
An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

NIA Glossary of Clinical Research Terms
Adverse Event (AE) – Any untoward or unfavorable medical occurrence in a clinical research study participant, including any abnormal sign (e.g. abnormal physical exam or laboratory finding), symptom, or disease, temporally associated with the participants’ involvement in the research, whether or not considered related to participation in the research.
Baseline – The initial time point in a clinical trial that provides a basis for assessing changes in subsequent assessments or observations. At this reference point, measurable values such as physical exam, laboratory tests, and outcome assessments are recorded.
Bias – A point of view or preference which prevents impartial judgment in the way in which a measurement, assessment, procedure, or analysis is carried out or reported.
Case Report Form (CRF) – A printed, optical, or electronic (eCRF) document designed to capture all protocol-required information for a study.
Coordinating Center (CC) – A group organized to coordinate the planning and operational aspects of a multi-center clinical trial. CCs may also be referred to as Data Coordinating Centers (DCCs) or Data Management Centers (DMCs).
Clinical Research or Study Coordinator (CRC) – An individual that handles the administrative and day-to-day responsibilities of a clinical trial and acts as a liaison for the clinical site. This person may collect the data or review it before it is entered into a study database.
Clinical Research – NIH defines clinical research as:
- Patient-oriented research. Research conducted with human subjects (or on material of human origin such as tissues, specimens and cognitive phenomena) for which an investigator directly interacts with human subjects. Excluded from this definition are in vitro studies that utilize human tissues that cannot be linked to a living individual. Patient-oriented research includes: (a) mechanisms of human disease, (b) therapeutic interventions, (c) clinical trials, or (d) development of new technologies.
- Epidemiologic and behavioral studies .
- Outcomes research and health services research.
Clinical Trial – NIH defines a clinical trial as a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. Clinical trials are used to determine whether new biomedical or behavioral interventions are safe, efficacious, and effective. Behavioral clinical trials involving an intervention to modify behavior (diet, physical activity, cognitive therapy, etc.) fit this definition of a clinical trial.
Concomitant Medication – Prescription and over-the-counter drugs and supplements a study participant has taken along with the study intervention. This information may be collected as a history item as well as during the study. Some studies may collect only those medications that may interact with the study or intervention or that may exclude an individual from participating in a study.
Conflict of Interest – A conflict of interest occurs when individuals involved with the conduct, reporting, oversight, or review of research also have financial or other interests, from which they can benefit, depending on the results of the research. Control Group – The group of individuals in a clinical trial assigned to a comparison intervention.
Controlled Clinical Trial – A clinical trial in which at least one group of participants is given a test intervention, while at least one other group concurrently receives a control intervention.
Data Management – The processes of handling the data collected during a clinical trial from development of the study forms/CRFs through the database locking process and transmission to statistician for final analysis.
Data Management Plan (DMP) – A plan that documents the processes for handling the flow of data from collection through analysis. Software and hardware systems along with quality control and validation of these systems, as relevant are described.
Data and Safety Monitoring Board (DSMB) –A group of individuals independent of the study investigators that is appointed by the NIA to monitor participant safety, data quality and to assess clinical trial progress.
Data and Safety Monitoring Plan (DSMP) – Plan included with the grant application for clinical trials which establishes the overall framework for data and safety monitoring, how adverse events will be reported to the IRB and the NIH and, when appropriate, how the NIH Guidelines and FDA regulations for INDs and IDEs will be satisfied.
Efficacy – Indication that the clinical trial intervention produces a desired therapeutic effect on the disease or condition under investigation.
Eligibility Criteria – List of criteria guiding enrollment of participants into a study. The criteria describe both inclusionary and exclusionary factors, (e.g. inclusion criterion - participants must be between 55 and 85 years old; exclusion criterion – must not take drug X three month prior to the study). Food and Drug Administration (FDA) – An agency within the U.S. Department of Health and Human Services (DHHS) responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs, biological products, medical devices, nation’s food supply, cosmetics, and products that emit radiation.
Good Clinical Practice – A standard for the design, conduct, performance, monitoring, auditing, recording, analyses, and reporting of clinical trials that provides assurance that the data and reported results are credible and accurate, and that the rights, integrity, and confidentiality of trial participants are protected.
Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule – The first comprehensive Federal protection for the privacy of personal health information. The Privacy Rule regulates the way certain health care groups, organizations, or businesses, called covered entities under the Rule, handle the individually identifiable health information known as protected health information (PHI).
Human Subject – A patient or healthy individual who is or becomes a participant in research, either as a recipient of the intervention or as a control.
Informed Consent – A process by which a participant or legal guardian voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the participant’s decision to take part in the clinical trial. Informed consent is usually documented by means of a written, signed, and dated informed consent form, which has been approved by an IRB/IEC .
Informed Consent Form – A document that describes the rights of a study participant and provides details about the study, such as its purpose, duration, required procedures, and key contacts. Risks and potential benefits are explained in the informed consent document. Institutional Review Board (IRB)/Independent Ethics Committee (IEC) – An independent body constituted of medical, scientific, and nonscientific members whose responsibility it is to ensure the protection of the rights, safety, and well-being of human subjects involved in a trial by, among other things, reviewing, approving, and providing continuing review of trials, protocols and amendments, and of the methods and material to be used to obtaining and documenting informed consent of the trial participant.
Intervention – A procedure or treatment such as a drug, nutritional supplement, gene transfer, vaccine, behavior or device modification that is performed for clinical research purposes.
Investigational New Drug Application (IND) – An IND is a request for authorization from the Food and Drug Administration (FDA) to administer an investigational drug or biological product to humans. Such authorization must be secured prior to interstate shipment and administration of any new drug or biological product that is not the subject of an approved New Drug Application or Biologics/Product License Application (21 CFR 312).
Masking/Blinding – A procedure in which the investigator administering the assessments and intervention as well as the participants in a clinical trial are kept unaware of the treatment assignment(s). Single blinding usually refers to the study participant(s) being unaware, and double blinding usually refers to the study participant(s) and any of the following being unaware of the treatment assignment(s): investigator(s), monitor, and data analyst(s).
Manual of Procedures (MOP) – A set of procedures describing study conduct. A MOP is developed to facilitate consistency in protocol implementation and data collection across study participants and clinical sites.
N ew Drug Application (NDA) – An application submitted by the manufacturer of a drug to the FDA, after the clinical trial has been completed, for a license to market the drug for a specified indication.
Observational Study Monitoring Board (OSMB) – The safety and data monitoring body for observational studies with large or vulnerable populations or risks associated with tests or standard of care.
Office for Human Research Protection (OHRP) – A federal government agency within the Department of Health and Human Services (DHHS) charged with the protection of human subjects participating in government funded research. It issues assurances and oversees compliance of regulatory guidelines by research institutions.
Open-Label Trial – A clinical trial in which investigators and participants know which intervention is being administered.
Pharmacokinetics – The process (in a living organism) of absorption, distribution, metabolism, and excretion of a drug or vaccine.
Phase I – clinical trials to test a new biomedical intervention in a small group of people (e.g., 20-80) for the first time to evaluate safety (e.g., to determine a safe dosage range and to identify side effects). It can include healthy participants or patients.
Phase II – clinical trials to study the biomedical or behavioral intervention in a larger group of people (several hundred) to determine efficacy and to further evaluate its safety. It is conducted in participants with the condition or disease under study and will determine common short-term side effects and risks.
Phase III – studies to investigate the efficacy of the biomedical or behavioral intervention in large groups of human subjects (from several hundred to several thousand) by comparing the intervention to other standard or experimental interventions as well as to monitor adverse effects, and to collect information that will allow the intervention to be used safely.
An NIH-defined Phase III clinical trial is a broadly based prospective Phase III clinical investigation, usually involving several hundred or more human subjects, for the purpose of evaluating an experimental intervention in comparison with a standard or controlled intervention or comparing two or more existing treatments. Often the aim of such investigation is to provide evidence leading to a scientific basis for consideration of a change in health policy or standard of care. The definition includes pharmacologic, non-pharmacologic, and behavioral interventions given for disease prevention, prophylaxis, diagnosis, or therapy. Community trials and other population-based intervention trials are also included.
Phase IV – studies conducted after the intervention has been marketed. These studies are designed to monitor effectiveness of the approved intervention in the general population and to collect information about any adverse effects associated with widespread use.
Placebo – A placebo is an inactive pill, liquid, powder, or other intervention that has no treatment value. In clinical trials, experimental treatments are often compared with placebos to assess the treatment's effectiveness.
Placebo Controlled Study – A method of investigation in which an inactive substance/treatment (the placebo) is given to one group of participants, while the test article is given to another group. The results obtained in the two groups are then compared to see if the investigational treatment is more effective in treating the condition.
Protocol – A document that describes the objective(s), design, methodology, statistical consideration, and organization of a trial.
Protocol Amendments – A written description of a change(s) to or formal clarification of a protocol.
Protocol Deviations – Failure to conduct a study as described in the protocol. The failure may be accidental or due to negligence and in either case, the protocol deviation should be documented. This also includes failure to comply with federal laws and regulations, the institution's commitments and policies, and standards of professional conduct and practice. Examples of noncompliance include:
- failure to obtain/maintain approval for research,
- failure to obtain informed consent when required,
- failure to file adverse event reports,
- performance of an unapproved study procedure,
- performance of research at an unapproved site,
- failure to file protocol modifications and
- failure to adhere to an approved protocol.
Protocol Deviations Report – Internal document created as part of the ongoing quality control process summarizing compliance with the protocol and listing protocol deviations and/or violations.
Prospectively Assigned – A pre-defined process (e.g., randomization) specified in an approved protocol that stipulates the assignment of research subjects (individually or in clusters) to one or more arms (e.g., intervention, placebo or other control) of the clinical trial.
Quality Assurance (QA) – Systematic approach to ensure that the data are generated, documented (recorded), and reported in compliance with the protocol and good clinical practice (GCP) standards.
Quality Control (QC) – The internal operational techniques and activities undertaken within the quality assurance system to verify that the requirements for quality of trial related activities have been fulfilled (e.g., data and form checks, monitoring by study staff, routine reports, correction actions, etc.).
Randomization – The process of assigning clinical trial participants to treatment or control groups using an element of chance to determine the assignments in order to reduce bias.
Recruitment Plan – The plan that outlines how individuals will be recruited for the study and how the study will reach the recruitment goal.
Retention Plan – The plan that details the methods in which the study will use in order to retain study participation in the clinical trial.
Safety Monitoring Plan – A plan that outlines the oversight of a clinical trial.
Safety Officer (SO) – An independent individual, often a clinician who is appointed by the NIA and performs data and safety monitoring activities in low-risk, single site clinical studies. The SO advises the NIA regarding participant safety, scientific integrity, and ethical conduct of a study. The SO is advisory to the Institute Director.
Screening Log – An essential document that records all individuals who entered the screening process. The screening log demonstrates the investigator’s attempt to enroll a representative sample of participants.
Screening Process – A process designed to determine individual’s eligibility for participation in a clinical research study.
Serious Adverse Event (SAE) – Any adverse event that:
- Results in death
- Is life threatening, or places the participant at immediate risk of death from the event as it occurred
- Requires or prolongs hospitalization
- Causes persistent or significant disability or incapacity
- Results in congenital anomalies or birth defects
- Is another condition which investigators judge to represent significant hazards
Source Document – Original documents, data, and records (e.g., hospital records, clinical and office charts, laboratory notes, memoranda, participant diaries, recorded data from automated instruments, x-rays, etc.) that are used in a clinical trial.
Standard Operating Procedure (SOPs) – Detailed written instructions to achieve uniformity of the performance of a specific function across studies and patients at an individual site.
Stopping Rules –Established safety criteria that would either pause or halt a study due to reasons including but not limited to futility or risk(s) to the participants.
Stratification – Separation of a study cohort into subgroups or strata according to specific characteristics such as age, gender, etc., so that factors which might affect the outcome of the study, can be taken into account.
Unanticipated Problems (UAPs) – Unanticipated problems involving risks to subjects or others, which meet all of the following criteria:
- Unexpected in terms of nature, severity, or frequency;
- Related or possibly related to participation in the research, and;
- Suggests that the research placces subjects or others at a greater risk of harm (including physical, psychological, economic, or social harm) than was previously known or recognized.
Unmasking/Unblinding – A procedure in which one or more parties to the trial are made aware of the treatment assignment(s).
Unanticipated Adverse Device Effects (UADEs) – Any serious adverse effect on health or safety or any life-threatening problem or death caused by, or associated with, a device, if that effect, problem, or death was not previously identified in a nature, severity, or degree of incidence in the investigational plan or application (including a supplementary plan or application) or any other unanticipated serious problem associated with a device that relates to the rights, safety, or welfare of subjects.
Glossary Sources:
Clinical Trials.gov NINDS Glossary of Clinical Research Terms CenterWatch, Inc. Patient Resources: Glossary. OHRP website NIH Definitions Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials (3 ed.). Missouri: Mosby-Year Book Inc., 1996. Meinert CL. Clinical Trials: Design, Conduct, and Analysis . New York: Oxford University Press, Inc., 1986.
Return to the NIA Clinical Research Investigator's Toolbox .
Related Content

Approved Concepts

Grants & Funding

Research Resources
nia.nih.gov
An official website of the National Institutes of Health
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, glossary of common terms, clinical research.
Clinical research is medical research that involves people to test new treatments and therapies.
Clinical Trial
A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
Healthy Volunteer
A Healthy volunteer is a person with no known significant health problems who participates in clinical research to test a new drug, device, or intervention.
Inclusion/Exclusion Criteria
Inclusion/Exclusion Criteria are factors that allow someone to participate in a clinical trial are inclusion criteria . Those that exclude or not allow participation are exclusion criteria .
Informed Consent
Informed consent explains risks and potential benefits about a clinical trial before someone decides whether to participate.
Patient Volunteer
A patient volunteer has a known health problem and participates in research to better understand, diagnose, treat, or cure that disease or condition.
Phases of Clinical Trials
Clinical trials are conducted in “phases.” The trials at each phase have a different purpose and help researchers answer different questions.
- Phase I trials — An experimental drug or treatment in a small group of people (20–80) for the first time. The purpose is to evaluate its safety and identify side effects.
- Phase II trials — The experimental drug or treatment is administered to a larger group of people (100–300) to determine its effectiveness and to further evaluate its safety.
- Phase III trials — The experimental drug or treatment is administered to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or equivalent treatments.
- Phase IV trials — After a drug is licensed and approved by the FDA researchers track its safety, seeking more information about its risks, benefits, and optimal use.
A placebo is a pill or liquid that looks like the new treatment but does not have any treatment value from active ingredients.
A Protocol is a carefully designed plan to safeguard the participants’ health and answer specific research questions.
Principal Investigator
A Principal Investigator is a doctor who leads the clinical research team and, along with the other members of the research team, regularly monitors study participants’ health to determine the study’s safety and effectiveness.
Randomization
Randomization is the process by which two or more alternative treatments are assigned to volunteers by chance rather than by choice.
Single- or Double-Blind Studies
Single- or double-blind studies (also called single- or double-masked studies) are studies in which the participants do not know which medicine is being used, so they can describe what happens without bias. In single-blind ("single-masked") studies, you are not told what is being given, but the research team knows. In a double-blind study, neither you nor the research team are told what you are given; only the pharmacist knows. Members of the research team are not told which participants are receiving which treatment, in order to reduce bias. If medically necessary, however, it is always possible to find out which treatment you are receiving.
Types of Clinical Trials
- Diagnostic trials determine better tests or procedures for diagnosing a particular disease or condition.
- Natural history studies provide valuable information about how disease and health progress.
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning.
- Quality of life trials (or supportive care trials) explore and measure ways to improve the comfort and quality of life of people with a chronic illness.
- Screening trials test the best way to detect certain diseases or health conditions.
- Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery or radiation therapy.
This page last reviewed on April 20, 2023
Connect with Us
- More Social Media from NIH
- Conference Coverage
- CRO/Sponsor
- 2023 Salary Survey

- Publications
- Conferences
CDISC Glossary of Clinical Research Terminology
- Helle Gawrylewski
- Steve Raymond
Applied Clinical Trials
Reference tool is a Rosetta Stone for clinical research. A look at the updates and enhancements in latest version.
The world of clinical research includes participants from many sectors: the pharmaceutical industry, government agencies, academia, healthcare providers, subjects, patients, technology providers, and many others. While there may be the perception that a lingua franca has evolved through common usage, this is not the case. We may find ourselves using common terminology, yet the strict definitions and interpretations of these words often differ markedly. This is not due solely to differences in native language or culture, but to the multiple “authoritative sources” of clinical research applicable definitions, including FDA, EMA, ICH, WHO, International Organization for Standardization (ISO), and specialty groups that code and use terminology for healthcare, payers, hospitals, and other stakeholders. While, in principle, the Glossary addresses global use, the primary sources of definitions are based on those derived from U.S. and European standards organizations and regulatory authorities.
Add to this scenario the need to ensure that information provided is both human- and machine-readable-a concept called “interoperability.” This really was the genesis point for CDISC-evolving, of necessity, as a standards organization at the beginning of the age of electronic data collection, analysis, and submission to regulatory authorities in support of new drug applications for marketing approval.
The long-standing Mission Statement for CDISC’s Glossary Group-the longest-serving working group under the CDISC umbrella (convening for the first time in 2002)-echoes these origins:
CDISC Glossary seeks to harmonize definitions (including acronyms, abbreviations, and initials) used in the various standards initiatives undertaken by CDISC in clinical research. Glossary also serves the community of clinical researchers by selecting and defining terms pertaining to clinical research, particularly eClinical investigations, sponsored by the pharmaceutical industry or a federal agency.
Recently, it has become apparent that the clarity and comprehension provided by the Glossary supports colocation and translation of the concepts in the many countries where research is done, and the resulting products are used.
Thus, the Glossary is intended to serve both novice and experienced users as an authoritative advisory resource in the context of clinical research and development. To accomplish this, it was necessary to identify recognized sources of definitions, clarify differing contextual interpretations, and, as much as possible, harmonize across a global landscape of word usage. It should be emphasized that the Glossary Group is not a standards development body but, rather, one that has developed an aid to better understanding the terminology.
Occasionally, the members of the group made modifications to definitions in order to better represent the most common use or, when no authoritative source was identified, defined the term, based on their extensive collective experience in the conduct of clinical studies; collection, analysis, and interpretation of data derived from those studies; and long-standing roles in the development of CDISC models for protocols, data standards, and collaboration with regulatory authorities worldwide.
The CDISC Glossary Version 11.0 adds to the four groups of new terms included in last year’s update: Milestones, eSource, Transparency, and Clinical Trial & Clinical Study. This year’s Glossary expands the Outcomes Assessment (and eCOA) and endpoints; and includes some new terms from ISO for the identification of medicinal products (IDMP), the ICH E6 update, and elements from the Common Protocol. The NIH-FDA BEST (Biomarkers, Endpoints, and other Tools) Resource, which contains FDA-NIH harmonized terms used in translational science, is included the Glossary’s Reference Citations section. Specific terms are included in this year’s update.
An additional technical update made this year is that the CDISC glossary content will be integrated into the NCI Thesaurus (NCIt; ncit.nci.nih.gov), an open source, publicly available biomedical coding terminology developed by the U.S. National Cancer Institute’s Enterprise vocabulary services (NCI-EVS).
Examples of operational issues addressed in the course of the Glossary development include:
• Providing useful resources beyond the term definition . Although the focus of the Glossary is to define commonly-used terms, the group determined that there would be great added value in providing links to associated information. These allow the user to further explore context and nuance that cannot be accommodated in the Glossary itself. Table 1 shows an example of the Excel table.
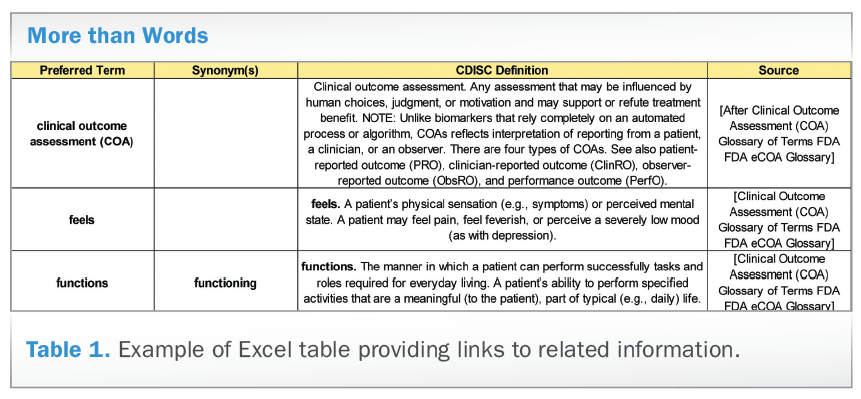
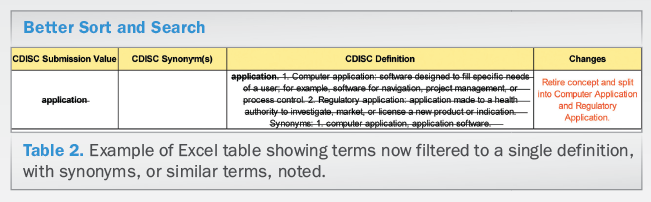
• Multiple interpretations. On occasion, there were multiple legitimate definitions that needed to be considered. These definitions usually differed due to associated context. Thus, in order to recognize this, we provided these definitions with explanatory notes that conveyed the rationale and appropriate use. Older versions of the Glossary were organized like a dictionary of terms, without supporting the sorting and searching utilities that enhance review of a tabular format. This year, for the first time, the Glossary and its many formats can be filtered and the terms are unique (see Table 2). Each unique term has a single definition and synonyms, or similar terms, are noted.
Examining the High Cost of the Drug Development Process
The drug development process, beginning with preclinical research up to when the product goes to market, requires time, risk, and high costs.
Clinical Trial Results Show Krystexxa Reduced Blood Pressure in Adults With Chronic Gout
Pegloticase (Krystexxa; Amgen) is approved to treat chronic gout in adults who fail to normalize serum uric acid and whose signs and symptoms are inadequately controlled with xanthine oxidase inhibitors.

Defining Quality Tolerance Limits and Key Risk Indicators that Detect Risks in a Timely Manner: Reflections from Early Adopters on Emerging Best Practices (Part 3)
Series Part 3—Methods for early detection of risk and summary.
Defining Quality Tolerance Limits and Key Risk Indicators that Detect Risks in a Timely Manner: Reflections from Early Adopters on Emerging Best Practices (Part 2)
Series Part 2—The process of defining QTLs.
Defining Quality Tolerance Limits and Key Risk Indicators that Detect Risks in a Timely Manner: Reflections from Early Adopters on Emerging Best Practices (Part 1)
Series Part 1—Introduction and the relationship between QTL and KRI.

Salary Survey: The Age of COVID
With large shift to decentralized strategies, industry roles appear set for change.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- Health Literacy Home

Clinical Research Glossary
- Best Practices
Using plain language definitions can make research materials easier to read and understand.
In Spring 2020, the MRCT Center launched a small pilot project with patients and participants and other stakeholders across the research industry, to develop plain language definitions of clinical research words.
The resulting Clinical Research Glossary is now available for use!
You can find other potentially helpful glossaries here .

Don’t forget about your verbs!
Phrasal verbs like “turn in your survey” can be confusing. What it means to “turn in” is often not clear and may be better stated as “submit” or “return” . Guidance and appropriate substitutions can be found here .
Welcome to the SOM Clinical Research Glossary
Quickly look up the meaning of words, acronyms, or abbreviations commonly used in clinical research:
- Narrow your search by typing the word you are looking for in the search box. Clear the search box to start a new search.
- Use the alphabet bar for quick filtering of words by first letter or select a word category to explore words that are related. Remove the letter or word filters by clicking the respective ALL button.
Return to the glossary index page after each word search using the « Back to Glossary Index link.
- Adverse Drug Reaction (ADR)
- Adverse Event (AE)
- Adverse Reaction (AR)
- Age of majority
- AIR (Activities Interests and Relationships)
- ALCOA (Attributable, Legible, Contemporaneous, Original, Accurate)
- ALCOA Plus, ALCOA+
- Ancillary review
- Applicable regulatory requirements
- Approved drug
- Aspirational benefit
- Association for Clinical and Translational Science (ACTS)
- Association for the Accreditation of Human Research Protection Programs (AAHRPP)
- Association of Clinical Research Professionals (ACRP)
- Audit report
- Audit trail
- Baseline assessment
- Billing Coverage Analysis (BCA)
- Biological Product
- Biological specimen
- Biologics License Application (BLA)
- Biomedical Research Imaging Center (BRIC)
- Biospecimen
- Biospecimen Processing Facility (BSP)
- Breach of confidentiality
- Budget justification
- Budget period
- Budget revision
- Business Associate
- Business Associate Agreement (BAA)
- Carolina Data Warehouse for Health (CDW-H)
- Case Report Form (CRF)
- Ceded review
- Centers for Disease Control and Prevention (CDC)
- Centers for Medicare & Medicaid Services (CMS)
- Centralized monitoring
- Certificate of Confidentiality (CoC)
- Certified copy
- Clinical and Translational Research Center (CTRC)
- Clinical and Translational Science Awards (CTSA) Program
- Clinical development
- Clinical investigation
- Clinical research
- Clinical Research Accountability Unit (CRAU)
- Clinical Research Associate (CRA)
- Clinical Research Coordinator (CRC)
- Clinical Research Management System (CRMS)
- Clinical Research Support Office (CRSO)
- Clinical significance
- Clinical study
- Clinical trial
- Clinical Trial Agreement (CTA)
- ClinicalTrials.gov
- Clinical Trials Quality Assurance (CTQA) Program
- Code of Federal Regulations (CFR)
- Co-investigator
- Collaborative Institutional Training Initiative (CITI)
- Collateral benefit
- Commercial Institutional Review Board (IRB)
- Common data model
- Common Rule
- Common Terminology Criteria for Adverse Events (CTCAE)
- Compensation
- Competitive Renewal
- Computable Phenotype
- Concomitant medication
- Confidential Disclosure Agreement (CDA)
- Confidentiality
- Conflict of Interest (COI)
- Conflict of Interest Office
- Consent capacity
- Continuing noncompliance
- Continuing review
- Contract Research Organization (CRO)
- Control group
- Coordinating Center (CC)
- Corrective and Preventive Action (CAPA) Plan
- Data acquisition
- Data and Safety Monitoring Board (DSMB)
- Data and Safety Monitoring Committee (DSMC)
- Data and Safety Monitoring Plan (DSMP)
- Database Management System (DBMS)
- Data encryption
- Data Integrity
- Data management
- Data Management and Sharing Plan (DMS Plan)
- Data Management Plan (DMP)
- Data Management System (DMS)
- Data Monitoring Committee (DMC)
- Data Use Agreement (DUA)
- Delegation of Authority (DOA) Log
- Demographic data
- Department of Health and Human Services (DHHS)
- Direct benefit
- Direct cost
- Disapproval
- Discontinue
- Disease registry
- Dosage regimen
- Dose Limiting Toxicity (DLT)
- Double blinding
- Drug toxicity
- ECRT (Effort Certification and Reporting Technology)
- Electronic Case Report Form (eCRF)
- Electronic Data Capture (EDC)
- Electronic Health Record (EHR)
- Electronic Informed Consent (eIC)
- Electronic Medical Record (EMR)
- Elements of informed consent
- Eligibility criteria
- Embryonic Stem Cell Research Oversight (ESCRO) Committee
- Encounter Level Data
- Environment, Health and Safety (EHS)
- Essential document
- Exclusion criteria
- Exculpatory language
- Exempt review
- Expanded access
- Expedited review
- Experimental drug
- Experimental group
- Export Compliance Office
- Export control
- Fabrication
- Falsification
- Family Educational Rights and Privacy Act (FERPA)
- FDA Form 482
- FDA Form 483
- FDA Form 1571
- FDA Form 1572
- Feasibility assessment
- Federalwide Assurance (FWA)
- Food and Drug Administration (FDA)
- Food, Drug and Cosmetics Act
- Free text data
- Full board review
- Generalizability, Generalization
- Good Clinical Practice (GCP)
- Grant application
- Grant Number
- Greater than minimal risk
- Health literacy
- Healthy volunteer
- HIPAA authorization
- HIPAA covered entity
- HIPAA (Health Insurance Portability and Accountability Act)
- HIPAA Privacy Rule
- Humanitarian Device Exemption (HDE)
- Humanitarian Use Device (HUD)
- Human Research Protection Program (HRPP)
- Human subject
- Human Subjects Research (HSR)
- Identifiable biospecimen
- Identifiable private information
- Impartial witness
- Inclusion criteria
- Inclusion/Exclusion (I/E) criteria
- Independent Ethics Committee (IEC)
- Independent IRB
- Indirect benefit
- Indirect Facility & Administrative (F&A) costs
- Individual Conflict of Interest (COI)
- Industry Contracting (IC)
- Industry sponsored study
- Informed Consent Form (ICF)
- Informed Consent (IC)
- Institution
- Institutional Biosafety Committee (IBC)
- Institutional Conflict of Interest (COI)
- Institutional Integrity and Risk Management (IIRM)
- Institutional Official (IO)
- Institutional Privacy Office (IPO)
- Institutional Privacy Officer
- Institutional Review Board Information System (IRBIS)
- Institutional Review Board (IRB)
- Interaction
- International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9CM)
- International Council for Harmonisation (ICH)
- Intervention
- Interventional study
- Investigation
- Investigational device
- Investigational Device Exemption (IDE)
- Investigational drug
- Investigational Drug Service (IDS)
- Investigational New Drug (IND)
- Investigational New Drug (IND) safety report
- Investigational plan
- Investigational product (IP)
- Investigator
- Investigator agreement
- Investigator-initiated study
- Investigator's Brochure (IB)
- IRB application
- IRB approval
- IRB Authorization Agreement (IAA)
- IRB expiration
- IRB of record
- Just-in-Time (JIT)
- Key information
- Legally Authorized Representative (LAR)
- Legally effective informed consent
- Letter of intent
- Life-threatening adverse event
- Limited dataset
- Lineberger Comprehensive Cancer Center (LCCC)
- Local considerations
- Longitudinal study
- Long Term Follow-Up (LTFU)
- Lost to Follow Up (LTFU)
- Manual of procedures (MOP)
- Material transfer agreement (MTA)
- Medical device
- Medical record
- Medical Record Number (MRN)
- Memorandum of understanding (MOU)
- Minimal risk
- Monitoring plan
- Monitoring report
- Multicenter study
- Multisite study
- National Institutes of Health (NIH)
- New Drug Application (NDA)
- NIH National Library of Medicine (NLM)
- NIH Public Access Policy
- No Cost Extension (NCE)
- Nonclinical study
- Noncompliance
- Nondisclosure agreement (NDA)
- Nonsignificant Risk (NSR) medical device
- Nonsignificant Risk (NSR) medical device study
- North Carolina Translational and Clinical Sciences (NC TraCS) Institute
- Not Human Subjects Research (NHSR)
- Notice of Award (NOA)
- Notice of privacy practices
- Not reasonably available (as it applies to locating a parent)
- Observational study
- Observational Study Monitoring Board (OSMB)
- Office for Human Research Protections (OHRP)
- Office for Innovation, Entrepreneurship and Economic Development (IEED)
- Office of Clinical Trials (OCT)
- Office of Human Research Ethics (OHRE)
- Office of Research Communications (ORC)
- Office of Research Development (ORD)
- Office of Research Information Systems (ORIS)
- Office of Research (OoR)
- Office of Research Support and Compliance (ORSC)
- Office of Sponsored Research (OSR)
- Office of Technology Commercialization (OTC)
- Office of the Vice Chancellor for Research (OVCR)
- Office of University Counsel (OUC)
- Off-label use
- On-site monitoring
- Open-label trial
- Organizational Conflict of Interest (COI)
- Package insert
- Parental permission
- Participant
- Participant engagement
- Participant partners
- Participating site
- Patient Centered Outcomes Research Network (PCORnet)
- Patient level data
- Patient registry
- Personnel Profile and Training System (PaTS)
- Pharmacogenomics
- Pharmacokinetics (PK)
- Pilot study
- Placebo effect
- Plain language
- Possibly related to the research
- Pragmatic trial
- Preclinical research
- Principal Investigator (PI)
- Privacy board
- Privacy Rule
- Private information
- Program announcement
- Program officer
- Promptly Reportable Information (PRI)
- Prospective study
- Protected Health Information (PHI)
- Protocol amendment
- Protocol deviation
- Protocol Review Committee (PRC)
- PubMed Central (PMC)
- Quality Assurance (QA)
- Quality Control (QC)
- Quality Improvement (QI)
- Questionnaire
- Radiation Safety Committee (RSC)
- Radiation Safety Subcommittee (RSS)
- Radioactive Drug Research Committee (RDRC)
- RAMSeS (Research Administration Management System & electronic Submission)
- Randomization
- Reasonable possibility
- Recruitment
- Recruitment period
- Recruitment plan
- Recruitment status
- REDCap (Research Electronic Data Capture)
- Registry study
- Regulatory binder
- Reliance agreement
- Relying Institution
- Research administrator
- Research Compliance Program (RCP)
- Research Coordination & Management Unit (RCMU)
- Research misconduct
- Research personnel
- Research plan
- Research registry
- Research study
- Research team
- Research team members
- Responsible conduct of research
- Retention plan
- Retrospective study
- Reviewing IRB
- Reviewing IRB Institution
- Risk-based monitoring
- Root Cause Analysis (RCA)
- Safety and Security Committee
- Schedule of activities (SOA)
- Schedule of Assessments
- School of Medicine (SOM)
- Scientific Review Committee (SRC)
- Screen failure
- Screening log
- Sensitive Information (SI)
- Serious Adverse Event (SAE)
- Serious and Unexpected Suspected Adverse Reaction (SUSAR)
- Serious noncompliance
- Short form consent
- Side effects
- Significant Risk (SR) medical device
- Significant Risk (SR) medical device study
- Single blinding
- Single IRB (sIRB)
- Site Initiation Visit (SIV)
- Site investigator
- Site Master File (SMF)
- Society of Clinical Research Associates (SoCRA)
- SOM Conflict of Interest Committee (COIC)
- Source data
- Source documents
- Sponsored Program Office (SPO)
- Sponsored Research Agreement (SRA)
- Sponsor-investigator
- Standard of Care (SOC)
- Standard Operating Procedure (SOP)
- Stopping rules
- Stratification
- Structured data
- Study Coordinator (SC)
- Study design
- Study documentation
- Study monitor
- Study population
- Sub-investigator
- Subject binder
- Subject chart
- Subject identification number/code
- Suspected Adverse Reaction
- Target enrollment
- Termination
- Test article
- Therapeutic misconception
- Tolerability
- Trial Master File (TMF)
- Unanticipated Adverse Device Effect (UADE)
- Unanticipated Problem Involving Risk to Subjects or Others (UPIRSO)
- UNC Health Network Entities
- UNC Health Privacy Office
- Underserved population
- Unexpected adverse event
- University of North Carolina at Chapel Hill
- Vulnerable populations
- Washout Period
- Work Instruction (WI)
We appreciate your input!
- UNC Chapel Hill
Clinical Research Glossary
Expand your clinical research word power with the som clinical research glossary.
Clinical research has a highly specialized vocabulary with a vast number of acronyms and abbreviations that help facilitate scientific communication. Whether you are new to clinical research or an experienced member of the research team, understanding and remembering the nuances of clinical research vocabulary can be challenging.
To ensure a consistent and clear clinical research communication, the CRSO has created the SOM Clinical Research Glossary , a comprehensive clinical research glossary in clear and plain language that aligns with industry and regulatory standards. As the glossary is intended to be broadly applicable to various types of research and audiences, its definitions provide nuances of usage. If there are variations in how key agencies are defining a term, each definition is provided.
We need your feedback
The SOM Clinical Research Glossary is intended to be a living resource that will be periodically updated with new terms and clarifications. Please let us know, if there is a word, acronym, or abbreviation you see regularly in clinical research that is not included in the glossary or if you want to propose an alternative definition.
- Description
- Knowledge Base
The Glossary effort was launched in 2002 to support other CDISC standards by clarifying and disambiguating key concepts in clinical research. Over time, the volunteers who serve on the Glossary team have enhanced its scope and utility by:
- When filling out the new term request form, please choose ‘CDISC-Glossary’ from the Codelist drop-down list
- Improving integration with terminology teams: Controlled Terminology Guiding Principles include expectation that the Glossary is an essential reference
- Harmonizing with terms developed by the CDISC Protocol Entities group
- Reviewing terms for accurate NCI C-codes and unique definitions
- Adding “clusters” to link groups of related terms and to highlight distinctions and similarities
- Proposing definitions of terms used in CDISC Standards, but not defined, like exposure, General Observation Classes.
The Glossary now includes more than 750 terms and is published in the National Cancer Institute’s Enterprise Vocabulary Services (NCI-EVS) on an NCI File Transfer Protocol (FTP) site in six formats: Excel , text , odm.xml , pdf , html, and OWL/RDF .
The Glossary team has worked with NCI-EVS to link Glossary terms with Controlled Terminology concepts. Each year, the team updates 50 - 100 existing terms, reviews new guidances and current topics for terms, and updates acronyms/abbreviations/initials, if needed. In addition, authoritative sources for definitions are provided as a tab in the Excel file for ease of use.

- Autoimmunity
- Immune system and PI
- Types of PI
- Laboratory tests
- Newborn screening
- Genetic testing
- Immunoglobulin replacement therapy
- Hematopoietic stem cell transplantation
- Gene therapy
- The immune system and genetics
- Think Zebra

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
Learn more about PI
- Addressing mental health
- Explaining your diagnosis
- Navigating flu season
- PI and COVID
- Peer and group support
- Advocating for your child
- Communicating with schools
- Caregiver mental health
- Managing workplace issues
- Choosing a plan
- Glossary of terms
- Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
Learn more about living with PI
- Corporate partnerships
- Walk for PI
- DIY fundraising
- Giving Circle
- Legacy giving
- Sustainer's Club
- Tribute gifts
- Workplace giving
- Share your story

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
Get involved today
- IDF surveys
- Participating in clinical trials
- Diagnosing PI
- Consulting immunologist
- Clinician education

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
View resource details
- Locate a PI specialist
- Find events
Ask the expert: PI and fatigue
Dr. Joud Hajjar provides an explanation of the causes and potential treatments for symptoms of fatigue with primary immunodeficiency.
The information, terminology, and opinions presented in this forum do not necessarily reflect the views of IDF, its Board of Trustees, sponsors, or donors.
TRANSCRIPT: primaryimmune.org/sites/default/fi…ue%20podcast.pdf
View a pdf of this presentation: primaryimmune.org/sites/default/fi…-IDF-Fatigue.pdf
Your Situation
- I might have PI
- I care for someone with PI
This page contains general medical and/or legal information that cannot be applied safely to any individual case. Medical and/or legal knowledge and practice can change rapidly. Therefore, this page should not be used as a substitute for professional medical and/or legal advice.
Related resources

Newly diagnosed kit for kids with PI

Newly diagnosed kit for adults with PI
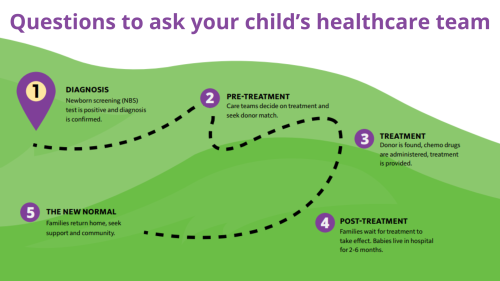
SCID: Questions for your child's healthcare team
Sign up for updates from idf.
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.

P: 410-321-6647

The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309
- Terms of Use & Policies

Feasibility and Acceptability of a Mobile App for Prolonged Grief Disorder Symptoms
Background: Mobile apps provide a unique platform for mental health assessment and monitoring. They can provide real time, accessible data on symptoms of mental disorders that may yield rich data for detailed clinical assessment and help individuals gain insight into their current mental state. We developed one of the first apps for tracking symptoms of prolonged grief disorder. Method: In this pilot feasibility study, we assess the feasibility and acceptability of a new mobile app mGAGE for use once a day for 3 weeks. 27 participants completed mental health assessments at t1 and t2. Results: Adherence to the app protocol was very high with 100% for the first two weeks of use. A surprising finding was the improvement of grief symptoms at t2. Debriefing interviews revealed general qualitative categories including positive feedback, negative feedback and specific recommendations. Overall, the app was found to be feasible for use for the first two weeks and acceptable for bereaved individuals. Conclusions: This app could provide valuable data for in depth clinical assessment, may support individuals to gain greater insight into their symptoms and may have a therapeutic effect in terms of improved grief symptoms. Implications for future studies including use in larger intervention studies are discussed.
Most read articles by the same author(s)
- Clare Killikelly, Mariia Merzhvynska, Ningning Zhou, Eva-Maria Stelzer, Philip Hyland, Jose Rocha, Menachem Ben-Ezra, Andreas Maercker, Examination of the New ICD-11 Prolonged Grief Disorder Guidelines Across Five International Samples , Clinical Psychology in Europe: Vol. 3 No. 1 (2021)
- Louisa Lorenz, Andreas Maercker, Rahel Bachem, The 12-Month Course of ICD-11 Adjustment Disorder in the Context of Involuntary Job Loss , Clinical Psychology in Europe: Vol. 2 No. 3 (2020)
- Eva Heim, Sebastian Burchert, Mirëlinda Shala, Marco Kaufmann, Arlinda Cerga Pashoja, Naser Morina, Michael P. Schaub, Christine Knaevelsrud, Andreas Maercker, Announcement of the Registered Report "Effect of Cultural Adaptation of a Smartphone-Based Self-Help Programme on its Acceptability and Efficacy" , Clinical Psychology in Europe: Vol. 2 No. 3 (2020)
- Clare Killikelly, Andreas Maercker, The Cultural Supplement: A New Method for Assessing Culturally Relevant Prolonged Grief Disorder Symptoms , Clinical Psychology in Europe: Vol. 5 No. 1 (2023)

COMMENTS
Glossary of Common Research Terms Term Definition Abstract This is a brief summary of a research study and its results. It should tell you why the study was done, how the researchers went about it and what they found. Action Research Action research is used to bring about improvement or practical change. A group of people who know about a
the research being conducted; and • Stress that enrolling in, and staying in, a clinical study is completely voluntary. Because giving consent to participate in research is not a contract, participants may leave a study at any time. The goal of the informed consent process is to protect participants. It begins when a potential
Clinical Research or Study Coordinator (CRC) - An individual that handles the administrative and day-to-day responsibilities of a clinical trial and acts as a liaison for the clinical site. This person may collect the data or review it before it is entered into a study database. Clinical Research - NIH defines clinical research as: Patient ...
Used in RevMan analyses to test the statistical signifi cance of the heterogeneity statistic. Clinical investigator A medical researcher in charge of carrying out a clinical trial ' s protocol. Clinical trial A research study to answer specifi c questions about new therapies or new ways of using known treatments.
health problems who participates in clinical research to test a new drug, device, or intervention.3 • Patient Volunteer: a person who has a known health problem and participates in research to better understand, diagnose, treat, or cure that disease or condition.3 Terms Related to Analyzing Clinical Trial Data
Dissemination is about making sure that the findings from a research study reach those who can benefit from them. It involves: Planned active efforts to communicate relevant research messages, in a timely way, to identified targeted audiences through appropriate channels. The researcher actively spreading key research messages.
Clinical Research. Clinical research is medical research that involves people to test new treatments and therapies. Clinical Trial. A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
commonly misunderstood research terms, followed by a comprehensive literature review and analysis of the identified research term s to create a research terminology consensus paper. The goal was not to create a comprehensive glossary of commonly understood research terms but to provide clarity on some of the common approaches to nursing research.
Glossary also serves the community of clinical researchers by selecting and defining terms pertaining to clinical research, particularly eClinical investigations, sponsored by the pharmaceutical industry or a federal agency. ... the Glossary has been updated to a PDF-based tabular format with searchable functionality. Terms can be sorted and ...
In Spring 2020, the MRCT Center launched a small pilot project with patients and participants and other stakeholders across the research industry, to develop plain language definitions of clinical research words. The resulting Clinical Research Glossary is now available for use! You can find other potentially helpful glossaries here. VISIT NOW.
encompass all the research that is carried out in the clinical setting (e.g., health services research). Clinical Research Associate (CRA) Person employed by the study sponsor or CRO to monitor a clinical study at all participating sites. See also, monitor. Clinical Research Coordinator (CRC) Site administer for the clinical study.
Pharmacokinetics (PK). The study of the processes of bodily absorption, distribution, metabolism, and excretion (ADME) of compounds and medicines. Pharmacovigilance. A European term similar to adverse event monitoring and reporting, increasingly used in the United States because of international harmonization efforts.
Short List of Commonly Used Acronyms in Clinical Research . ACRP Association of Clinical Research Professionals AE Adverse Event ADR Adverse Drug Reaction AMA American Medical Association BAA Business Associate Agreement BIND Biological IND CA Coverage Analysis CAP College of American Pathologists ...
Welcome to the SOM Clinical Research Glossary Quickly look up the meaning of words, acronyms, or abbreviations commonly used in clinical research: Narrow your search by typing the word you are looking for in the search box. Clear the search box to start a new search. Use the alphabet bar for quick filtering of words […]
Expand your clinical research word power with the SOM Clinical Research Glossary Clinical research has a highly specialized vocabulary with a vast number of acronyms and abbreviations that help facilitate scientific communication. Whether you are new to clinical research or an experienced member of the research team, understanding and remembering the nuances of clinical research … Read more
A variable that pertains to the efficacy or safety evaluations of a trial. An endpoint is more specific as compared to an outcome since it relate to the planned objective of the study. Enrollment ...
clinical research handbook will be available for physicians and PIs starting in January 2021. This. clinical handbook starts by discussing various ways for the clinical studies to be organized and. executed, including a step-by-step approach to research documentation while managing. regulatory and ethical concerns in research.
The Glossary effort was launched in 2002 to support other CDISC standards by clarifying and disambiguating key concepts in clinical research. Over time, the volunteers who serve on the Glossary team have enhanced its scope and utility by: ... (NCI-EVS) on an NCI File Transfer Protocol (FTP) site in six formats: Excel, text, odm.xml, pdf, html, ...
Clinical Research Basics Clinical Trials and Observational Studies Clinical research, a type of medical research, uses human volunteers. The terminologies of clinical research and clinical trials are often used interchangeability, with some researchers often using a narrow de nition of clinical research as being clinical trials. Clinical research
A clinical trial where groups of volunteers are administered two or more interventions in a specific order. For example, a "two-by-two" cross-over trial design is where one group receives drug A at the beginning of the trial and then receives drug B for the rest of the trial. In the second group, participants receive drug B first and then ...
Good Clinical Research Practice (GCP) is a process that incorporates established ethical and scientifi c quality standards for the design, conduct, recording and reporting of clinical research involving the participation of human subjects. Compliance with GCP provides public assurance that the rights, safety, and well-being of research
Clinical research may be defined as investigations involving human subjects or the use of patient samples. The scientific practices described here are generally accepted by investigators conducting both multi-center and single-institution clinical studies and help ensure both the quality and integrity of scientific findings in clinical research.
Description. Clinical research is vital for advancement in medicine, yet in most medical specialties, and in many countries, its tools are used inappropriately, resulting in invalid results. Furthermore, many clinicians cannot critically evaluate research findings. The purpose of our program is to offer a highly interactive learning environment ...
The IDF Podcast · Ask the expert: PI and fatigue. Dr. Joud Hajjar provides an explanation of the causes and potential treatments for symptoms of fatigue with primary immunodeficiency. The information, terminology, and opinions presented in this forum do not necessarily reflect the views of IDF, its Board of Trustees, sponsors, or donors.
Background: Mobile apps provide a unique platform for mental health assessment and monitoring. They can provide real time, accessible data on symptoms of mental disorders that may yield rich data for detailed clinical assessment and help individuals gain insight into their current mental state. We developed one of the first apps for tracking symptoms of prolonged grief disorder.