2024
- Power List Methodology
- Submit a Recommendation
- 2024 NJBIZ Power 100
- 2024 Health Care Power 50
- 2023 Accounting Power 50
- 2023 Law Power 50
- 2023 Education Power 50
- 2023 Commercial Real Estate Power 50
- Add My Business
- Contact Leads & Data Center
- About Leads & Data Center
NJBIZ Conversations
- Photo Galleries
- Advertising & Marketing Tips
- Free Growth Guide
- Editorial and Special Products Calendar
- Special Sections
- Digital Edition
- Food & Hospitality
- Health Care
- Manufacturing
- Real Estate
- Sports & Entertainment
- Transportation
- Reader Rankings
- This Week’s Issue
- Digital Editions
- Movers & Shakers
- Advertise With NJBIZ
- Event Sponsorship
- Leads & Data
- 2023 NJBIZ Power 100
Colgate opens Volpe Clinical Research Center in Piscataway
Gabrielle Saulsbery // June 3, 2022 //
Share this!
Related Content

Unilever receives $20.9M to cool carbon emissions at ice cream factories
Four ice cream factories will utilize the funding, which could create a model that leads to further decarboniz[...]
March 28, 2024

Gannett plans to close Cherry Hill printing press, lay off 139 workers
The move comes as more media outlets shut down or consolidate such operations amid the ongoing shift to digita[...]
March 26, 2024

OPINION: How WBE certification can help women make their mark
This Women's History Month, BFI Furniture President and CEO Kate Kerpchar explores how the Women Business Ente[...]

Mars invests $70M to support innovation, manufacturing in Hackettstown (PHOTOS)
The candymaker's new, state-of-the-art research & development facility will focus on fan favorites, such as M&[...]
March 21, 2024

NJMEP passes torch for annual major manufacturing event
“Handing over the reins of the State-of-the-State of Manufacturing Summit to NJBIA marks a pivotal moment in[...]

Cambrex a year ahead of schedule on $100M expansion projects
In total, the initiative adds more than 150,000 square feet of capacity and expanded capability across 70% of [...]
March 20, 2024
NJBIZ Daily Newsletter
Sign up for your daily digest of New Jersey News.
- By signing up you agree to our
- Privacy Policy
Latest Headlines

Luxury dog-boarding brand fetches $10M investment
Fanwood-based K9 Resorts Luxury Pet Hotel received the funding from its largest multiunit franchisee, Partners Pacific Resort[...]

Husband-wife duo preps South Jersey dispensary launch
Located in a former TD Bank branch in Blackwood, Flower & Flame will begin welcoming customers March 29.

Unilever receives $20.9M to cool carbon emissions at ice cream factori[...]
Four ice cream factories will utilize the funding, which could create a model that leads to further decarbonization throughou[...]

Saker ShopRites to donate $30K bonus from sale of $1.13B Mega Millions[...]
The lucky purchase was made at the retailer's wine and liquor store on Route 66 in Neptune.

$3.7M announced for NJ offshore wind research, monitoring
The funding aims to ensure the ecologically responsible development of the sector, especially following pushback and rash of [...]

NJBIZ Conversations: Winter4Kids CEO Schone Malliet
The organization's founder talks about why it's so important, how he keeps it running and his plans for t[...]
Introducing the 2024 NJBIZ Leaders in Law honorees
NJBIZ unveils 2024 Leaders in Finance Lifetime Achievement honoree
NJBIZ unveils 2024 Leaders in Finance honorees (updated)
NJBIZ introduces new honors for 2024 events
Video Replay: NJBIZ Leaders in Real Estate, Construction and Design 2023
Privacy Overview
MTA Missing Math Clock
Release: as part of new “project women’s health” initiative, gottheimer announces legislation to combat rare diseases impacting women.
Multi-front effort to boost clinical trials and find treatments and cures
Women often left out or underrepresented in clinical trials

Above: Gottheimer at Cooperman Barnabas Medical Center.
LIVINGSTON, NJ — Today, March 19, 2024, U.S. Congressman Josh Gottheimer (NJ-5) announced new legislation, the Securing Equal Access to Research, Care, and Health, or SEARCH Act, to help fund new efforts to increase the number of women in clinical research trials, leading to treatments and cures for rare diseases and blood disorders which affect millions of women in New Jersey and across our nation. The legislation is part of Gottheimer’s new, multi-front legislative initiative: “Project Women’s Health.”
Video of the announcement can be found here .
Gottheimer also highlighted his support for the President’s State of the Union Address earlier this month, which called on Congress to provide $12 billion to research health conditions and diseases that disproportionately affect women. In Congress, Gottheimer plans to work to help make this investment a reality.
“There is a clear gap in research, awareness, diagnosis, management, treatment, and cures of diseases and disorders affecting women. We must address the inequality between men and women in research, clinical trials, and awareness. It must be taken seriously and fixed and there’s no better time than the present,” said Congressman Josh Gottheimer (NJ-5), Rare Disease Caucus Member. “Even with all of the challenges we are facing, I remain optimistic and hopeful. I hope that you are, too. Built on collaborative work with experts and advocates, I’m confident that the SEARCH Act will ultimately lead to life changing medication, treatment, and cures for women impacted by rare diseases and bleeding disorders.”
“The Congressman is an advocate for ensuring equal access to care for all residents in New Jersey. He is focused on addressing disparities and improving healthcare outcomes across the state. We share a deep commitment with the Congressman to the most vulnerable among us,” said RWJBarnabas Health President and CEO Mark E. Manigan. “While we’re very proud of the awesome clinical services we offer here at Cooper and across the system, we are equally proud of that commitment to the most vulnerable as this state’s largest provider of charity care and as the state’s largest providers of care to beneficiaries to the medicare program by two times.”
“Thank you congressman for giving me the opportunity to speak this morning and for leading the charge on the SEARCH Act. It’s an important piece of legislation that champions the health of women, especially those affected by rare disease. The importance of the SEARCH act goes beyond my professional role and in fact connects deeply and is personal to me. In 1999, my family’s life changed forever when my niece who was ten years old at the time was diagnosed with Friedreich’s ataxia — a rare, debilitating, degenerative, neuromuscular disease. Sadly, in 2020, my niece’s battle ended just months after her 30th birthday… just last year… almost three years to the day after she died, the FDA approved a new medication to slow the progression for Friedreich’s ataxia. A breakthrough that arrived too late for my niece, but marked a significant step forward for others. The SEARCH Act represents a beacon of hope for women suffering from rare diseases,” said RWJBarnabas Health Vice President of Women’s Services Suzanne Spernal.
“I have witnessed the disparities women face and have faced for decades. Women are overlooked, misdiagnosed, or not diagnosed at all and it has become clear that legislation of this kind is not only necessary but critical for women, not only with bleeding disorders but also for women in the rare disease community at large,” said Hemophilia Association of New Jersey Executive Director Stephanie Lapidow.
“Although a disease may be rare, thus impacting a small number of people, it does not change the profound impact a condition has on those afflicted, as well as their families. Not uncommonly, due to the limited understanding of these diseases and the small number of people affected, funding for research is often scarce, making it difficult to find treatments,” said RWJBarnabas Director of Cell Therapy and Bone Marrow Transplantation Northern Regions Dr. Adrienne Phillips . “For the individual or family with a disease, getting to a diagnosis may be a long and frustrating road fraught with prolonged diagnosis and treatment delays.”
Challenges Facing Women’s Health Related to Rare Diseases and Blood Disorders:
- Since 1994, fewer than 4% of adults in the United States participate in clinical trials despite increasingly prolonged recruitment periods.
- Up to 85% of clinical trials fail to recruit or retain a sufficient sample size, leading to failures to meet targets in four out of every five trials. For rare diseases more commonly impacting women, like rett syndrome and multiple sclerosis, it can be even harder to start and maintain proper trials.
- The CDC estimates that up to 1 in 100 women and girls in the United States — more than 1.5 million Americans — have a bleeding disorder, many of whom aren’t even aware of their condition.
- The most common bleeding disorder affecting women is von Willebrand disease, or VWD, which results from a deficiency or defect in the body’s ability to produce a certain protein that helps blood clot.
- Reports indicate that women are directed to a hospital and specialists later than men following the onset of symptoms, which delays diagnosis and care. This can often lead to a rapid progression of the disease.
Gottheimer’s New Legislation, the Securing Equal Access to Research, Care, and Health or SEARCH Act will:
- There are many diseases that disproportionately impact women and are often swept under the research and funding mat.
- Fund recruitment campaigns for women in NIH clinical research trials for rare diseases by increasing advertising at hospitals, doctors’ offices, health centers, and healthcare clinics. This can have a direct and immediate impact on helping Jersey families.
- Require the CDC and HHS to increase the number of women in federal bleeding disorder programs that provide funding for research, surveillance, prevention, and services through public awareness campaigns.
- Create a task force between the HHS, NIH, FDA, CMS, and the private sector , including hospitals and labs in New Jersey, to produce a report on the rare diseases that disproportionately impact women, helping ensure more funding goes toward diseases and conditions that impact women.
Gottheimer was joined by RWJBarnabas Health President and CEO Mark E. Manigan, Cooperman Barnabas Medical Center President and CEO Richard L. Davis, RWJBarnabas Health Senior Vice President Dr. Balpreet Grewal-Virk, RWJBarnabas Health Vice President of Women’s Services, Director of Cell Therapy and Bone Marrow Transplantation Northern Regions Adrienne Phillips, and Hemophilia Association of New Jersey Executive Director Stephanie Lapidow.
Below: Gottheimer with rare disease doctors, nurses, and advocates.

Gottheimer’s remarks as prepared for delivery:
Good afternoon, and thank you again to Mark and Rick, Dr. Phillips, Stephanie, and Suzanne. It’s great to be back here in Livingston at Cooperman Barnabas Medical Center. I say back because I was born here and grew up down the road in North Caldwell. I also had surgery here after I broke my elbow as a kid. I can’t tell you the exact room. It’s a little different than what I remember — and the hospital looks fantastic. I do remember my mom sneaking me apple pancakes from the Ritz diner.
Before I begin, I want to thank all our nurses, doctors, frontline health care workers, our great North Jersey bio and life sciences leaders, and of course our rare disease researchers and advocates for the incredible work they do each and every day for our families. I cannot thank all of you enough for everything you do for our community to help improve the health and lives of countless Americans.
There are a few reasons it’s appropriate that we’re here this morning. March not only is it Women’s History Month, but it’s Blood Disorder Month, and it was just recently Rare Disease week.
Just yesterday, recognizing the importance of this month, the President signed an Executive order directing federal agencies to deploy federal funds to research health conditions and diseases that disproportionately affect women. As a member of the Rare Disease Caucus in Congress, I couldn’t agree more.
That is why today, I’m here to announce new legislation to help fund a new effort to increase the number of women in clinical research trials, leading to treatments and cures for rare diseases and blood disorders which affect millions of women across our nation.
It’s all part of my new, multi-front legislative initiative project women’s health which builds on my years of work on this front.
When it comes to research on rare and blood diseases, including those that disproportionately affect women, there is a shortage of women in clinical trials. My new legislation — the Securing Equal Access to Research, Care, and Health or SEARCH Act — will take that on.
As a quick refresher, a rare disease is one that impacts fewer than 200,000 people and far too often has no FDA-approved treatments. We just heard from Suzanne about her niece who was diagnosed with Friedreich Ataxia and sadly passed away at the age of 30, just three years before an FDA medication was approved to help delay the effects of her condition. As Suzanne mentioned, if we had had more investments for recruitment and public awareness campaigns for clinical trials, maybe the medication to delay the effects of her niece’s condition would have been discovered in time.
Personally, I lost my mom, of blessed memory, to a rare disease, Sarcoidosis, four and a half years ago.
While we are making progress, and have had some big breakthroughs, it’s far too slow, and we still have a long way to go. In fact, 95 percent of the more than 10,000 rare diseases that impact more than 30 million Americans have no FDA-approved treatment.
Rare disease research is critical. Not only can it lead to a cure or treatment for those afflicted, but the research can also be a gateway to breakthroughs for other treatments and cures.
Here in New Jersey, we are lucky to have some of the best, hospitals and medical facilities, labs, and R&D in the country, the best doctors, medical school graduates, nurses, and life sciences leaders.
With fourteen of the world’s top twenty research-based biopharmaceutical companies here in our state, we not only have cutting-edge hospitals, but our pharmaceutical and medical device companies have led the way on some of the biggest medical breakthroughs. New Jersey ranks second in the nation for cancer medicines in development and first for heart and stroke drugs and development. In 2017, 50 percent of all new FDA approvals came from companies with a New Jersey footprint.
What’s great is we already have some success stories from rare disease and bleeding disorder research.
In 2019, there were new approvals for the first triple combination therapy for patients with cystic fibrosis and a new gene therapy to treat pediatric patients under two-years-old with spinal muscular atrophy.
And just last year, the FDA approved gene therapies for hemophilia A and hemophilia B — an important advancement in providing treatment options and reducing the need for ongoing, routine therapy.
And Allergan, right here in New Jersey, announced FDA approval for an existing drug application that can now be used to treat lower-limb spasticity in children ages two to seventeen.
I want us to double down, so that we expand in the years ahead. We can lead the way in helping to develop the next cures for orphan diseases and blood disorders too commonly leaving women without the support and treatments they need.
And when we find those cures, it’ll help the amazing medical professionals — like here at Cooperman Barnabas Medical Center — care for women too often left without the help they need.
As Suzanne intimated, there are clear challenges and obstacles — especially when it comes to research.
First, participation in clinical trials is poor. Since 1994, fewer than four percent of adults in the United States participate in clinical trials despite increasingly prolonged recruitment periods. What’s worse, up to 85 percent of clinical trials fail to recruit or retain a sufficient sample size, leading to failures to meet targets in four out of every five trials. For rare diseases more commonly impacting women, like rett syndrome and multiple sclerosis, it can be even harder to start and maintain proper trials.
Second, like rare diseases, women with bleeding disorders have often been underrecognized and underdiagnosed. The CDC even estimates that up to 1 of every 100 women and girls in the United States — more than 1.5 million Americans — have a bleeding disorder, many of whom aren’t even aware of their condition. The most common bleeding disorder affecting women is von Willebrand disease, or VWD, which results from a deficiency or defect in the body’s ability to produce a certain protein that helps blood clot. Women with bleeding disorders have historically been excluded from research.
Third, because women tend to have rare diseases diagnosed later than men, bias in cures or treatments start early and leave lasting impacts. Reports indicate that women are directed to a hospital and specialists later than men following the onset of symptoms, which delays diagnosis and care. This can often lead to a rapid progression of the disease.
The bottom line: There is a clear gap in research, awareness, diagnosis, management, treatment, and cures of diseases and disorders affecting women. It must be taken seriously and fixed. We must do more to provide hope and support to the families who struggle every day to manage a rare disease or bleeding disorder — or any disease, for that matter. We must address the inequality between men and women in research, clinical trials, and awareness. And there’s no better time than the present.
That’s why, today, as I mentioned earlier, I’m here with medical professionals, advocates, and family members of those taken from rare diseases to announce the Securing Equal Access to Research, Care, and Health or SEARCH Act. This new legislation I’m introducing in Congress will support new research and clinical studies — and hopefully new treatments and cures — for diseases impacting women, including rare diseases and blood disorders.
First, the legislation will require the National Institute of Health, or NIH, to produce an action plan within 180 days to highlight the rare disease and health complications that uniquely impact women. As we laid out today, there are many diseases like blood disorders that disproportionately impact women and are often swept under the research and funding mat.
Second, the SEARCH Act will fund recruitment campaigns for women in NIH clinical research trials for rare diseases by increasing advertising at hospitals, doctors’ offices, health centers, and healthcare clinics. This can have a direct and immediate impact on helping Jersey families.
Third, the new legislation requires the CDC and HHS to increase the number of women in federal bleeding disorder programs that provide funding for research, surveillance, prevention, and services through public awareness campaigns.
Fourth, the SEARCH Act will create a task force between the HHS, NIH, FDA, CMS, and the private sector, including hospital and labs in New Jersey, to produce a report on the rare diseases that disproportionately impact women, helping ensure more funding goes toward diseases and conditions that impact women.
Finally, in his State of the Union Address earlier this month, the President called on Congress to provide $12 billion to research health conditions and diseases that disproportionately affect women. In Congress, I plan to work overtime in the coming months to make this investment a reality. We must pass legislation to create a new fund for Women’s Health Research at the NIH that will support a nationwide network of cutting-edge research centers in women’s health care.
Today, we’re here as part of my new, legislative fight on multiple fronts to protect women’s health care – whether that’s about research, clinical trials, treatments, and finding cures, or doing everything we can to protect a woman’s fundamental right to make her own personal, private healthcare decisions.
Even with all of the challenges we are facing, I remain optimistic and hopeful. I hope that you are, too. Built on collaborative work with experts and advocates, I’m confident that the SEARCH Act will ultimately lead to life changing medication, treatment, and cures for women impacted by rare diseases and bleeding disorders.
In the greatest country in the world, if we can come together to solve problems and support women’s health, I know that our best days will always be ahead of us. Once again, thank you for joining me here today.
May God bless you, your families, and may God continue to bless the United States of America.
Recent Posts
Release: gottheimer statement on the passing of former united states senator joe lieberman.
NORTH JERSEY — Today, March 27, 2024, U.S. Congressman Josh Gottheimer (NJ-5) released the following statement on the passing of former United States Senator Joe Lieberman: “Today, America lost one of our best. My friend and mentor, former Senator Joe Lieberman, served our nation in Congress for more than two decades. He was a trailblazer — […]
RELEASE: Gottheimer Statement on MTA Vote Moving Forward with Congestion Tax, Despite Significant Public Opposition
NORTH JERSEY — Today, March 27, 2024, U.S. Congressman Josh Gottheimer (NJ-5) released the following statement on the latest Congestion Tax announcements from the MTA: “Today’s vote was just a rubber stamp on the MTA’s unprecedented cash grab. It just proves what we knew all along — the MTA doesn’t care about less traffic, helping […]
RELEASE: As Part of New “Pedestrian Safety Strategy,” Gottheimer Announces Federal Investments, Legislation to Keep Pedestrians Safe
Follows increase of pedestrian deaths across North Jersey New Jersey most dangerous state in the nation for pedestrians Above: Gottheimer announcing his new “Pedestrian Safety Strategy.” RIDGEWOOD, NJ — Today, March 26, 2024, U.S. Congressman Josh Gottheimer announced a new “Pedestrian Safety Strategy” which includes a three-pronged approach to protecting pedestrians walking across our communities. Video […]
Read our research on: Abortion | Podcasts | Election 2024
Regions & Countries
What the data says about abortion in the u.s..
Pew Research Center has conducted many surveys about abortion over the years, providing a lens into Americans’ views on whether the procedure should be legal, among a host of other questions.
In a Center survey conducted nearly a year after the Supreme Court’s June 2022 decision that ended the constitutional right to abortion , 62% of U.S. adults said the practice should be legal in all or most cases, while 36% said it should be illegal in all or most cases. Another survey conducted a few months before the decision showed that relatively few Americans take an absolutist view on the issue .
Find answers to common questions about abortion in America, based on data from the Centers for Disease Control and Prevention (CDC) and the Guttmacher Institute, which have tracked these patterns for several decades:
How many abortions are there in the U.S. each year?
How has the number of abortions in the u.s. changed over time, what is the abortion rate among women in the u.s. how has it changed over time, what are the most common types of abortion, how many abortion providers are there in the u.s., and how has that number changed, what percentage of abortions are for women who live in a different state from the abortion provider, what are the demographics of women who have had abortions, when during pregnancy do most abortions occur, how often are there medical complications from abortion.
This compilation of data on abortion in the United States draws mainly from two sources: the Centers for Disease Control and Prevention (CDC) and the Guttmacher Institute, both of which have regularly compiled national abortion data for approximately half a century, and which collect their data in different ways.
The CDC data that is highlighted in this post comes from the agency’s “abortion surveillance” reports, which have been published annually since 1974 (and which have included data from 1969). Its figures from 1973 through 1996 include data from all 50 states, the District of Columbia and New York City – 52 “reporting areas” in all. Since 1997, the CDC’s totals have lacked data from some states (most notably California) for the years that those states did not report data to the agency. The four reporting areas that did not submit data to the CDC in 2021 – California, Maryland, New Hampshire and New Jersey – accounted for approximately 25% of all legal induced abortions in the U.S. in 2020, according to Guttmacher’s data. Most states, though, do have data in the reports, and the figures for the vast majority of them came from each state’s central health agency, while for some states, the figures came from hospitals and other medical facilities.
Discussion of CDC abortion data involving women’s state of residence, marital status, race, ethnicity, age, abortion history and the number of previous live births excludes the low share of abortions where that information was not supplied. Read the methodology for the CDC’s latest abortion surveillance report , which includes data from 2021, for more details. Previous reports can be found at stacks.cdc.gov by entering “abortion surveillance” into the search box.
For the numbers of deaths caused by induced abortions in 1963 and 1965, this analysis looks at reports by the then-U.S. Department of Health, Education and Welfare, a precursor to the Department of Health and Human Services. In computing those figures, we excluded abortions listed in the report under the categories “spontaneous or unspecified” or as “other.” (“Spontaneous abortion” is another way of referring to miscarriages.)
Guttmacher data in this post comes from national surveys of abortion providers that Guttmacher has conducted 19 times since 1973. Guttmacher compiles its figures after contacting every known provider of abortions – clinics, hospitals and physicians’ offices – in the country. It uses questionnaires and health department data, and it provides estimates for abortion providers that don’t respond to its inquiries. (In 2020, the last year for which it has released data on the number of abortions in the U.S., it used estimates for 12% of abortions.) For most of the 2000s, Guttmacher has conducted these national surveys every three years, each time getting abortion data for the prior two years. For each interim year, Guttmacher has calculated estimates based on trends from its own figures and from other data.
The latest full summary of Guttmacher data came in the institute’s report titled “Abortion Incidence and Service Availability in the United States, 2020.” It includes figures for 2020 and 2019 and estimates for 2018. The report includes a methods section.
In addition, this post uses data from StatPearls, an online health care resource, on complications from abortion.
An exact answer is hard to come by. The CDC and the Guttmacher Institute have each tried to measure this for around half a century, but they use different methods and publish different figures.
The last year for which the CDC reported a yearly national total for abortions is 2021. It found there were 625,978 abortions in the District of Columbia and the 46 states with available data that year, up from 597,355 in those states and D.C. in 2020. The corresponding figure for 2019 was 607,720.
The last year for which Guttmacher reported a yearly national total was 2020. It said there were 930,160 abortions that year in all 50 states and the District of Columbia, compared with 916,460 in 2019.
- How the CDC gets its data: It compiles figures that are voluntarily reported by states’ central health agencies, including separate figures for New York City and the District of Columbia. Its latest totals do not include figures from California, Maryland, New Hampshire or New Jersey, which did not report data to the CDC. ( Read the methodology from the latest CDC report .)
- How Guttmacher gets its data: It compiles its figures after contacting every known abortion provider – clinics, hospitals and physicians’ offices – in the country. It uses questionnaires and health department data, then provides estimates for abortion providers that don’t respond. Guttmacher’s figures are higher than the CDC’s in part because they include data (and in some instances, estimates) from all 50 states. ( Read the institute’s latest full report and methodology .)
While the Guttmacher Institute supports abortion rights, its empirical data on abortions in the U.S. has been widely cited by groups and publications across the political spectrum, including by a number of those that disagree with its positions .
These estimates from Guttmacher and the CDC are results of multiyear efforts to collect data on abortion across the U.S. Last year, Guttmacher also began publishing less precise estimates every few months , based on a much smaller sample of providers.
The figures reported by these organizations include only legal induced abortions conducted by clinics, hospitals or physicians’ offices, or those that make use of abortion pills dispensed from certified facilities such as clinics or physicians’ offices. They do not account for the use of abortion pills that were obtained outside of clinical settings .
(Back to top)
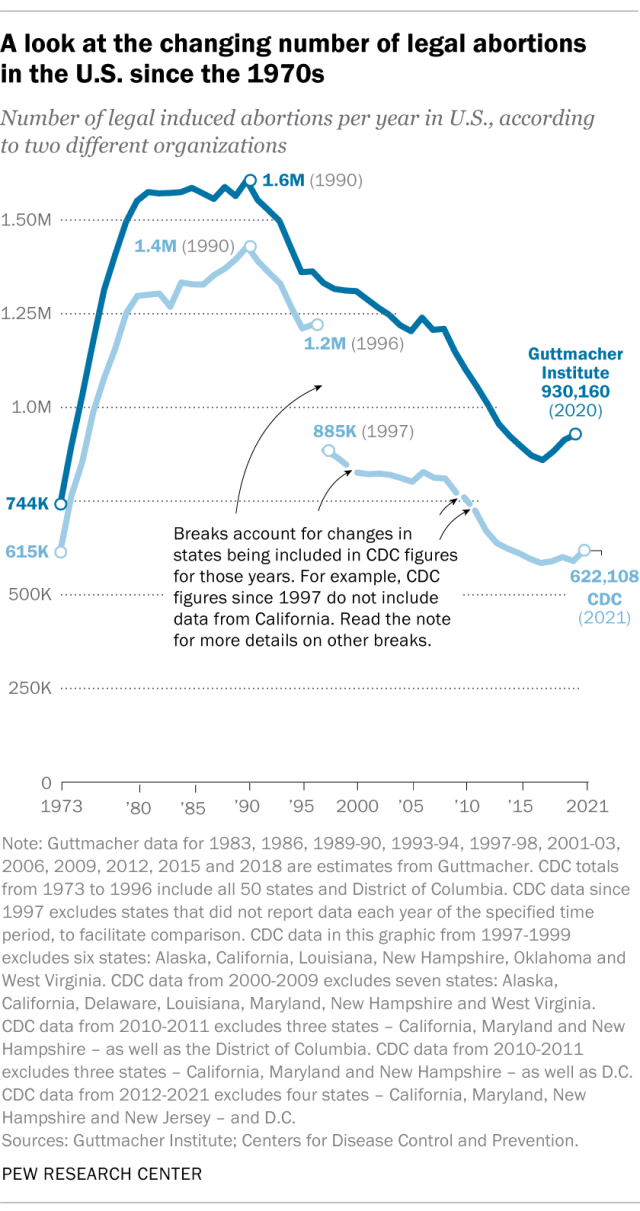
The annual number of U.S. abortions rose for years after Roe v. Wade legalized the procedure in 1973, reaching its highest levels around the late 1980s and early 1990s, according to both the CDC and Guttmacher. Since then, abortions have generally decreased at what a CDC analysis called “a slow yet steady pace.”
Guttmacher says the number of abortions occurring in the U.S. in 2020 was 40% lower than it was in 1991. According to the CDC, the number was 36% lower in 2021 than in 1991, looking just at the District of Columbia and the 46 states that reported both of those years.
(The corresponding line graph shows the long-term trend in the number of legal abortions reported by both organizations. To allow for consistent comparisons over time, the CDC figures in the chart have been adjusted to ensure that the same states are counted from one year to the next. Using that approach, the CDC figure for 2021 is 622,108 legal abortions.)
There have been occasional breaks in this long-term pattern of decline – during the middle of the first decade of the 2000s, and then again in the late 2010s. The CDC reported modest 1% and 2% increases in abortions in 2018 and 2019, and then, after a 2% decrease in 2020, a 5% increase in 2021. Guttmacher reported an 8% increase over the three-year period from 2017 to 2020.
As noted above, these figures do not include abortions that use pills obtained outside of clinical settings.
Guttmacher says that in 2020 there were 14.4 abortions in the U.S. per 1,000 women ages 15 to 44. Its data shows that the rate of abortions among women has generally been declining in the U.S. since 1981, when it reported there were 29.3 abortions per 1,000 women in that age range.
The CDC says that in 2021, there were 11.6 abortions in the U.S. per 1,000 women ages 15 to 44. (That figure excludes data from California, the District of Columbia, Maryland, New Hampshire and New Jersey.) Like Guttmacher’s data, the CDC’s figures also suggest a general decline in the abortion rate over time. In 1980, when the CDC reported on all 50 states and D.C., it said there were 25 abortions per 1,000 women ages 15 to 44.
That said, both Guttmacher and the CDC say there were slight increases in the rate of abortions during the late 2010s and early 2020s. Guttmacher says the abortion rate per 1,000 women ages 15 to 44 rose from 13.5 in 2017 to 14.4 in 2020. The CDC says it rose from 11.2 per 1,000 in 2017 to 11.4 in 2019, before falling back to 11.1 in 2020 and then rising again to 11.6 in 2021. (The CDC’s figures for those years exclude data from California, D.C., Maryland, New Hampshire and New Jersey.)
The CDC broadly divides abortions into two categories: surgical abortions and medication abortions, which involve pills. Since the Food and Drug Administration first approved abortion pills in 2000, their use has increased over time as a share of abortions nationally, according to both the CDC and Guttmacher.
The majority of abortions in the U.S. now involve pills, according to both the CDC and Guttmacher. The CDC says 56% of U.S. abortions in 2021 involved pills, up from 53% in 2020 and 44% in 2019. Its figures for 2021 include the District of Columbia and 44 states that provided this data; its figures for 2020 include D.C. and 44 states (though not all of the same states as in 2021), and its figures for 2019 include D.C. and 45 states.
Guttmacher, which measures this every three years, says 53% of U.S. abortions involved pills in 2020, up from 39% in 2017.
Two pills commonly used together for medication abortions are mifepristone, which, taken first, blocks hormones that support a pregnancy, and misoprostol, which then causes the uterus to empty. According to the FDA, medication abortions are safe until 10 weeks into pregnancy.
Surgical abortions conducted during the first trimester of pregnancy typically use a suction process, while the relatively few surgical abortions that occur during the second trimester of a pregnancy typically use a process called dilation and evacuation, according to the UCLA School of Medicine.
In 2020, there were 1,603 facilities in the U.S. that provided abortions, according to Guttmacher . This included 807 clinics, 530 hospitals and 266 physicians’ offices.
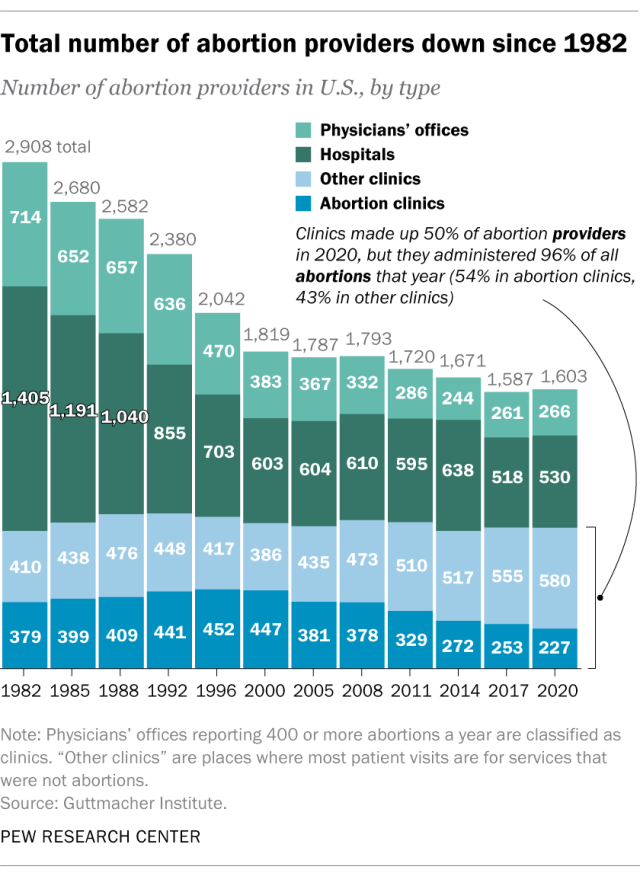
While clinics make up half of the facilities that provide abortions, they are the sites where the vast majority (96%) of abortions are administered, either through procedures or the distribution of pills, according to Guttmacher’s 2020 data. (This includes 54% of abortions that are administered at specialized abortion clinics and 43% at nonspecialized clinics.) Hospitals made up 33% of the facilities that provided abortions in 2020 but accounted for only 3% of abortions that year, while just 1% of abortions were conducted by physicians’ offices.
Looking just at clinics – that is, the total number of specialized abortion clinics and nonspecialized clinics in the U.S. – Guttmacher found the total virtually unchanged between 2017 (808 clinics) and 2020 (807 clinics). However, there were regional differences. In the Midwest, the number of clinics that provide abortions increased by 11% during those years, and in the West by 6%. The number of clinics decreased during those years by 9% in the Northeast and 3% in the South.
The total number of abortion providers has declined dramatically since the 1980s. In 1982, according to Guttmacher, there were 2,908 facilities providing abortions in the U.S., including 789 clinics, 1,405 hospitals and 714 physicians’ offices.
The CDC does not track the number of abortion providers.
In the District of Columbia and the 46 states that provided abortion and residency information to the CDC in 2021, 10.9% of all abortions were performed on women known to live outside the state where the abortion occurred – slightly higher than the percentage in 2020 (9.7%). That year, D.C. and 46 states (though not the same ones as in 2021) reported abortion and residency data. (The total number of abortions used in these calculations included figures for women with both known and unknown residential status.)
The share of reported abortions performed on women outside their state of residence was much higher before the 1973 Roe decision that stopped states from banning abortion. In 1972, 41% of all abortions in D.C. and the 20 states that provided this information to the CDC that year were performed on women outside their state of residence. In 1973, the corresponding figure was 21% in the District of Columbia and the 41 states that provided this information, and in 1974 it was 11% in D.C. and the 43 states that provided data.
In the District of Columbia and the 46 states that reported age data to the CDC in 2021, the majority of women who had abortions (57%) were in their 20s, while about three-in-ten (31%) were in their 30s. Teens ages 13 to 19 accounted for 8% of those who had abortions, while women ages 40 to 44 accounted for about 4%.
The vast majority of women who had abortions in 2021 were unmarried (87%), while married women accounted for 13%, according to the CDC , which had data on this from 37 states.
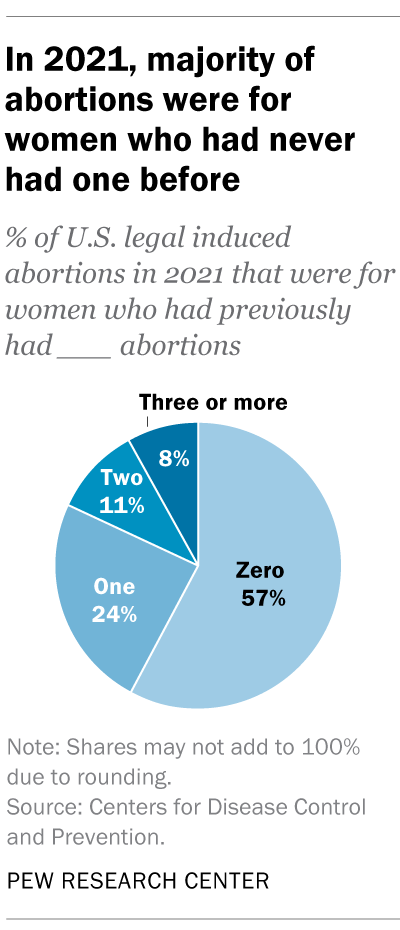
In the District of Columbia, New York City (but not the rest of New York) and the 31 states that reported racial and ethnic data on abortion to the CDC , 42% of all women who had abortions in 2021 were non-Hispanic Black, while 30% were non-Hispanic White, 22% were Hispanic and 6% were of other races.
Looking at abortion rates among those ages 15 to 44, there were 28.6 abortions per 1,000 non-Hispanic Black women in 2021; 12.3 abortions per 1,000 Hispanic women; 6.4 abortions per 1,000 non-Hispanic White women; and 9.2 abortions per 1,000 women of other races, the CDC reported from those same 31 states, D.C. and New York City.
For 57% of U.S. women who had induced abortions in 2021, it was the first time they had ever had one, according to the CDC. For nearly a quarter (24%), it was their second abortion. For 11% of women who had an abortion that year, it was their third, and for 8% it was their fourth or more. These CDC figures include data from 41 states and New York City, but not the rest of New York.
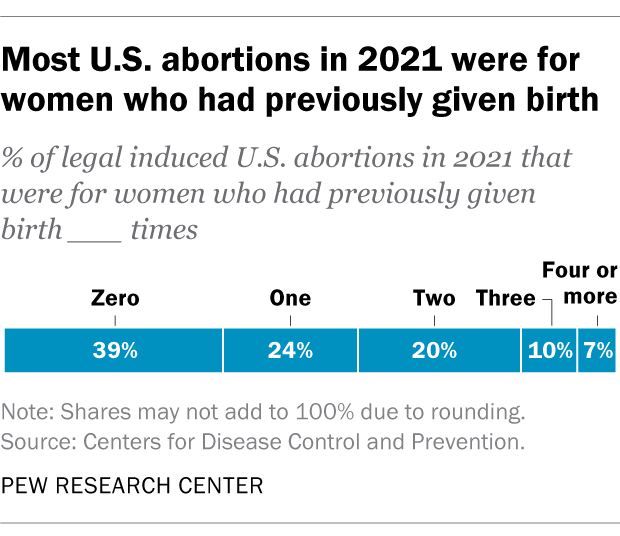
Nearly four-in-ten women who had abortions in 2021 (39%) had no previous live births at the time they had an abortion, according to the CDC . Almost a quarter (24%) of women who had abortions in 2021 had one previous live birth, 20% had two previous live births, 10% had three, and 7% had four or more previous live births. These CDC figures include data from 41 states and New York City, but not the rest of New York.
The vast majority of abortions occur during the first trimester of a pregnancy. In 2021, 93% of abortions occurred during the first trimester – that is, at or before 13 weeks of gestation, according to the CDC . An additional 6% occurred between 14 and 20 weeks of pregnancy, and about 1% were performed at 21 weeks or more of gestation. These CDC figures include data from 40 states and New York City, but not the rest of New York.
About 2% of all abortions in the U.S. involve some type of complication for the woman , according to an article in StatPearls, an online health care resource. “Most complications are considered minor such as pain, bleeding, infection and post-anesthesia complications,” according to the article.
The CDC calculates case-fatality rates for women from induced abortions – that is, how many women die from abortion-related complications, for every 100,000 legal abortions that occur in the U.S . The rate was lowest during the most recent period examined by the agency (2013 to 2020), when there were 0.45 deaths to women per 100,000 legal induced abortions. The case-fatality rate reported by the CDC was highest during the first period examined by the agency (1973 to 1977), when it was 2.09 deaths to women per 100,000 legal induced abortions. During the five-year periods in between, the figure ranged from 0.52 (from 1993 to 1997) to 0.78 (from 1978 to 1982).
The CDC calculates death rates by five-year and seven-year periods because of year-to-year fluctuation in the numbers and due to the relatively low number of women who die from legal induced abortions.
In 2020, the last year for which the CDC has information , six women in the U.S. died due to complications from induced abortions. Four women died in this way in 2019, two in 2018, and three in 2017. (These deaths all followed legal abortions.) Since 1990, the annual number of deaths among women due to legal induced abortion has ranged from two to 12.
The annual number of reported deaths from induced abortions (legal and illegal) tended to be higher in the 1980s, when it ranged from nine to 16, and from 1972 to 1979, when it ranged from 13 to 63. One driver of the decline was the drop in deaths from illegal abortions. There were 39 deaths from illegal abortions in 1972, the last full year before Roe v. Wade. The total fell to 19 in 1973 and to single digits or zero every year after that. (The number of deaths from legal abortions has also declined since then, though with some slight variation over time.)
The number of deaths from induced abortions was considerably higher in the 1960s than afterward. For instance, there were 119 deaths from induced abortions in 1963 and 99 in 1965 , according to reports by the then-U.S. Department of Health, Education and Welfare, a precursor to the Department of Health and Human Services. The CDC is a division of Health and Human Services.
Note: This is an update of a post originally published May 27, 2022, and first updated June 24, 2022.

Sign up for our weekly newsletter
Fresh data delivered Saturday mornings
Key facts about the abortion debate in America
Public opinion on abortion, three-in-ten or more democrats and republicans don’t agree with their party on abortion, partisanship a bigger factor than geography in views of abortion access locally, do state laws on abortion reflect public opinion, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .

IMAGES
VIDEO
COMMENTS
BIO P069 Dandruff Hair Study (New Groups Added + Referral Bonus $) Posted February 12, 2024 . 03/26/2024. ... CRL Research Labs 371 Hoes Lane Suite 100 Piscataway NJ 08854 732-562-1010 [email protected]. Quick links. New Referral Bonus! Facebook Notifications;
NJ 08854 Piscataway USA Phone: 001-732-981-1616 Fax: 001-732-981-0520 ... www.crlresearchlabs.com. Get Directions. From . Eurofins CRL, Inc. was first incorporated in 1992 as Clinical Research Laboratories, Inc. Today, Eurofins CRL, Inc. operates as a contract laboratory dedicated to providing a wide range of in vitro and in vivo clinical ...
Colgate-Palmolive is opening the Volpe Clinical Research Center in Piscataway, NJ, which will accelerate development of the Company's cutting-edge technology and product formulas, fulfilling our purpose to reimagine a healthier future for all. ... Colgate conducts over 200 clinical studies each year with the help of academic partners ...
The University of Tennessee Health Science Center in Memphis, TN has a research opportunity for people to participate in the study: Pain Sensitivity and Unpleasantness in People with Alzheimer's Disease and Cancer. Specifically, they are looking for individuals over the age of 60 for 1 of 3 groups: (1) those ….
The Company focuses its clinical expertise in the areas of dermatology, photobiology, ophthalmology, bioinstrumentation, microbiology, cosmetology, clinical trials, and associated services. ECRL operates a 22,000 square foot clinical testing facility with approximately 75 employees in Piscataway, NJ along with two additional facilities in ...
Eurofins CRL Inc is a premier product testing facility located in Piscataway, N.J. Here at Eurofins CRL, we conduct product tests for cosmetics, toiletries, fragrances and other products manufactured by the world's foremost cosmetics companies. Most of the time, you'll be offered panels that test moisturizers, makeup, hair coloring, nail ...
NJ 08854 Piscataway USA Phone: 001-732-981-1616 Fax: 001-732-981-0520 ... www.crlresearchlabs.com. Get Directions. From . Eurofins CRL, Inc. was first incorporated in 1992 as Clinical Research Laboratories, Inc. ... bioinstrumentation, microbiology, cosmetology, clinical trials, and associated services. Services: *Safety/Clinical Trials. HRIPT
Clinical trials for Piscataway, New Jersey. ... Healthy Volunteers for Cancer Treatment Study - Secaucus NJ: Healthy Volunteers for Parkinson's Disease Study 18 to 45 - Secaucus NJ ... ClinicalConnection.com is a resource that provides information on clinical trials and other research opportunities worldwide.
The Rutgers Cancer Institute of New Jersey Clinical Research Program is organized based on disease teams, mainly related to specific diagnoses. Some of the Disease Specific Groups are more thematic: Early Drug Development (Phase 1), Precision Medicine, and Cellular Therapies. Each disease team reviews available clinical trials or develops their own trials to meet the clinical needs of patients ...
Specialties: Join us at Clinical Research Laboratories! Here at CRL, Panelists receive compensation to participate in a variety of different clinical studies that involve testing new products from numerous clients. Established in 1992. Clinical Research Laboratories (CRL) was incorporated in 1992. Today, CRL operates as a GCP certified contract laboratory providing a wide range of clinical ...
RBHS boasts five Clinical Research Units (CRUs), located in New Brunswick, Piscataway and Newark. As Core services, the CRUs provide Rutgers investigators with access to trained clinical research clinicians as well as clinical space to conduct study visits and procedures. As part of the overall mission to improve the efficiency and quality of ...
RBHS boasts five Clinical Research Units (CRUs), located in New Brunswick, Piscataway and Newark. ... the CRUs provide Rutgers investigators with access to trained clinical research clinicians as well as clinical space to conduct study visits and procedures. Clinical Research Unit ... New Jersey Alliance For Clinical and Translational Science ...
Clinical trials are research studies that require the participation of both patients and healthy volunteers. Ultimately, clinical studies expand the treatment options of patients by providing access to the most promising therapies and advanced technologies. ... New Jersey Alliance For Clinical and Translational Science (NJ ACTS) Contact RU ...
Advanced Clinical Research Program; Masters Courses. ... STUDY Debt-free. Sollers supports you with student-friendly financial options that clear a path for your future. KNOW MORE. EXPERIENCE Real-world. ... 33 Wills Way Building, 9 Piscataway, NJ 08854. PHONE. IT Admission: (848) 279-1736.
Eurofins CRL, Inc. is a highly specialized clinical testing lab focused on the evaluation of topical antiseptics such as hand sanitizers, surgical site preps, and surgical scrubs. Previously the ECRL Texas location operated as Vivo Clinical Testing, a joint venture between Microchem Laboratory based in Round Rock, TX and Clinical Research ...
Eurofins CRL Inc 371 Hoes Lane, Suite #100, Piscataway, NJ 08854 Phone: 732-981-1616 - Fax: 732-981-0520
Colgate-Palmolive opened the Volpe Clinical Research Center in Piscataway on June 1. The new clinical research and innovation testing center is named after Colgate-Palmolive's vice president of ...
606 Clinical Research jobs available in Piscataway, NJ on Indeed.com. Apply to Clinical Research Coordinator, Clinical Research Associate, Clinical Study Manager and more! ... Clinical Operations, duties and responsibilities relative to the management of Clinical Research studies will include, but will not be limited to:
3,598 Clinical Research Clinical Research jobs available in Piscataway, NJ on Indeed.com. Apply to Clinical Research Coordinator, Clinical Research Associate, Clinical Technician and more! ... Piscataway, NJ 08854. ... Participate in clinical research studies as needed.
CRL has completed over 20,000 clinical studies for more than 300 different clients. CRL routinely conducts studies in safety, efficacy and claims substantiation. Contact information: Suncare Research Laboratories, LLC [email protected] Voice: (336) 725-6501. Clinical Research Laboratories, LLC [email protected] Voice: (732) 981-1616.
Above: Gottheimer at Cooperman Barnabas Medical Center. LIVINGSTON, NJ — Today, March 19, 2024, U.S. Congressman Josh Gottheimer (NJ-5) announced new legislation, the Securing Equal Access to Research, Care, and Health, or SEARCH Act, to help fund new efforts to increase the number of women in clinical research trials, leading to treatments and cures for rare diseases and blood disorders ...
The CDC says that in 2021, there were 11.6 abortions in the U.S. per 1,000 women ages 15 to 44. (That figure excludes data from California, the District of Columbia, Maryland, New Hampshire and New Jersey.) Like Guttmacher's data, the CDC's figures also suggest a general decline in the abortion rate over time.