Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 07 January 2021

Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance
- Darren Shu Jeng Ting ORCID: orcid.org/0000-0003-1081-1141 1 , 2 na1 ,
- Charlotte Shan Ho ORCID: orcid.org/0000-0003-1131-1448 2 na1 ,
- Rashmi Deshmukh 2 ,
- Dalia G. Said 1 , 2 &
- Harminder S. Dua ORCID: orcid.org/0000-0002-4683-6917 1 , 2
Eye volume 35 , pages 1084–1101 ( 2021 ) Cite this article
20k Accesses
170 Citations
22 Altmetric
Metrics details
- Corneal diseases
Epidemiology
- Risk factors
A Correction to this article was published on 11 May 2021
This article has been updated
Corneal opacity is the 5th leading cause of blindness and visual impairment globally, affecting ~6 million of the world population. In addition, it is responsible for 1.5–2.0 million new cases of monocular blindness per year, highlighting an ongoing uncurbed burden on human health. Among all aetiologies such as infection, trauma, inflammation, degeneration and nutritional deficiency, infectious keratitis (IK) represents the leading cause of corneal blindness in both developed and developing countries, with an estimated incidence ranging from 2.5 to 799 per 100,000 population-year. IK can be caused by a wide range of microorganisms, including bacteria, fungi, virus, parasites and polymicrobial infection. Subject to the geographical and temporal variations, bacteria and fungi have been shown to be the most common causative microorganisms for corneal infection. Although viral and Acanthamoeba keratitis are less common, they represent important causes for corneal blindness in the developed countries. Contact lens wear, trauma, ocular surface diseases, lid diseases, and post-ocular surgery have been shown to be the major risk factors for IK. Broad-spectrum topical antimicrobial treatment is the current mainstay of treatment for IK, though its effectiveness is being challenged by the emergence of antimicrobial resistance, including multidrug resistance, in some parts of the world. In this review, we aim to provide an updated review on IK, encompassing the epidemiology, causative microorganisms, major risk factors and the impact of antimicrobial resistance.
角膜混浊是全球致盲和视力障碍的第五大原因, 世界范围内大约600万人受其影响。此外, 角膜混浊每年导致的单眼失明约150-200万例, 突显其对人类健康造成的持久性负担。在感染、创伤、炎症、变性和营养缺乏等所有的致病因素中, 感染性角膜炎 (IK) 是发达国家和发展中国家角膜病致盲的主要原因, 大概每100000人口中2.5-799人罹患此病。IK可由多种微生物引起, 包括细菌、真菌、病毒、寄生虫和多重感染。受地理和时间变化的影响, 细菌和真菌已被证明是角膜感染最常见的病原微生物。虽然病毒性角膜炎和棘阿米巴角膜炎并不常见, 但在发达国家, 它们是角膜病致盲的重要原因。接触镜的佩戴、外伤、眼表疾病、眼睑疾病以及眼部手术已证实是IK的主要危险因素。广谱抗生素是目前IK治疗的主要选择, 但是在世界某些地区, 其有效性正在受到抗菌药物耐药性的挑战, 其中包含多重耐药性等。本篇综述旨在提供有关IK的最新进展, 包括流行病学、病原微生物、主要危险因素以及抗生素耐药性对于疗效的影响。
Similar content being viewed by others
Analysis of microbial keratitis incidence, isolates and in-vitro antimicrobial susceptibility in the East of England: a 6-year study
Malik Moledina, Harry W. Roberts, … James Myerscough

Predisposing factors, microbiological features and outcomes of patients with clinical presumed concomitant microbial and herpes simplex keratitis
Maria Cabrera-Aguas, Pauline Khoo, … Stephanie L. Watson
Infectious keratitis: trends in microbiological and antibiotic sensitivity patterns
Mohammad Soleimani, Seyyed Ali Tabatabaei, … S. Saeed Mohammadi
Introduction
Corneal opacity represents the 5th leading cause of blindness globally, accounting for ~3.2% of all cases [ 1 ]. The recent World Health Organisation (WHO) report highlighted that ~6 million of the world population are affected by cornea-related blindness or moderate/severe visual impairment, including 2 million of those who are affected by trachoma [ 1 , 2 ]. In addition, corneal opacity is estimated to be responsible for 1.5–2.0 million cases of unilateral blindness annually, highlighting an ongoing unchecked burden on human health [ 3 , 4 ].
Any significant insult to the cornea such as infection, trauma, inflammation, degeneration, or nutritional deficiency can result in corneal opacity with visual impairment. Among all, infectious keratitis (IK) has been shown to be the most common cause for corneal blindness in both developed and developing countries [ 5 ]. According to a nationwide study, IK was shown to be the most common cause of all corneal blindness in China, primarily attributed to increased risk of trauma, low socioeconomic status and illiteracy [ 6 ]. IK is a common yet potentially vision-threatening ophthalmic condition, characterised by acute ocular pain, decreased vision, corneal ulceration, and/or stromal infiltrates [ 5 ]. Previously, it has been recognised as a “silent epidemic” in the developing world [ 3 ], and recently, a consortium-led proposal has suggested the designation of IK as a “neglected tropical disease (NTD)” [ 7 ], adding on to the list of NTDs in ophthalmology (i.e. trachoma, onchocerciasis and leprosy). The proposal to attain status of an NTD aims to draw concerted global effort to tackle IK in under-resourced tropical countries, to ameliorate the societal and humanistic burden of IK.
IK can be caused by a wide variety of pathogens including bacteria, fungi, protozoa and viruses. In addition, polymicrobial infection has shown to be accountable for ~2–15% of all IK cases [ 8 , 9 , 10 , 11 ]. As the ocular surface is equipped with highly regulated innate and adaptive defense mechanisms [ 12 ], IK rarely occurs in the absence of predisposing factors such as contact lens (CL) wear, trauma, ocular surface diseases (OSDs), and post-corneal surgery, which are some of the common risk factors implicated in IK [ 13 ].
IK not only causes visual impairment, but also negatively impacts on the quality of life (QOL) of the affected individuals. A study from Uganda reported that IK affected both vision-related QOL (attributed to vision loss) and health-related QOL (attributed to pain in the acute phase) [ 14 ]. The psychological impact on these patients was related to the fear of losing the eye and the social stigma attached. Even when the visual recovery was complete, the individuals affected by IK displayed a lower QOL score than the unaffected controls [ 14 ]. Apart from the impact on the individuals which can affect their economic productivity, IK is also responsible for a huge economic burden on society. According to a report in 2010, the US spent an estimated 175 million dollars on the treatment of IK [ 15 ]. Furthermore, complications of IK such as corneal perforations and scarring form the major indications of corneal transplants in developing countries such as India, Thailand and China [ 13 ], placing additional burden on the limited pool of donor corneas.
Considering that most parts of the world affected by IK are under-resourced, it is highly likely that the actual burden of IK is underestimated due to the lack of surveillance and under-reporting. In view of the global burden of IK, this review aims to provide an updated and comprehensive overview of the epidemiology, causative microorganisms, risk factors and the impact of antimicrobial resistance in relation to IK.
B.1. Incidence
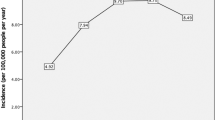
To date, there are limited studies available in the literature that examined the incidence of IK and the majority of studies were conducted more than a decade ago [ 5 ]. Depending on the geographical location and study design, the incidence of IK has been estimated to be in the range of 2.5–799 cases per 100,000 population/year [ 16 , 17 ], particularly more prevalent in the low-income countries. Previous IK studies reported an estimated incidence of 2.5–27.6 per 100,000 population-year in the US [ 16 , 18 ] and 2.6–40.3 per 100,000 population-year in the UK [ 19 , 20 ]. Our recent Nottingham IK Study concurred with the findings of these older studies. We observed a relatively stable incidence of 34.7 per 100,000 population-year in Nottingham, UK, between 2007 and 2019 [ 8 ], highlighting a persistent burden of IK in the developed countries. Another recent study conducted in Australia similarly demonstrated a low IK incidence of 6.6 per 100,000 population-year during the period of 2005–2015 [ 21 ]. However, it is noteworthy that the incidence reported in these two studies is likely to be underestimated as the numbers were based on IK patients who underwent corneal scraping.
In contrast, a substantially higher rate of IK has been reported in under-resourced countries such as South India (113 per 100,000 population-year) [ 22 ] and Nepal (799 per 100,000 population-year) [ 17 ]. The higher incidence observed in these regions was primarily attributable to the poorer environmental and personal hygiene, lower level of education, agricultural industry, increased risk to work-related corneal trauma and poorer access to sanitation and healthcare facility.
The epidemiological patterns and risk factors have been found to vary with demographic factors such as age, gender and socioeconomic status. A tabulated summary of the demographic factors and microbiological profiles of IK is provided in Table 1 [ 8 , 9 , 10 , 13 , 21 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ].
IK has been shown to affect individuals across all age groups. Based on large-scale studies (>500 patients), IK most commonly affected people aged between 30 and 55 years (Table 1 ) [ 8 , 9 , 10 , 13 , 21 , 24 , 25 , 29 , 31 , 35 , 37 , 39 , 42 , 43 ], primarily attributed to the underlying risk factors such as CL wear and ocular trauma associated with the working age group. Patients affected by trauma-related IK secondary to agricultural products and foreign bodies are usually around 45–55 years old [ 18 , 44 ]. The employed workforce of some developing countries is mainly composed of farmers and manual labourers, rendering them more susceptible to IK of traumatic aetiology [ 13 , 45 ]. On the other hand, patients affected by CL-related IK are usually between 25 and 40 years old [ 18 , 44 , 46 , 47 ].
Although prevalence of IK is generally low in the extremes of age [ 18 , 48 , 49 , 50 , 51 ], IK may serve as a major contributor to childhood blindness in some countries. For instance, IK was shown to be the second most common cause of visual impairment in children aged <15 years in Uganda [ 52 ]. Ophthalmia neonatorum, defined as conjunctivitis occurring in newborns within 28 days of life, is another important cause of childhood corneal blindness in developing countries, particularly when it is affected by Neisseria gonorrhoea where bilateral ocular involvement is common [ 4 ].
In addition, some studies have demonstrated that elderly patients affected by IK were associated with poor visual outcome (around 40–75% with visual acuity of <6/60) and higher rate of complications such as corneal melting, perforation and loss of eye (i.e. evisceration or enucleation) [ 11 , 53 , 54 ]. This might be related to the higher rate of ocular co-morbidities and the delay in presentation and/or diagnosis of IK as elderly patients are usually dependent on spouse or family when seeking medical care and they may relate their condition to “normal” age-related changes [ 55 , 56 ].
B.3. Gender
The majority of studies did not observe any gender predilection in IK (Table 1 ). However, when gender difference or predominance exists, it is usually attributed to the underlying risk factors in different regions. For instance, CL-related IK has been shown to exhibit a female predominance of 57–69% [ 18 , 44 , 46 , 57 ], whereas trauma-related IK is associated with a male predominance of 74–78% [ 18 , 44 , 46 ], correlating with a high male prevalence (58–75%) of IK in the under-resourced regions such as South America [ 29 , 32 ], Asia [ 13 , 45 , 49 , 58 ], and Africa [ 51 , 59 , 60 ]. Interestingly, a study in Nepal [ 49 ] found that there are significantly more male than female patients across all the age groups. This might be due to a combination of higher rate of trauma, lower number of CL wear, and reduced opportunities among the females to access medical services due to cultural customs.
B.4. Socioeconomic status and level of education
Low socioeconomic status has been shown to increase the risk of developing IK, primarily attributed to poor education, lack of ocular protection and personal hygiene, and limited access to eye care in rural communities [ 6 , 13 , 45 , 51 , 61 ]. In Asia and Africa, amongst those who were diagnosed with IK, ~45–71% of the patients were illiterate and 62–79% of them resided in rural areas with a poorer access to healthcare facilities [ 51 , 60 , 62 ]. In addition, it was found that farmers, rural residents and illiterates were at a higher risk of refractory IK with poorer outcomes [ 51 ].
In some countries such as Nigeria and Malawi, residents in rural communities were shown to be more likely to self-medicate or approach village healers for traditional eye medicine [ 59 , 63 ]. Although it would be unfair to conclude that all therapies performed by traditional healers are inimical, common beliefs or practises of applying breast milk or plant products directly to the eye may actually worsen their keratitis [ 63 ]. In addition, patients who had prior use of traditional eye medicine tended to present later to the eye care professionals, resulting in delayed treatment and poorer visual outcome [ 63 ]. Another study conducted in Nepal reported almost half of the patients with keratitis did not use any medication, self-medicated or treated with undocumented medicine [ 61 ].
Causative microorganisms
A wide range of microorganisms, including bacteria, fungi, protozoa (particularly Acanthamoeba), and viruses, are capable of causing IK. Recently, Ung et al [ 5 ]. have provided a comprehensive summary of the literature concerning the causative microorganisms of IK (up to June 2018). In view of the recent growing literature, this section aimed to summarise the evidence based on large IK studies (>500 sample size) published during 2010–2020 (Table 1 ) [ 8 , 9 , 10 , 13 , 21 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ].
C.1. Bacteria
Bacteria are commonly categorised into Gram-positive and Gram-negative bacteria based on the difference in the compositions of bacterial cell envelope. In addition to the universal structure of inner/cytoplasmic membrane, Gram-positive bacteria possess a thick outer cell wall, which is composed of layers of peptidoglycan interspersed with teichoic acids and lipotechoic acids, whereas Gram-negative bacteria consist of a thin middle-layer peptidoglycan and an additional outer membrane primarily made of lipopolysaccharide, which has been shown to play an important role in the pathogenesis of infection (including IK) and the contribution to host inflammatory responses [ 64 , 65 ].
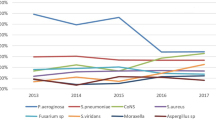
Bacterial keratitis represents the most common type of IK in most regions, including the UK (91–93%) [ 8 , 9 , 10 , 24 ], North America (86–92%) [ 25 ], South America (79–88%) [ 29 , 30 , 31 ], Middle East (91.8%) [ 42 ], and Australasia (93–100%) [ 21 , 43 ]. In terms of specific bacterial strains, coagulase negative staphylococci (CoNS), which are a group of common ocular commensal [ 66 ], were shown to be the most commonly isolated organisms (24–46%) in about half of the included studies [ 9 , 10 , 21 , 23 , 24 , 25 , 29 , 31 , 32 , 35 , 39 , 40 , 41 , 42 , 43 ]. Other common bacteria implicated in IK included S. aureus (5–36%) , Streptococci spp. (7–16%), Pseudomonas aeruginosa (5–24%), Enterobacteriaceae spp . (15%), Corynebacterium spp . (14%), and Propionibacterium spp . (9%; see Table 1 ). Over the past decade, there were several studies in the UK documenting a significant increase in Moraxella keratitis, which are often associated with longer corneal healing time [ 8 , 9 , 10 ]. Interestingly, Nocardia keratitis, a rare cause of IK, was identified as the third most common microorganism (11% of all cases) in the Steroids for Corneal Ulcers Trial (SCUT), and the outcome was found to be negatively influenced by the use of topical steroids [ 67 , 68 ]. Acid-fast bacilli such as non-tuberculous mycobacteria (NTM) serve as another important group of pathogens that are capable of causing IK [ 69 ]. NTM keratitis is commonly associated with refractive surgery and trauma, and it often requires prolonged and aggressive treatment for complete eradication, largely attributed to their propensity to form biofilms [ 69 , 70 ].
Fungi can be broadly divided into two categories, namely filamentous and yeast or yeast-like fungi. Filamentous fungi such as Fusarium spp . and Aspergillus spp . normally thrives in tropical climates whereas yeast-like fungi such as Candida spp. were more commonly observed in temperate regions [ 71 ]. Several studies have demonstrated that Fusarium spp . (13–24%) and Aspergillus spp . (8–30%) were the main causes of IK in Asia, particularly India and China (Table 1 ) [ 13 , 33 , 34 , 36 , 37 , 41 ]. In 2018, the Asian Cornea Society Infectious Keratitis Study (ACSIKS) included more than 6000 patients from eight Asian countries and re-confirmed the dominance of Fusarium spp . keratitis within China (26%) and India (31%) established two decades ago [ 72 , 73 , 74 ]. Although the prevalence of fungal keratitis in temperate regions such as the UK, Europe and North America was reportedly lower, the growth of yeast-like fungi such as Candida spp . is relatively common in patients with history of corneal transplantation or OSDs [ 44 ]. In view of the recent improvement in the diagnostic techniques, rare pathogens such as Cryptococcus curvatus , Arthrographis kalrae , Pythium spp ., and many others are increasingly being identified and reported as rare causes of fungal keratitis [ 75 , 76 , 77 ].
C.3. Protozoa
Acanthamoeba is a free-living protozoan that is found ubiquitously in the environment such as water, soil, air and dust [ 78 ]. Although not as common as bacterial or fungal keratitis, Acanthamoeba keratitis serves as another important cause of IK as it is often associated with prolonged treatment course and poor visual outcome [ 78 ]. It was estimated that Acanthamoeba keratitis affects 1–33 per million CL wearers per year [ 78 ]. In the UK, Carnt et al [ 79 ]. recently confirmed an outbreak of Acanthamoeba keratitis in the South East England during 2010–2016, with an approximately threefold increase compared to the preceding decade.
Based on recent large studies, Acanthamoeba keratitis accounts for ~0–5% of all IK (Table 1 ). Most of the Acanthamoeba keratitis were observed in CL wearer (71–91%) [ 32 , 60 , 80 ]. However, non-CL wearers can also develop this infection if their eyes are exposed to contaminated water, soil or dust, [ 81 , 82 ]. One of the Indian studies reported that only 4% of Acanthamoeba keratitis cases were associated with CL wear and the remainder were associated with trauma and/or exposure to contaminated water [ 82 ]. In addition, the clinical features of non-CL related Acanthamoeba keratitis may differ from CL-related cases [ 82 ]. Moreover, Acanthamoeba sclerokeratitis may manifest as a rare but difficult-to-treat clinical entity that is usually associated with poor clinical outcomes [ 83 ].
Microsporidial keratitis represents another type of parasitic IK that accounts for ~0.4% cases of all IK [ 84 ]. It is mainly observed in Asian countries and may manifest as superficial keratoconjunctivitis or stromal keratitis. It is commonly associated with ocular trauma, exposure to contaminated water/soil, and potentially acquired immunodeficiency syndrome [ 84 , 85 ].
C.4. Viruses
Viral keratitis, most commonly in the form of herpes simplex keratitis (HSK) and herpes zoster keratitis (HZK), represents a common cause of IK [ 86 , 87 ]. However, as viral keratitis cases are commonly treated based on their typical clinical appearance (e.g. dendritic corneal ulcer in HSK) and/or previous ocular history, the majority of cases did not require any microbiological investigation and hence were not captured in many IK studies. Nonetheless, the ACSIKS study demonstrated that viral keratitis represented the most common cause (46%) of IK in China, primarily attributed to HSK (24%) and HZK (17%) [ 13 ]. Another two studies, conducted in Egypt and China, respectively, observed that 15–21% of IK were caused by herpetic keratitis [ 51 , 58 ]. Based on these results, it is likely that viral keratitis represents an important and common cause of IK in many other regions, though further studies are required to elucidate this. Herpetic keratitis is often associated with neurotrophic keratopathy, which can result in poor corneal healing, increased risk of further IK and other corneal complications such as melting and perforation [ 86 , 88 ].
C.5. Polymicrobial infection
Polymicrobial keratitis (IK caused by two or more causative microorganisms) has been reported in around 2–15% of all IK cases [ 8 , 9 , 10 , 11 , 21 ]. Depending on the study design and the definition used, polymicrobial keratitis may include two or more types of organisms from the same category (e.g. bacteria-bacteria, fungus-fungus) or different categories (bacteria-fungus, fungus-protozoan). Polymicrobial keratitis often poses significant diagnostic and therapeutic challenges, and usually fares worse than monomicrobial keratitis [ 11 , 75 , 89 ]. Khoo et al [ 11 ]. observed that patients affected by polymicrobial keratitis (median of 6/60 vision) had a significantly worse visual outcome as compared to those affected by bacterial keratitis (median of 6/18 vision) or culture negative IK (median of 6/9 vision). In another retrospective comparative study, Lim et al [ 89 ]. demonstrated that medical therapy was sufficient to resolve all monomicrobial IK cases but only 81% of polymicrobial IK. In view of the relatively common occurrence of polymicrobial keratitis and variably low culture yield of current microbiological investigation, clinicians should always maintain a low threshold of repeating corneal scraping if patients are not responding to either antibacterial or antifungal therapy, even in the presence of positive culture results.
C.6. Seasonal variations
Pathogens are tremendously adaptive to climate and seasonality. Many studies have shown that IK was most prevalent during the summer season, with P. aeruginosa being one of the most frequently isolated microbes [ 34 , 90 , 91 ]. P. aeruginosa is a well-recognised organisms associated with environmental water as in swimming pools [ 92 ] and CL [ 44 , 46 , 48 , 93 , 94 ]. The seasonal predilection of IK during summer is attributed to the likely increased use of CL wear and engagement in water activities. On the other hand, several studies have shown that the incidence of fungal keratitis in India peaked during the windy and harvest seasons, primarily related to a higher risk of trauma secondary to agricultural activities and agricultural debris being blown in the eyes by the wind [ 34 , 62 ].
Seasonal variation was similarly observed in Acanthamoeba keratitis, though with conflicting results. Lin et al [ 34 ]. observed that Acanthamoeba keratitis occurred more commonly during summer in South India, potentially related to the higher temperature and increased risk of corneal trauma during windy seasons, whereas Walkden et al [ 91 ]. reported an increase in Acanthamoeba keratitis during the winter in the UK.
Major risk factors
In the majority of IK cases, local and/or systemic risk factors are usually present. The most common risk factors include CL wear, ocular trauma, OSDs (e.g. dry eye diseases (DEDs), neurotrophic keratopathy, rosacea, etc.), lid diseases, post-corneal surgery (e.g. keratoplasty, corneal cross-linking (CXL)), and systemic diseases (e.g. diabetes, immunosuppression), amongst others. A tabulated summary of large IK studies reporting the risk factors of IK is provided in Table 2 [ 11 , 13 , 18 , 29 , 32 , 35 , 44 , 45 , 46 , 48 , 49 , 50 , 51 , 58 , 59 , 60 , 61 , 62 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 ].
D.1. Contact lens (CL) wear
CL wear has been recognised as one of the most common risk factors of IK, particularly in developed countries. A study conducted in Northern California reported that the incidence of IK among CL wearers was ~9.3 times higher than the non-CL wearers (130.4 vs. 14.0 per 100,000 person-years) [ 18 ]. Based on the large studies (>200 patients) published in the recent literature, CL wear was shown to be the main predisposing factor (29–64%) of IK in developed countries like Portugal [ 48 ], France [ 93 ], Sweden [ 95 ], the US [ 18 , 44 , 97 ], Singapore[ 46 ] and Australia [ 11 ]. On the contrary, CL-related IK was considerably less common (0–18%) in developing countries due to less number of CL wearers [ 13 , 35 , 50 , 59 , 60 ], highlighting the geographical disparity in the risk factors as well as the causative microorganisms of IK between high income and low-income countries (Table 2 ).
The pathogenesis of CL-related IK is complex and multifactorial. Although it is commonly believed that CL-related IK is triggered by superficial injury secondary to CL wear, several studies had refuted this hypothesis as it was shown that the presence or absence of epithelial injury did not influence the risk or severity of IK [ 65 ]. Plausible mechanisms of CL-related IK include reduction of tear exchange during blinking (which leads to potential degradation of protective components at ocular surface), tear stagnation under CL (particularly soft CL) resulting in accumulation and adherence of microbes to the cornea, reduced corneal epithelial cell desquamation, and alteration of tear fluid biochemistry [ 65 ]. In addition, multiple predisposing factors of CL-related IK have been identified, including the types of CL used (higher risk in soft CL than rigid gas permeable CL), poor CL and CL case hygiene, overnight wear, use of expired CL, types of CL solution used, and CL being prescribed/dispensed by non-ophthalmologists or non-opticians [ 93 , 102 , 103 , 104 , 105 , 106 ]. Reports of IK secondary to the use of cosmetic lens and orthokeratology lens have also been highlighted [ 107 , 108 ].
In terms of underlying aetiologies, CL-related keratitis is most commonly associated with P. aeruginosa and Acanthamoeba spp ., which are both free-living microorganisms that are ubiquitously present in the environment, including water and CL solutions [ 47 ]. As noted above, Pseudomonas keratitis is one of the most common causes of IK, especially in the developed countries where there is increased prevalence of CL wear. Yildiz et al [ 102 ]. and Tong et al [ 46 ]. observed that P. aeruginosa was responsible for 63% and 70% of the CL-related IK, respectively. While Acanthamoeba keratitis is uncommon, most of these cases (71–91%) were observed in CL wearers [ 32 , 60 , 80 ]. Yu et al [ 32 ]. observed that more than 90% of the Acanthamoeba keratitis were associated with CL use. In a 32-year Brazilian study of over 6000 IK cases, Cariello et al [ 29 ]. reported that CL wearers had a 1.7 times higher risk of developing Acanthamoeba-positive culture than non-CL wearers. Interestingly, CL wear was also shown to be a major risk factor for fungal keratitis in a US study [ 44 ].
D.2. Trauma
Trauma serves as another common risk factor for IK in both developed and developing countries. Based on the IK studies reported in the literature, farmers (54–70%) and manual labour workers (11–17%) constituted the main occupations in Asia [ 13 , 45 , 49 , 51 , 58 , 59 , 109 ]. These groups of workers were at a high risk of developing IK due to the increased occupational exposure to plant materials and foreign bodies, which was frequently compounded by the lack of eye protection [ 45 , 51 , 58 , 98 , 109 ].
Fungal keratitis is by far the most common cause (47–83%) of trauma-related IK, especially in regions such as Asia and Africa which are dominated by agricultural communities [ 45 , 51 , 58 , 60 , 94 ]. Occupational exposures to vegetative matter, organic materials and animal products, predominantly in males in the working age group, are the main causes in these regions. The risk of fungal keratitis is further magnified by tropical climates, which are conducive to fungal growth [ 51 , 60 ]. Cariello et al [ 29 ]. observed that the risk of developing culture-proven fungal keratitis was increased by four times if the patients suffered from plant-related trauma. In addition, some studies demonstrated that trauma-related IK fared worse than non-traumatic cases [ 46 , 58 ]. Pan et al [ 58 ]. conducted a 10-year study in China and revealed that patients who presented with trauma-related IK were at a high risk of developing fungal keratitis and requiring surgical interventions (89%), including therapeutic keratoplasty and evisceration/enucleation.
On the other hand, the majority of trauma-related IK reported in European countries were caused by Gram-positive bacteria, including CoNS, S . aureus, Streptococci , and Corynebacterium [ 48 , 95 ]. These are common ocular surface commensals, which have the ability to tolerate hot and dry climates in temperate and sub-tropical zones [ 51 , 110 , 111 ]. Corneal trauma resulting from non-vegetative matter with consequent secondary opportunistic infection with ocular surface commensals could explain the high rate of Gram-positive infection in trauma-related IK in this region.
D.3. Ocular surface and eyelid diseases
Ocular surface diseases (OSDs), encompassing DEDs, blepharitis, neurotrophic keratopathy, Steven–Johnson syndrome, ocular cicatricial pemphigoid and bullous keratopathy, have been identified as one of the main risk factors for IK in both developed and developing countries [ 18 , 44 , 49 , 60 , 97 , 112 ]. OSD-related IK is most commonly caused by Gram-positive bacteria (around 60–80%) [ 11 , 60 , 95 , 112 ], which constitute the main group of ocular surface commensals. In particular, CoNS and S. aureus were shown to be the main culprits in OSD-related IK [ 95 , 112 ].
DED is the most common OSD that is characterised by “ a loss of tear film homeostasis with ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles ” [ 113 ]. The dysregulated ocular surface health can lead to breakdown of the corneal epithelium, a vital ocular surface defence, and ocular surface inflammation, consequently increasing the risk of IK [ 60 , 114 ].
Posterior blepharitis or meibomian gland disease (MGD) is a common eyelid disease, which is difficult to cure. It can lead to an array of ocular surface complications, including evaporative DED, marginal keratitis and IK, amongst others [ 115 ]. Meibomian gland abnormalities (e.g. gland dropout and hyperkeratinisation), alteration of the secreted lipid products, and the dysregulation of bacterial populations and their corresponding lipase or esterase activity are believed to contribute to the ocular surface inflammation and infection. In a 5-year Australian study, MGD was shown to be the most common cause (79%) of OSD implicated in IK [ 112 ]. In addition, nasolacrimal duct obstruction (NLDO) can also increase the risk of IK, primarily attributed to tear stagnation and reduction of tear exchange, resulting in the accumulation of microbes and debris on the ocular surface with increased risk of IK. Chidambaram et al [ 45 ]. showed that NLDO could increase the risk of fungal and bacterial IK, particularly S. pneumonia keratitis.
D.5. Post-ocular surgery
IK may occur following various ocular surgeries, including corneal transplant, refractive surgery, CXL, pterygium surgery, cataract surgery, and others [ 29 , 51 , 116 , 117 ]. Corneal transplant serves as the main sight-restoring surgery for a wide range of corneal diseases, though postoperative complications such as graft failure and IK may develop. In a retrospective study of over 2000 corneal transplants, Dohse et al [ 116 ]. reported an incidence of post-keratoplasty IK of 4%, with loose and broken sutures being reported as one of the most common risk factors (24%) [ 116 ]. Cariello et al [ 29 ]. demonstrated that 22% of the IK cases were associated with prior ocular surgery, particularly corneal graft (56%). In addition, the paradigm shift of penetrating keratoplasty to lamellar keratoplasty has created a new array of host-graft interface complications such as interface infectious keratitis (IIK), which often causes diagnostic and therapeutic challenges due to the deep-seated location of the infection [ 118 , 119 ]. We have recently highlighted a clinically challenging case of post-endothelial keratoplasty interface fungal keratitis, which required in vivo confocal microscopy for confirmatory diagnosis in the absence of positive culture results [ 118 ]. Fortunately the interface infection resolved quickly after the discontinuation of topical steroids and initiation of appropriate antifungal treatment.
Although IK rarely develops after refractive surgery, the significant amount of refractive surgeries performed globally render this an important clinical entity [ 120 ]. This was supported by a Brazilian study where refractive surgery was shown to be the second commonest surgery associated with IK [ 29 ]. Post-refractive surgery IK is most commonly caused by Gram-positive bacteria and NTM, though fungal and Acanthamoeba infection may also occur [ 120 ]. The high rate of Gram-positive bacterial IK following other types of ocular surgeries (e.g. cataract surgery, pterygium surgery) were also observed, most likely as a result of opportunistic infection secondary to ocular surface commensals [ 51 , 93 , 95 ].
In the recent years, CXL has emerged as a therapeutic modality for managing corneal ectactic conditions [ 121 , 122 ] and moderate-to-severe IK [ 123 , 124 , 125 ]. However, the intraoperative removal of corneal epithelium and postoperative insertion of bandage CL (which is the current standard practice in most institutes) can increase the risk of IK following CXL, particularly in patients with OSD such as vernal or atopic keratoconjunctivitis [ 117 , 126 , 127 ]. Post-CXL IK may be further complicated by the reactivation of herpetic keratitis [ 126 ] and manifestation of acute hydrops [ 127 ] and corneal melt/perforation [ 117 ].
D.6. Use of topical steroids
Steroids are commonly used in ophthalmology as a topical immunosuppressive/immunomodulatory agent to manage a wide range of intraocular and ocular surface inflammatory diseases, including DED, allergic eye disease, non-IK, chemical eye injury, cicatricial conjunctivitis and many others [ 128 , 129 ]. The recent SCUT study also demonstrated the benefit of adjuvant topical steroids in improving the visual outcome in patients with severe and central bacterial keratitis [ 67 ]. In addition to managing OSDs, topical steroids are also frequently used as postoperative topical treatment following intraocular and ocular surface surgeries, including corneal transplantation [ 130 ].
However, topical steroids can sometimes act as a double-edge sword. Studies have shown that topical steroids can increase the risk of IK, particularly fungal keratitis and/or polymicrobial keratitis [ 11 , 44 , 118 ]. In a study of 733 fungal keratitis, Keay et al [ 44 ]. reported that 13% of the cases were associated with chronic use of topical steroids. In addition, a study has shown that previous use of topical steroid could negatively impact on the clinical outcome of IK, with 73% ending with poor outcome (defined as worse than 6/60 vision, decreased vision during treatment, or perforation) [ 11 ]. While topical steroids serve as an effective treatment for stromal HSK, which is primarily an immune-related keratitis [ 131 ], its use can potentially exacerbate epithelial HSK and culminate in geographic ulcer [ 132 ]. Interestingly, an Indian study showed that 41% of the Acanthamoeba keratitis cases were associated with the use of topical steroid [ 45 ]. The high rate of prior steroid use might be related to the fact that Acanthamoeba keratitis often presents with non-specific corneal epithelial changes and is mismanaged as viral keratitis [ 104 ].
D.7. Systemic immunosuppression
Systemic immunosuppression, either secondary to diseases or immunosuppressive agents, has been shown to increase the risk of IK. Diabetes mellitus serves as one of the most important systemic risk factors for IK. Hyperglycaemia has been shown to facilitate microbial growth and alter the microbiota of ocular surface, including an upregulation of Pseudomonas spp . and Acinetobacter spp . [ 133 ], as well as affect the homeostasis, corneal sensation and wound healing of the corneal epithelium, thereby increasing the risk of IK [ 134 ]. Sub-basal corneal nerve plexus of patients with diabetic neuropathy is often affected and can lead to neuropathic keratopathy with complications such as corneal melt and IK [ 135 ].
Several large studies have highlighted the association between diabetes and IK (around 8–16%), particularly fungal and bacterial keratitis [ 45 , 58 , 60 , 136 , 137 ]. Zbiba et al [ 60 ]. observed that diabetes was relatively common in patients with bacterial keratitis (15%) and fungal keratitis (16%) as well as mixed bacterial and fungal keratitis (29%). In addition, viral keratitis was also reported to have a high prevalence amongst patients with diabetes [ 138 ]. Viruses, particularly HSV, are omnipresent in the general population, with an estimated prevalence of 1.5 per 1000 population [ 139 ]. Kaiserman et al [ 140 ]. demonstrated that patients with diabetes had a significantly higher incidence and recurrence rate of ocular surface herpetic eye diseases when compared to non-diabetic patients. Pan et al [ 58 ]. observed that 17% patients with diabetes had a substantially higher rate of HSK as compared to bacterial or fungal keratitis. Another study described that all patients with diabetes presented with IK were of viral origin, though the sample size was small [ 51 ]. The heterogeneity in the subtypes of microorganisms associated with diabetes observed in different studies was likely related to the disparity in the ocular predisposing factors of the studied cohort since more than one risk factor is often present in patients with IK [ 11 ].
Apart from diabetes, Jeng et al [ 18 ]. observed an approximately tenfold increased risk of IK in individuals affected by human immunodeficiency viruses compared to healthy individuals (238.1 vs. 27.6 per 100,000 population-year), highlighting the importance of host immunity in ocular surface defence. Intriguingly, a study demonstrated that 55% of the patients with HSK had a history of upper respiratory tract infection prior to the infection or recurrence [ 58 ]. This could be potentially explained by the mechanism linked to a host cell enzyme called heparanase [ 141 ], which is a known contributing factor to the pathogenesis of several viruses, including HSV, respiratory syncytial virus, human papilloma virus, and others. End-stage renal disease, particularly associated with diabetes, was also shown to be a risk factor for IK [ 142 ].
Antimicrobial resistance (AMR)
E.1. overview.
AMR has been recognised as a major public health crisis in the past two decades, with many infectious organisms developing resistance against previously effective antimicrobial agents [ 143 ]. The development of AMR is largely driven by a multitude of factors, including the overuse/abuse of antimicrobial agents in agricultural sectors due to commercial pressure, uncertainty in diagnosis (e.g. bacterial infection vs. viral infection) leading to inappropriate use of antibiotics, financial incentives for prescribing antibiotic, and use of non-prescription antibiotics among the general public, particularly in low- and middle-income countries [ 143 , 144 ]. From the genetic point of view, bacteria primarily develop AMR through two strategies, namely genetic mutational resistance and horizontal gene transfer. The genetic and mechanistic basis of AMR can be referred to a recent excellent review provided by Munita and Arias [ 144 ].
E.2. AMR in the context of IK
Broad-spectrum topical antibiotic therapy is the gold standard treatment for IK. Depending on the disease severity and clinicians’ preference, antibiotic therapy is commonly administered in the form of dual therapy using cephalosporin and aminoglycoside or monotherapy using fluoroquinolone [ 145 ]. As intensive topical antibiotics are applied directly and frequently during the treatment of IK, high concentration of antibiotics can be effectively achieved at the target site (i.e. the infected cornea), which could potentially reduce the risk of AMR in ocular infections. However, a few recent IK studies have highlighted the emergence of AMR in ocular infections, particularly in the US [ 28 ], China [ 41 ] and India [ 40 ]. The driving force is likely to be multifactorial, including the injudicious widespread use of antibiotics in both ocular and systemic infections [ 146 ], incorrect dosing regimen [ 147 ], and representations of the community prevalence of drug resistance, with consequent colonisation of ocular surface by drug resistant pathogens [ 148 ]. For instance, in the SCUT trial, there was a 3.5-fold higher MIC for bacteria isolated from patients who had previous treatment with fluoroquinolones compared to treatment naive patients [ 149 ].
A tabulated summary of the literature concerning the in vitro antibiotic susceptibility and resistance of IK-related bacteria is provided in Table 3 [ 8 , 9 , 21 , 24 , 25 , 26 , 28 , 31 , 35 , 38 , 40 , 41 , 42 , 43 , 150 ]. Overall, fluoroquinolone-resistant, methicillin-resistant and multidrug resistant (MDR; i.e. resistant to 3 or more antibiotics) infections are being increasingly reported in IK [ 28 , 31 , 35 , 40 , 41 , 150 ]. Geographical and temporal factors play a role in the variation of AMR pattern in ocular infections. Reports from Southern India demonstrated that MDR was commonly observed among S. pneumoniae (44%), S. epidermidis (14.8%), S. aureus (14%), and P. aeruginosa (6%). However, gatifloxacin—a fourth-generation fluoroquinolone—was effective against the majority of Gram-negative bacteria (~90%), including P. aeruginosa and Acinetobacter spp ., thus its use as a monotherapy in Gram-negative IK was recommended in that region [ 35 ]. Another study from Southern China similarly reported an increase in MDR among Gram-positive cocci from 2010 to 2018, while susceptibility to fluoroquinolone and aminoglycoside among Gram-negative bacilli remained stable [ 41 ]. In contrast, a Northern India study reported a high rate of resistance of P. aeruginosa against ciprofloxacin (57%), moxifloxacin (47%), and aminoglycoside (52–60%) [ 40 ], highlighting the geographical disparity in the AMR pattern and the importance of region-specific interrogation of the AMR profile in ocular infections.
An increasing trend of MRSA-related ocular infection has also been reported in several studies in the past decade [ 28 , 31 , 41 ]. The Antibiotic Resistance Among Ocular Microorganisms study in the US observed that a high rate of AMR, specifically methicillin resistance, was observed among Staphylococci spp . and Streptococci spp . and the risk increased with age [ 28 ]. More worryingly, ~75% of the MRSA and MR-CoNS were MDR. Another US study demonstrated an increased rate of MRSA-related IK as well as resistance against fluoroquinolones, which questioned their ongoing use as primary monotherapy [ 26 ]. Similarly, a 10-year Mexico study showed that 21–79% of the S. aureus and 48–71% of the CoNS were resistant to oxacillin (or methicillin). P. aeruginosa and other Gram-negative infections displayed resistance against oxacillin (86% and 90%, respectively) and vancomycin (97% and 70%, respectively), with an increasing trend of resistance to ceftazidime observed over time [ 31 ]. Another study conducted in Taiwan also highlighted the emerging issue of methicillin resistance, with MRSA accounting for 43% of all Gram-positive IK [ 38 ]. On the other hand, an increase in voriconazole resistance was observed in the Mycotic Ulcer Treatment Trial (MUTT)-I for fungal keratitis, with a 2.1-fold increase in the mean MIC per year after adjustment for causative organism [ 151 ].
Reassuringly, reports from the UK showed that Gram-positive bacteria exhibited a high susceptibility to cephalosporin (87–100%), but a moderate susceptibility to fluoroquinolone (61–81%). However, Gram-negative bacteria were highly susceptible to both aminoglycoside (97–100%) and fluoroquinolone (91–100%) [ 8 , 9 , 24 ], suggesting that current antibiotic regimen (fluoroquinolone monotherapy or cephalosporin-aminoglycoside dual therapy) could safely remain as the first-line treatment in the UK. In our recent 12-year Nottingham IK Study, we observed an increasing trend of resistance against penicillin over time in both Gram-positive and Gram-negative isolates but a generally good susceptibility to aminoglycosides and fluoroquinolones was maintained; therefore, no change of antibiotic regimen was required [ 8 ].
E.3. Clinical impact
AMR represents a global challenge with a huge impact on morbidity and mortality. It was estimated that 2 million people/year in USA are infected with antimicrobial resistant organisms, with a $20 billion cost incurred on the healthcare system. A recent UK report also predicted a global loss of $100 trillion by 2050 related to AMR [ 152 ].
Within the context of IK, AMR was found to negatively affect the clinical outcome of IK. Kaye et al [ 153 ]. observed that the corneal healing time of IK was prolonged with the increase of minimum inhibitory concentration (MIC; i.e. antibiotic resistance) of the causative organisms, including P. aeruginosa , S. aureus and Enterobacteriaceae spp ., against fluoroquinolone monotherapy. In addition, Lalitha et al [ 154 ]. demonstrated that higher level of MIC was associated with a significantly increase risk of corneal perforation in fungal keratitis.
AMR is continuing to increase in an alarming way. There is a pressing need to increase the awareness amongst prescribers on judicious use of antimicrobials, to tighten the control of ‘over the counter (OTC)” antimicrobials in many countries, and to develop novel therapeutic modalities and strategies for IK, including therapeutic CXL and host defence peptides (or previously known as antimicrobial peptides), which hold great promises as a new class of antimicrobials in the future [ 123 , 155 , 156 , 157 ].
Conclusions
IK represents a persistent burden on human health in both developed and developing countries. As the incidence of IK is likely to be underestimated in the recent studies, well-designed prospective studies including all types of microorganisms (i.e. bacteria, fungi, protozoa and viruses) are required to truly ascertain the incidence and impact of IK. Understanding of the major risk factors for IK in different regions, particularly CL wear, trauma, OSD, and post-ocular surgery, will facilitate a more effective public health intervention to modify and reduce the risk of IK. The increase rate of AMR in ocular infection in several countries, including the US, China, and India, over the past decade highlights the need for judicious use of antimicrobials, tighter control of OTC antimicrobials and development of new antimicrobials and strategies for therapy. Improvement in the diagnostic yield of microbiological investigations of IK with emerging technologies such as next-generation sequencing and artificial intelligence-assisted platforms could also provide a better guidance on the appropriate use of antimicrobial therapy in the future, ultimately reducing the risk of AMR [ 158 , 159 ].
Methods of literature review
Two authors (DSJT and CSH) searched the PubMed (January 1980–May 2020) for relevant articles related to IK. Keywords such as “corneal infection”, “corneal ulcer”, “IK”, “microbial keratitis”, “incidence”, “prevalence”, “epidemiology”, “risk factors”, “antibiotic resistance” and “antimicrobial resistance” were used. There was no restriction to the language used. Bibliographies of included articles were manually screened to identify further relevant studies. The final search was updated on 15 June 2020.
A web application designed for systematic reviews, Rayyan (Qatar), was used to help collate the potential studies and expedite the initial screening of abstracts and titles [ 160 ]. The titles and abstracts obtained from the searches were independently screened by two authors (DSJT and CSH) to include studies that fulfilled the eligibility criteria. The authors then independently assessed the full-text version of all selected articles and extracted data onto a standardised data collection form for data synthesis. The extracted data included the authors, year of publication, country, sample size, demographic factors, culture results, risk factors and in vitro antibiotic susceptibility. Discrepancies were resolved by group consensus and independent adjudication (HSD) if consensus could not be reached. The summary of literature search is detailed in the PRISMA flow chart (Fig. 1 ).
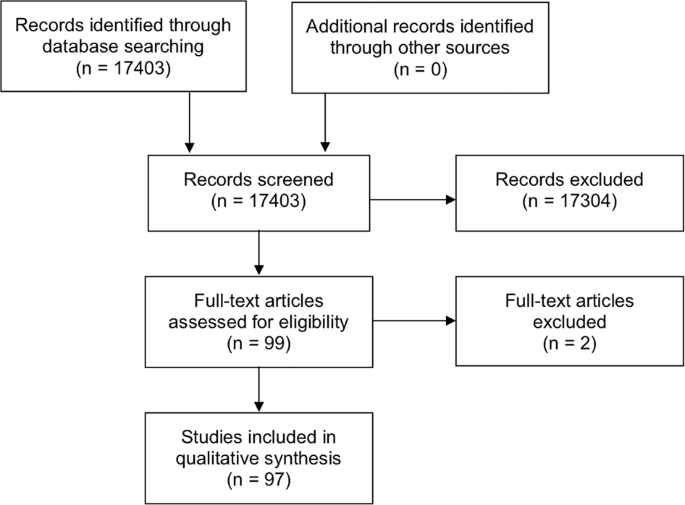
The PRISMA flow chart detailing the process and results of literature search for articles related to infectious keratitis.
Corneal opacity represents the 5th leading cause of blindness globally, with infectious keratitis (IK) being the main culprit.
IK can be caused by a wide variety of pathogens, including bacteria, fungi, viruses, parasites and polymicrobial infection.
Contact lens wear, trauma and ocular surface diseases are the three most common risk factors of IK.
Several studies have highlighted the emerging trends in antimicrobial resistance in ocular infections, particularly in the US, China and India.
Change history
11 may 2021.
A Correction to this paper has been published: https://doi.org/10.1038/s41433-021-01568-0
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e34.
Article PubMed Google Scholar
https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment . [accessed on 1st May 2020]
Whitcher JP, Srinivasan M. Corneal ulceration in the developing world-a silent epidemic. Br J Ophthalmol. 1997;81:622–3.
Article CAS PubMed PubMed Central Google Scholar
Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–21.
CAS PubMed PubMed Central Google Scholar
Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019;64:255–71.
Song X, Xie L, Tan X, Wang Z, Yang Y, Yuan Y, et al. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS ONE. 2014;9:e113843.
Article PubMed PubMed Central Google Scholar
Ung L, Acharya NR, Agarwal T, Alfonso EC, Bagga B, Bispo PJ, et al. Infectious corneal ulceration: a proposal for neglected tropical disease status. Bull World Health Organ. 2019;97:854–6.
Ting DSJ, Ho CS, Cairns J, Elsahn A, Al-Aqaba M, Boswell T, et al. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: the Nottingham Infectious Keratitis Study. Br J Ophthalmol. 2020; https://doi.org/10.1136/bjophthalmol-2020-316128 .
Tan SZ, Walkden A, Au L, Fullwood C, Hamilton A, Qamruddin A, et al. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye (Lond). 2017;31:1229–36.
Article CAS Google Scholar
Ting DSJ, Settle C, Morgan SJ, Baylis O, Ghosh S. A 10-year analysis of microbiological profiles of microbial keratitis: the North East England Study. Eye (Lond). 2018;32:1416–7.
Article Google Scholar
Khoo P, Cabrera-Aguas MP, Nguyen V, Lahra MM, Watson SL. Microbial keratitis in Sydney, Australia: risk factors, patient outcomes, and seasonal variation. Graefes Arch Clin Exp Ophthalmol. 2020; https://doi.org/10.1007/s00417-020-04681-0 .
Foulsham W, Coco G, Amouzegar A, Chauhan SK, Dana R. When clarity is crucial: regulating ocular surface immunity. Trends Immunol. 2018;39:288–301.
Article CAS PubMed Google Scholar
Khor WB, Prajna VN, Garg P, Mehta JS, Xie L, Liu Z, et al. The Asia Cornea Society Infectious Keratitis Study: a Prospective Multicenter Study of Infectious Keratitis in Asia. Am J Ophthalmol. 2018;195:161–70.
Arunga S, Wiafe G, Habtamu E, Onyango J, Gichuhi S, Leck A, et al. The impact of microbial keratitis on quality of life in Uganda. BMJ Open Ophthalmol. 2019;4:e000351.
Collier SA, Gronostaj MP, MacGurn AK, Cope JR, Awsumb KL, Yoder JS, et al. Estimated burden of keratitis-United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63:1027–30.
PubMed PubMed Central Google Scholar
Erie JC, Nevitt MP, Hodge DO, Ballard DJ. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol. 1993;111:1665–71.
Upadhyay MP, Karmacharya PC, Koirala S, Shah DN, Shakya S, Shrestha JK, et al. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br J Ophthalmol. 2001;85:388–92.
Jeng BH, Gritz DC, Kumar AB, Holsclaw DS, Porco TC, Smith SD, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128:1022–8.
Seal DV, Kirkness CM, Bennett HG, Peterson M. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens Anterior Eye. 1999;22:49–57.
Ibrahim YW, Boase DL, Cree IA. Incidence of Infectious Corneal Ulcers, Portsmouth Study, UK. J Clin Exp Ophthalmol. 2012;S6:001.
Google Scholar
Green M, Carnt N, Apel A, Stapleton F. Queensland Microbial Keratitis Database: 2005-2015. Br J Ophthalmol. 2019;103:1481–6.
Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996;3:159–66.
Kaye R, Kaye A, Sueke H, Neal T, Winstanley C, Horsburgh M, et al. Recurrent bacterial keratitis. Investig Ophthalmol Vis Sci. 2013;54:4136–9.
Tavassoli S, Nayar G, Darcy K, Grzeda M, Luck J, Williams OM, et al. An 11-year analysis of microbial keratitis in the South West of England using brain-heart infusion broth. Eye (Lond). 2019;33:1619–25.
Tam ALC, Côté E, Saldanha M, Lichtinger A, Slomovic AR. Bacterial Keratitis in Toronto: a 16-Year Review of the Microorganisms Isolated and the Resistance Patterns Observed. Cornea. 2017;36:1528–34.
Peng MY, Cevallos V, McLeod SD, Lietman TM, Rose-Nussbaumer J. Bacterial keratitis: isolated organisms and antibiotic resistance patterns in San Francisco. Cornea. 2018;37:84–7.
Kowalski RP, Nayyar SV, Romanowski EG, Shanks RMQ, Mammen A, Dhaliwal DK, et al. The Prevalence of Bacteria, Fungi, Viruses, and Acanthamoeba From 3,004 Cases of Keratitis, Endophthalmitis, and Conjunctivitis. Eye Contact Lens. 2019; https://doi.org/10.1097/ICL.0000000000000642 .
Asbell PA, Sanfilippo CM, Sahm DF, DeCory HH. Trends in Antibiotic Resistance Among Ocular Microorganisms in the United States From 2009 to 2018. JAMA Ophthalmol. 2020;138:1–12.
Article PubMed Central Google Scholar
Cariello AJ, Passos RM, Yu MC, Hofling-Lima AL. Microbial keratitis at a referral center in Brazil. Int Ophthalmol. 2011;31:197–204.
Marujo FI, Hirai FE, Yu MC, Hofling-Lima AL, Freitas D, Sato EH. [Distribution of infectious keratitis in a tertiary hospital in Brazil]. Arq Bras Oftalmol. 2013;76:370–3.
Hernandez-Camarena JC, Graue-Hernandez EO, Ortiz-Casas M, Ramirez-Miranda A, Navas A, Pedro-Aguilar L, et al. Trends in Microbiological and Antibiotic Sensitivity Patterns in Infectious Keratitis: 10-Year Experience in Mexico City. Cornea 2015;34:778–85.
Yu MC, Höfling-Lima AL, Furtado GH. Microbiological and epidemiological study of infectious keratitis in children and adolescents. Arq Bras Oftalmol. 2016;79:289–93.
Rautaraya B, Sharma S, Kar S, Das S, Sahu SK. Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern India. BMC Ophthalmol. 2011;11:39.
Lin CC, Lalitha P, Srinivasan M, Prajna NV, McLeod SD, Acharya NR, et al. Seasonal trends of microbial keratitis in South India. Cornea. 2012;31:1123–7.
Kaliamurthy J, Kalavathy CM, Parmar P, Nelson Jesudasan CA, Thomas PA. Spectrum of bacterial keratitis at a tertiary eye care centre in India. Biomed Res Int. 2013;2013:181564.
Lalitha P, Prajna NV, Manoharan G, Srinivasan M, Mascarenhas J, Das M, et al. Trends in bacterial and fungal keratitis in South India, 2002-2012. Br J Ophthalmol. 2015;99:192–4.
Wang L, Han L, Yin W. Study of Pathogens of Fungal Keratitis and the Sensitivity of Pathogenic Fungi to Therapeutic Agents with the Disk Diffusion Method. Curr Eye Res. 2015;40:1095–101.
Hsiao CH, Sun CC, Yeh LK, Ma DH, Chen PY, Lin HC, et al. Shifting Trends in Bacterial Keratitis in Taiwan: a 10-Year Review in a Tertiary-Care Hospital. Cornea. 2016;35:313–7.
Zhang Y, Wang ZQ, Sun XG. [Etiological analysis and in vitro drug sensitivity of bacterial keratitis in northern China in the period of 2006-2015]. Zhonghua Yan Ke Za Zhi. 2017;53:662–7.
CAS PubMed Google Scholar
Acharya M, Farooqui JH, Singh A, Gandhi A, Mathur U. Bacterial isolates in microbial keratitis: three-year trend analysis from North India. Indian J Ophthalmol. 2019;67:1508–9.
Lin L, Duan F, Yang Y, Lou B, Liang L, Lin X. Nine-year analysis of isolated pathogens and antibiotic susceptibilities of microbial keratitis from a large referral eye center in southern China. Infect Drug Resist. 2019;12:1295–302.
Politis M, Wajnsztajn D, Rosin B, Block C, Solomon A. Trends of Bacterial Keratitis Culture Isolates in Jerusalem; a 13- Years Analysis. PLoS ONE. 2016;11:e0165223.
Cabrera-Aguas M, Khoo P, George CRR, Lahra MM, Watson S. Antimicrobial resistance trends in bacterial keratitis over 5 years in Sydney, Australia. Clin Exp Ophthalmol. 2019;48:183–91.
Keay LJ, Gower EW, Iovieno A, Oechsler RA, Alfonso EC, Matoba A, et al. Clinical and microbiological characteristics of fungal keratitis in the United States, 2001-2007: a multicenter study. Ophthalmology. 2011;118:920–6.
Chidambaram JD, Venkatesh Prajna N, Srikanthi P, Lanjewar S, Shah M, Elakkiya S, et al. Epidemiology, risk factors, and clinical outcomes in severe microbial keratitis in South India. Ophthalmic Epidemiol. 2018;25:297–305.
Tong W, Chen D, Chai C, Tan AM, Manotosh R. Disease patterns of microbial keratitis in Singapore: a retrospective case series. Cont Lens Anterior Eye. 2019;42:455–61.
Stapleton F. Contact lens-related corneal infection in Australia. Clin Exp Optom. 2020;103:408–17.
Ferreira CS, Figueira L, Moreira-Gonçalves N, Moreira R, Torrão L, Falcão-Reis F. Clinical and Microbiological Profile of Bacterial Microbial Keratitis in a Portuguese Tertiary Referral Center-Where Are We in 2015? Eye Contact Lens. 2018;44:15–20.
Ganguly S, Salma KC, Kansakar I, Sharma M, Bastola P, Pradhan R. Pattern of fungal isolates in cases of corneal ulcer in the western periphery of Nepal. Nepal J Ophthalmol. 2011;3:118–22.
Al-Ghafri A, Al-Raisi A. The epidemiology of nonviral microbial keratitis in a tertiary care center in Muscat, Oman. Oman J Ophthalmol. 2018;11:213–9.
Mandour SS, Marey HM, Farahat HG. Resistant Microbial Keratitis in South Nile Delta, Egypt: influence of Regional Risk Factors. Semin Ophthalmol. 2016;31:473–8.
PubMed Google Scholar
Waddell KM. Childhood blindness and low vision in Uganda. Eye (Lond). 1998;12:184–92.
Butler TK, Spencer NA, Chan CC, Singh Gilhotra J, McClellan K. Infective keratitis in older patients: a 4 year review, 1998-2002. Br J Ophthalmol. 2005;89:591–6.
Kunimoto DY, Sharma S, Garg P, Gopinathan U, Miller D, Rao GN. Corneal ulceration in the elderly in Hyderabad, south India. Br J Ophthalmol. 2000;84:54–9.
Barua K, Borah M, Deka C, Kakati R. Morbidity pattern and health-seeking behavior of elderly in urban slums: a cross-sectional study in Assam, India. J Fam Med Prim Care. 2017;6:345–50.
Srivastava S, Gill A. Untreated morbidity and treatment-seeking behaviour among the elderly in India: analysis based on National Sample Survey 2004 and 2014. SSM Popul Health. 2020;10:100557.
Green M, Sara S, Hughes I, Apel A, Stapleton F. Trends in contact lens microbial keratitis 1999 to 2015: a retrospective clinical review. Clin Exp Ophthalmol. 2019;47:726–32.
Pan XJ, Jiang T, Zhu H, Liu PP, Zhou ZY, Mao AJ. Corneal infection in Shandong peninsula of China: a 10-year retrospective study on 578 cases. Int J Ophthalmol. 2016;9:53–7.
Oladigbolu K, Rafindadi A, Abah E, Samaila E. Corneal ulcers in a tertiary hospital in Northern Nigeria. Ann Afr Med. 2013;12:165–70.
Zbiba W, Abdesslem NB. Acanthamoeba keratitis: an emerging disease among microbial keratitis in the Cap Bon region of Tunisia. Exp Parasitol. 2018;192:42–5.
Gautam V, Chaudhary A, Singh SK, Rai PG. Profile of Corneal Ulcer in a Month of harvesting Season in a Tertiary Level Eye Hospital of Eastern Nepal. Nepal J Ophthalmol. 2018;10:32–8.
Kumar A, Pandya S, Kavathia G, Antala S, Madan M, Javdekar T. Microbial keratitis in Gujarat, Western India: findings from 200 cases. Pan Afr Med J. 2011;10:48.
Courtright P, Lewallen S, Kanjaloti S, Divala DJ. Traditional eye medicine use among patients with corneal disease in rural Malawi. Br J Ophthalmol. 1994;78:810–2.
Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414.
Fleiszig SMJ, Kroken AR, Nieto V, Grosser MR, Wan SJ, Metruccio MME, et al. Contact lens-related corneal infection: intrinsic resistance and its compromise. Prog Retin Eye Res. 2020;76:100804.
Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27:870–926.
Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, Glidden DV, et al. The steroids for corneal ulcers trial: study design and baseline characteristics. Arch Ophthalmol. 2012;130:151–7.
Lalitha P, Srinivasan M, Rajaraman R, Ravindran M, Mascarenhas J, Priya JL, et al. Nocardia keratitis: clinical course and effect of corticosteroids. Am J Ophthalmol. 2012;154:934–9.e1.
Chu HS, Hu FR. Non-tuberculous mycobacterial keratitis. Clin Microbiol Infect. 2013;19:221–6.
Faria S, Joao I, Jordao L. General Overview on Nontuberculous Mycobacteria, Biofilms, and Human Infection. J Pathog. 2015;2015:809014.
Castano G, Elnahry AG, Mada PK. Fungal Keratitis. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2020.
Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–5.
Sharma S, Kunimoto DY, Gopinathan U, Athmanathan S, Garg P, Rao GN. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: a survey of eight years of laboratory experience. Cornea. 2002;21:643–7.
Sun XG, Zhang Y, Li R, Wang ZQ, Luo SY, Jin XY, et al. Etiological analysis on ocular fungal infection in the period of 1989 - 2000. Chin Med J (Engl). 2004;117:598–600.
Ting DSJ, Bignardi G, Koerner R, Irion LD, Johnson E, Morgan SJ, et al. Polymicrobial Keratitis With Cryptococcus curvatus, Candida parapsilosis, and Stenotrophomonas maltophilia After Penetrating Keratoplasty: a Rare Case Report With Literature Review. Eye Contact Lens. 2019;45:e5–e10.
Ting DSJ, McKenna M, Sadiq SN, Martin J, Mudhar HS, Meeney A, et al. Arthrographis kalrae Keratitis Complicated by Endophthalmitis: a Case Report With Literature Review. Eye Contact Lens. 2020; https://doi.org/10.1097/ICL.0000000000000713 .
Sahay P, Goel S, Nagpal R, Maharana PK, Sinha R, Agarwal T, et al. Infectious Keratitis Caused by Rare and Emerging Micro-Organisms. Curr Eye Res. 2020;45:761–73.
Somani SN, Ronquillo Y, Moshirfar M. Acanthamoeba Keratitis. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2020.
Carnt N, Hoffman JJ, Verma S, Hau S, Radford CF, Minassian DC, et al. Acanthamoeba keratitis: confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factors. Br J Ophthalmol. 2018;102:1621–8.
Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148:487–99.e2.
Brown AC, Ross J, Jones DB, Collier SA, Ayers TL, Hoekstra RM, et al. Risk Factors for Acanthamoeba Keratitis-A Multistate Case-Control Study, 2008-2011. Eye Contact Lens. 2018;44:S173–S8.
Garg P, Kalra P, Joseph J. Non-contact lens related Acanthamoeba keratitis. Indian J Ophthalmol. 2017;65:1079–86.
Iovieno A, Gore DM, Carnt N, Dart JK. Acanthamoeba sclerokeratitis: epidemiology, clinical features, and treatment outcomes. Ophthalmology. 2014;121:2340–7.
Moshirfar M, Somani SN, Shmunes KM, Espandar L, Gokhale NS, Ronquillo YC, et al. A Narrative Review of Microsporidial Infections of the Cornea. Ophthalmol Ther. 2020;9:265–78.
Friedberg DN, Stenson SM, Orenstein JM, Tierno PM, Charles NC. Microsporidial keratoconjunctivitis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1990;108:504–8.
Ting DSJ, Ghosh N, Ghosh S. Herpes zoster ophthalmicus. BMJ. 2019;364:k5234.
Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. Herpes keratitis. Prog Retin Eye Res. 2013;32:88–101.
Tuli S, Gray M, Shah A. Surgical management of herpetic keratitis. Curr Opin Ophthalmol. 2018;29:347–54.
Lim NC, Lim DK, Ray M. Polymicrobial versus monomicrobial keratitis: a retrospective comparative study. Eye Contact Lens. 2013;39:348–54.
Gorski M, Genis A, Yushvayev S, Awwad A, Lazzaro DR. Seasonal Variation in the Presentation of Infectious Keratitis. Eye Contact Lens. 2016;42:295–7.
Walkden A, Fullwood C, Tan SZ, Au L, Armstrong M, Brahma AK, et al. Association Between Season, Temperature and Causative Organism in Microbial Keratitis in the UK. Cornea. 2018;37:1555–60.
Shankar J, Sueke H, Wiehlmann L, Horsburgh MJ, Tuft S, Neal TJ, et al. Genotypic analysis of UK keratitis-associated Pseudomonas aeruginosa suggests adaptation to environmental water as a key component in the development of eye infections. FEMS Microbiol Lett. 2012;334:79–86.
Dethorey G, Daruich A, Hay A, Renard G, Bourges JL. [Severe bacterial keratitis referred to ophthalmology emergency departments: a retrospective study of 268 cases]. J Fr Ophtalmol. 2013;36:129–37.
Khor HG, Cho I, Lee K, Chieng LL. Spectrum of Microbial Keratitis Encountered in the Tropics. Eye Contact Lens. 2020;46:17–23.
Sagerfors S, Ejdervik-Lindblad B, Söderquist B. Infectious keratitis: isolated microbes and their antibiotic susceptibility pattern during 2004-2014 in Region Örebro County, Sweden. Acta Ophthalmol. 2020;98:255–60.
French DD, Margo CE. Demographic patterns of ED patients diagnosed as having corneal ulcer. Am J Emerg Med. 2013;31:1082–5.
Truong DT, Bui MT, Memon P, Cavanagh HD. Microbial Keratitis at an Urban Public Hospital: a 10-Year Update. J Clin Exp Ophthalmol. 2015;6:498.
Dhakhwa K, Sharma MK, Bajimaya S, Dwivedi AK, Rai S. Causative organisms in microbial keratitis, their sensitivity pattern and treatment outcome in western Nepal. Nepal. J Ophthalmol. 2012;4:119–27.
CAS Google Scholar
Hussain I, Khan BS, Soni M, Iqbal M. Habibullah. Non-viral microbial keratitis: etiology, clinical features and visual outcome. J Coll Physicians Surg Pak. 2012;22:151–4.
Deorukhkar S, Katiyar R, Saini S. Epidemiological features and laboratory results of bacterial and fungal keratitis: a five-year study at a rural tertiary-care hospital in western Maharashtra, India. Singap Med J. 2012;53:264–7.
Sitoula RP, Singh SK, Mahaseth V, Sharma A, Labh RK. Epidemiology and etiological diagnosis of infective keratitis in eastern region of Nepal. Nepal J Ophthalmol. 2015;7:10–5.
Yildiz EH, Airiani S, Hammersmith KM, Rapuano CJ, Laibson PR, Virdi AS, et al. Trends in contact lens-related corneal ulcers at a tertiary referral center. Cornea. 2012;31:1097–102.
Sauer A, Greth M, Letsch J, Becmeur PH, Borderie V, Daien V, et al. Contact Lenses and Infectious Keratitis: from a Case-Control Study to a Computation of the Risk for Wearers. Cornea. 2020;39:769–74.
Carnt N, Robaei D, Minassian DC, Dart JKG. Acanthamoeba keratitis in 194 patients: risk factors for bad outcomes and severe inflammatory complications. Br J Ophthalmol. 2018;102:1431–5.
Stapleton F, Naduvilath T, Keay L, Radford C, Dart J, Edwards K, et al. Risk factors and causative organisms in microbial keratitis in daily disposable contact lens wear. PLoS ONE. 2017;12:e0181343.
Hoddenbach JG, Boekhoorn SS, Wubbels R, Vreugdenhil W, Van Rooij J, Geerards AJ. Clinical presentation and morbidity of contact lens-associated microbial keratitis: a retrospective study. Graefes Arch Clin Exp Ophthalmol. 2014;252:299–306.
Sauer A, Meyer N, Bourcier T, Keratitis FSGfCLRM. Risk Factors for Contact Lens-Related Microbial Keratitis: a Case-Control Multicenter Study. Eye Contact Lens. 2016;42:158–62.
Scanzera AC, Tu EY, Joslin CE. Acanthamoeba Keratitis in Minors With Orthokeratology (OK) Lens Use: a Case Series. Eye Contact Lens. 2020; https://doi.org/10.1097/ICL.0000000000000728 .
Kumar A, Khurana A, Sharma M, Chauhan L. Causative fungi and treatment outcome of dematiaceous fungal keratitis in North India. Indian J Ophthalmol. 2019;67:1048–53.
Suzuki T, Sutani T, Nakai H, Shirahige K, Kinoshita S. The Microbiome of the Meibum and Ocular Surface in Healthy Subjects. Investig Ophthalmol Vis Sci. 2020;61:18.
Graham JE, Moore JE, Jiru X, Goodall EA, Dooley JS, Hayes VE, et al. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Investig Ophthalmol Vis Sci. 2007;48:5616–23.
Khoo P, Cabrera-Aguas M, Robaei D, Lahra MM, Watson S. Microbial Keratitis and Ocular Surface Disease: a 5-Year Study of the Microbiology, Risk Factors and Clinical Outcomes in Sydney, Australia. Curr Eye Res. 2019;44:1195–202.
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276–83.
Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510.
McCulley JP, Shine WE. Changing concepts in the diagnosis and management of blepharitis. Cornea. 2000;19:650–8.
Dohse N, Wibbelsman TD, Rapuano SB, Hammersmith KM, Nagra PK, Rapuano CJ, et al. Microbial keratitis and clinical outcomes following penetrating and endothelial keratoplasty. Acta Ophthalmol. 2020; https://doi.org/10.1111/aos.14404 .
Maharana PK, Sahay P, Sujeeth M, Singhal D, Rathi A, Titiyal JS, et al. Microbial Keratitis After Accelerated Corneal Collagen Cross-Linking in Keratoconus. Cornea. 2018;37:162–7.
Ting DSJ, Said DG, Dua HS. Interface Haze After Descemet Stripping Automated Endothelial Keratoplasty. JAMA Ophthalmol. 2019;137:1201–2.
Fontana L, Moramarco A, Mandarà E, Russello G, Iovieno A. Interface infectious keratitis after anterior and posterior lamellar keratoplasty. Clinical features and treatment strategies. A review. Br J Ophthalmol. 2019;103:307–14.
Randleman JB, Shah RD. LASIK interface complications: etiology, management, and outcomes. J Refract Surg. 2012;28:575–86.
Elmassry A, Said Ahmed OI, Abdalla MF, Gaballah K. Ten years experience of corneal collagen cross-linking: an observational study of 6120 cases. Eur J Ophthalmol. 2020:1120672120928921.
Ting DSJ, Rana-Rahman R, Chen Y, Bell D, Danjoux JP, Morgan SJ, et al. Effectiveness and safety of accelerated (9 mW/cm(2)) corneal collagen cross-linking for progressive keratoconus: a 24-month follow-up. Eye (Lond). 2019;33:812–8.
Ting DSJ, Henein C, Said DG, Dua HS. Photoactivated chromophore for infectious keratitis - Corneal cross-linking (PACK-CXL): a systematic review and meta-analysis. Ocul Surf. 2019;17:624–34.
Prajna NV, Radhakrishnan N, Lalitha P, Austin A, Ray KJ, Keenan JD, et al. Cross-Linking-Assisted Infection Reduction: a Randomized Clinical Trial Evaluating the Effect of Adjuvant Cross-Linking on Outcomes in Fungal Keratitis. Ophthalmology. 2020;127:159–66.
Said DG, Elalfy MS, Gatzioufas Z, El-Zakzouk ES, Hassan MA, Saif MY, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121:1377–82.
Dhawan S, Rao K, Natrajan S. Complications of corneal collagen cross-linking. J Ophthalmol. 2011;2011:869015.
Ting DSJ, Bandyopadhyay J, Patel T. Microbial keratitis complicated by acute hydrops following corneal collagen cross-linking for keratoconus. Clin Exp Optom. 2019;102:434–6.
Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017;15:575–628.
Tempest-Roe S, Joshi L, Dick AD, Taylor SR. Local therapies for inflammatory eye disease in translation: past, present and future. BMC Ophthalmol. 2013;13:39.
Di Zazzo A, Kheirkhah A, Abud TB, Goyal S, Dana R. Management of high-risk corneal transplantation. Surv Ophthalmol. 2017;62:816–27.
Wilhelmus KR, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, et al. Herpetic Eye Disease Study: a Controlled Trial of Topical Corticosteroids for Herpes Simplex Stromal Keratitis. Ophthalmology. 2020;127:S5–s18.
Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13.
Li S, Yi G, Peng H, Li Z, Chen S, Zhong H, et al. How Ocular Surface Microbiota Debuts in Type 2 Diabetes Mellitus. Front Cell Infect Microbiol. 2019;9:202.
Zhu L, Titone R, Robertson DM. The impact of hyperglycemia on the corneal epithelium: molecular mechanisms and insight. Ocul Surf. 2019;17:644–54.
Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res. 2019;73:100762.
Dan J, Zhou Q, Zhai H, Cheng J, Wan L, Ge C, et al. Clinical analysis of fungal keratitis in patients with and without diabetes. PLoS ONE. 2018;13:e0196741.
Wang B, Yang S, Zhai HL, Zhang YY, Cui CX, Wang JY, et al. A comparative study of risk factors for corneal infection in diabetic and non-diabetic patients. Int J Ophthalmol. 2018;11:43–7.
Kaiserman I, Kaiserman N, Nakar S, Vinker S. Herpetic eye disease in diabetic patients. Ophthalmology. 2005;112:2184–8.
Liesegang TJ. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1160–5.
Vadoothker S, Andrews L, Jeng BH, Levin MR. Management of Herpes Simplex Virus Keratitis in the Pediatric Population. Pediatr Infect Dis J. 2018;37:949–51.
Lobo AM, Agelidis AM, Shukla D. Pathogenesis of herpes simplex keratitis: the host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019;17:40–9.
Jan RL, Tai MC, Weng SF, Chang C, Wang JJ, Chang YS. Risk of corneal ulcer in patients with end-stage renal disease: a retrospective large-scale cohort study. Br J Ophthalmol. 2018;102:868–72.
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–98.
Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016;4. https://doi.org/10.1128/microbiolspec.VMBF-0016-2015 .
McDonald EM, Ram FS, Patel DV, McGhee CN. Topical antibiotics for the management of bacterial keratitis: an evidence-based review of high quality randomised controlled trials. Br J Ophthalmol. 2014;98:1470–7.
Brown L. Resistance to ocular antibiotics: an overview. Clin Exp Optom. 2007;90:258–62.
Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother. 2012;56:2795–805.
Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006;104:322–45.
Ray KJ, Prajna L, Srinivasan M, Geetha M, Karpagam R, Glidden D, et al. Fluoroquinolone treatment and susceptibility of isolates from bacterial keratitis. JAMA Ophthalmol. 2013;131:310–3.
Vola ME, Moriyama AS, Lisboa R, Vola MM, Hirai FE, Bispo PJ, et al. Prevalence and antibiotic susceptibility of methicillin-resistant Staphylococcus aureus in ocular infections. Arq Bras Oftalmol. 2013;76:350–3.
Prajna NV, Lalitha P, Rajaraman R, Krishnan T, Raghavan A, Srinivasan M, et al. Changing Azole Resistance: a Secondary Analysis of the MUTT I Randomized Clinical Trial. JAMA Ophthalmol. 2016;134:693–6.
O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. Rev Antimicrobial Resist. 2016:1–81.
Kaye S, Tuft S, Neal T, Tole D, Leeming J, Figueiredo F, et al. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Investig Ophthalmol Vis Sci. 2010;51:362–8.
Lalitha P, Prajna NV, Oldenburg CE, Srinivasan M, Krishnan T, Mascarenhas J, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea. 2012;31:662–7.
Ting DSJ, Beuerman RW, Dua HS, Lakshminarayanan R, Mohammed I. Strategies in Translating the Therapeutic Potentials of Host Defense Peptides. Front Immunol. 2020;11:983.
Mayandi V, Xi Q, Leng Goh ET, Koh SK, Jie Toh TY, Barathi VA, et al. Rational Substitution of ε-Lysine for α-Lysine Enhances the Cell and Membrane Selectivity of Pore-Forming Melittin. J Med Chem. 2020;63:3522–37.
Mohammed I, Said DG, Nubile M, Mastropasqua L, Dua HS. Cathelicidin-Derived Synthetic Peptide Improves Therapeutic Potential of Vancomycin Against Pseudomonas aeruginosa. Front Microbiol. 2019;10:2190.
Ung L, Bispo PJM, Doan T, Van Gelder RN, Gilmore MS, Lietman T, et al. Clinical metagenomics for infectious corneal ulcers: Rags to riches? Ocul Surf. 2020;18:1–12.
Ting DSJ, Foo VH, Yang LWY, Sia JT, Ang M, Lin H, et al. Artificial intelligence for anterior segment diseases: emerging applications in ophthalmology. Br J Ophthalmol. 2020; https://doi.org/10.1136/bjophthalmol-2019-315651 .
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
Download references
DSJT acknowledges support from the Medical Research Council/Fight for Sight (FFS) Clinical Research Fellowship (MR/T001674/1) and the FFS/John Lee, Royal College of Ophthalmologists Primer Fellowship (24CO4).
Author information
These authors contributed equally: Darren Shu Jeng Ting, Charlotte Shan Ho
Authors and Affiliations
Academic Ophthalmology, Division of Clinical Neuroscience, School of Medicine, University of Nottingham, Nottingham, UK
Darren Shu Jeng Ting, Dalia G. Said & Harminder S. Dua
Department of Ophthalmology, Queen’s Medical Centre, Nottingham, UK
Darren Shu Jeng Ting, Charlotte Shan Ho, Rashmi Deshmukh, Dalia G. Said & Harminder S. Dua
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Harminder S. Dua .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ting, D.S.J., Ho, C.S., Deshmukh, R. et al. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 35 , 1084–1101 (2021). https://doi.org/10.1038/s41433-020-01339-3
Download citation
Received : 20 July 2020
Revised : 22 October 2020
Accepted : 24 November 2020
Published : 07 January 2021
Issue Date : April 2021
DOI : https://doi.org/10.1038/s41433-020-01339-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Potential applications of artificial intelligence in image analysis in cornea diseases: a review.
- Kai Yuan Tey
- Ezekiel Ze Ken Cheong
Eye and Vision (2024)
A comparison of antimicrobial regimen outcomes and antibiogram development in microbial keratitis: a prospective cohort study in Alexandria, Egypt
- Amira A. Nayel
- Noha A. Hamdy
- Nelly M. Mohamed
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
Monitoring the transition from corneal ulceration to healed scar using a Scheimpflug tomography–based densitometry
- Yen-Cheng Chen
- Yu-Ting Hsiao
- Ming-Tse Kuo
A comparative study on two methods of ocular surface microbial sampling
- Yanyan Chen
BMC Ophthalmology (2023)
Next-generation nanomaterials: advancing ocular anti-inflammatory drug therapy
Journal of Nanobiotechnology (2023)

Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Update on the management of fungal keratitis
- Published: 30 April 2021
- Volume 41 , pages 3249–3256, ( 2021 )
Cite this article
- Xiao-Yuan Sha 1 ,
- Lian Liu 1 &
- Jing-Xiang Zhong 1
1274 Accesses
9 Citations
Explore all metrics
The aim of this article is to introduce the recent advance on the studies of fungal keratitis published over past 5 years.
We performed literature review of articles published on PubMed, Google Scholar, CNKI and Web of Science relevant to the diagnosis, pathogenesis and novel treatment of fungal keratitis.
Excessive inflammation can lead to stromal damage and corneal opacification, hence the research on immune mechanism provides many potential therapeutic targets for fungal keratitis. Many researchers discussed the importance of earlier definitive diagnosis and were trying to find rapid and accurate diagnostic methods of pathogens. Develop new drug delivery systems and new routes of administration with better corneal penetration, prolonged ocular residence time, and better mucoadhesive properties is also one of the research hotspots. Additionally, many novel therapeutic agents and methods have been gradually applied in clinical ophthalmology.
The diagnosis and treatment of fungal keratitis are still a challenge for ophthalmologist, and many researches provide new methods to conquer these problems.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Ocular toxoplasmosis: a review of the current diagnostic and therapeutic approaches
Dimitrios Kalogeropoulos, Hercules Sakkas, … Chris Kalogeropoulos

Dual-Atom Nanozyme Eye Drops Attenuate Inflammation and Break the Vicious Cycle in Dry Eye Disease
Dandan Chu, Mengyang Zhao, … Jingguo Li
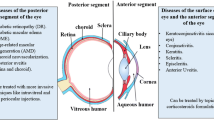
Corticosteroids in ophthalmology: drug delivery innovations, pharmacology, clinical applications, and future perspectives
Sherif A. Gaballa, Uday B. Kompella, … Hamdy Abdelkader
Cao J, Yang Y, Yang W et al (2014) Prevalence of infectious keratitis in Central China. BMC Ophthalmol. https://doi.org/10.1186/1471-2415-14-43
Article PubMed PubMed Central Google Scholar
Parthiban N, Sampath NL, JeyaMaheshwari J et al (2019) Quantitative profiling of tear proteome reveals down regulation of zinc alpha-2 glycoprotein in Aspergillus flavus keratitis patients. Exp Eye Res. https://doi.org/10.1016/j.exer.2019.107700
Article PubMed Google Scholar
Xu Q, Hu L-T, Wang Q et al (2019) Expression of macrophage migration inhibitory factor in Aspergillus fumigatus keratitis. Int J Ophthalmol 12:711–716. https://doi.org/10.18240/ijo.2019.05.03
Khames A, Khaleel MA, El-Badawy MF, El-Nezhawy AOH (2019) Natamycin solid lipid nanoparticles - sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: preparation and optimization. Int J Nanomedicine 14:2515–2531. https://doi.org/10.2147/IJN.S190502
Article CAS PubMed PubMed Central Google Scholar
Garg P, Roy A, Roy S (2016) Update on fungal keratitis. Curr Opin Ophthalmol 27:333–339
Article Google Scholar
Wang L, Sun S, Jing Y et al (2009) Spectrum of fungal keratitis in central China. Clin Exp Ophthalmol. https://doi.org/10.1111/j.1442-9071.2009.02155.x
Prajna NV, Srinivasan M, Lalitha P et al (2013) Differences in clinical outcomes in keratitis due to fungus and bacteria. JAMA Ophthalmol 131:1088–1089. https://doi.org/10.1001/jamaophthalmol.2013.1612
Verma A, Sharma G, Jain A et al (2019) Systematic optimization of cationic surface engineered mucoadhesive vesicles employing Design of Experiment (DoE): a preclinical investigation. Int J Biol Macromol 133:1142–1155. https://doi.org/10.1016/j.ijbiomac.2019.04.118
Article CAS PubMed Google Scholar
Cheng M, Lin J, Li C et al (2019) Wedelolactone suppresses IL-1β maturation and neutrophil infiltration in Aspergillus fumigatus keratitis. Int Immunopharmacol 73:17–22. https://doi.org/10.1016/j.intimp.2019.04.050
Gu L, Lin J, Wang Q et al (2020) Dimethyl itaconate protects against fungal keratitis by activating the Nrf2/HO-1 signaling pathway. Immunol Cell Biol 98:229–241. https://doi.org/10.1111/imcb.12316
Liu Y, Li J, Liu Y et al (2016) Inhibition of zymosan-induced cytokine and chemokine expression in human corneal fibroblasts by triptolide. Int J Ophthalmol 9:9–14. https://doi.org/10.18240/ijo.2016.01.02
Xu Q, Zhao G, Lin J et al (2015) Role of Dectin-1 in the innate immune response of rat corneal epithelial cells to Aspergillus fumigatus . BMC Ophthalmol 15:126. https://doi.org/10.1186/s12886-015-0112-1
Xu S, Hazlett LD (2019) MicroRNAs in ocular infection. Microorganisms 7:359. https://doi.org/10.3390/microorganisms7090359
Article CAS PubMed Central Google Scholar
Boomiraj H, Mohankumar V, Lalitha P, Devarajan B (2015) Human corneal microRNA expression profile in fungal keratitis. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.15-17619
Yang H, Wang Q, Han L et al (2020) Nerolidol inhibits the LOX-1 / IL-1β signaling to protect against the Aspergillus fumigatus keratitis inflammation damage to the cornea. Int Immunopharmacol 80:106118. https://doi.org/10.1016/j.intimp.2019.106118
Jiang N, Zhao G-Q, Lin J et al (2016) Expression of indoleamine 2,3-dioxygenase in a murine model of Aspergillus fumigatus keratitis. Int J Ophthalmol 9:491–496. https://doi.org/10.18240/ijo.2016.04.03
Qin X-H, Ma X, Fang S-F et al (2019) IL-17 produced by Th17 cells alleviates the severity of fungal keratitis by suppressing CX43 expression in corneal peripheral vascular endothelial cells. Cell Cycle 18:274–287. https://doi.org/10.1080/15384101.2018.1556059
Li C, Li C, Lin J et al (2020) The role of autophagy in the innate immune response to fungal keratitis caused by Aspergillus fumigatus infection. Invest Ophthalmol Vis Sci 61:25. https://doi.org/10.1167/iovs.61.2.25
Tang Q, Che C, Lin J et al (2019) Maresin1 regulates neutrophil recruitment and IL-10 expression in Aspergillus fumigatus keratitis. Int Immunopharmacol 69:103–108. https://doi.org/10.1016/j.intimp.2019.01.032
Yin M, Li C, Peng X-D et al (2019) Expression and role of calcitonin gene-related peptide in mouse Aspergillus fumigatus keratitis. Int J Ophthalmol 12:697–704. https://doi.org/10.18240/ijo.2019.05.01
Tian R, Zou H, Wang L et al (2020) Analysis of differentially expressed genes in bacterial and fungal keratitis. Indian J Ophthalmol 68:39–46. https://doi.org/10.4103/ijo.IJO_65_19
Mahmoudi S, Masoomi A, Ahmadikia K et al (2018) Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses 61:916–930. https://doi.org/10.1111/myc.12822
Niu L, Liu X, Ma Z et al (2020) Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb Pathog 138:103802
Wang YE, Tepelus TC, Vickers LA et al (2019) Role of in vivo confocal microscopy in the diagnosis of infectious keratitis. Int Ophthalmol 39:2865–2874. https://doi.org/10.1007/s10792-019-01134-4
Thomas PA (2003) Fungal infections of the cornea. Eye 17:852–862. https://doi.org/10.1038/sj.eye.6700557
Liu Z, Cao Y, Li Y et al (2020) Automatic diagnosis of fungal keratitis using data augmentation and image fusion with deep convolutional neural network. Comput Methods Programs Biomed 187:105019. https://doi.org/10.1016/j.cmpb.2019.105019
Thomas PA, Kaliamurthy J (2013) Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect 19:210–220. https://doi.org/10.1111/1469-0691.12126
Patel A (2013) Ocular drug delivery systems: an overview. World J Pharmacol. https://doi.org/10.5497/wjp.v2.i2.47
Zhang Z-H, Teng F, Sun Q-X et al (2019) Rapamycin liposome gutta inhibiting fungal keratitis of rats. Int J Ophthalmol 12:536–541. https://doi.org/10.18240/ijo.2019.04.02
El-Nabarawi MA, Abd El Rehem RT, Teaima M et al (2019) Natamycin niosomes as a promising ocular nanosized delivery system with ketorolac tromethamine for dual effects for treatment of candida rabbit keratitis; in vitro/in vivo and histopathological studies. Drug Dev Ind Pharm 45:922–936. https://doi.org/10.1080/03639045.2019.1579827
Chhonker YS, Prasad YD, Chandasana H et al (2015) Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int J Biol Macromol 72:1451–1458. https://doi.org/10.1016/j.ijbiomac.2014.10.014
Younes NF, Abdel-Halim SA, Elassasy AI (2018) Corneal targeted Sertaconazole nitrate loaded cubosomes: preparation, statistical optimization, in vitro characterization, ex vivo permeation and in vivo studies. Int J Pharm 553:386–397. https://doi.org/10.1016/j.ijpharm.2018.10.057
Tavakoli N, Taymouri S, Saeidi A, Akbari V (2019) Thermosensitive hydrogel containing sertaconazole loaded nanostructured lipid carriers for potential treatment of fungal keratitis. Pharm Dev Technol 24:891–901. https://doi.org/10.1080/10837450.2019.1616755
Guo Y, Karimi F, Fu Q et al (2020) Reduced administration frequency for the treatment of fungal keratitis: a sustained natamycin release from a micellar solution. Expert Opin Drug Deliv 17:407–421. https://doi.org/10.1080/17425247.2020.1719995
Huang J-F, Zhong J, Chen G-P et al (2016) A hydrogel-based hybrid theranostic contact lens for fungal keratitis. ACS Nano 10:6464–6473. https://doi.org/10.1021/acsnano.6b00601
Roy G, Galigama RD, Thorat VS et al (2019) Amphotericin B containing microneedle ocular patch for effective treatment of fungal keratitis. Int J Pharm 572:118808. https://doi.org/10.1016/j.ijpharm.2019.118808
Frei R, Breitbach AS, Blackwell HE (2012) 2-Aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa biofilms. Angew Chem Int Ed Engl 51:5226–5229. https://doi.org/10.1002/anie.201109258
Ganegoda N, Rao SK (2004) Antifungal therapy for keratomycoses. Expert Opin Pharmacother 5:865–874. https://doi.org/10.1517/14656566.5.4.865
Eckert R (2011) Road to clinical efficacy: Challenges and novel strategies for antimicrobial peptide development. Future Microbiol 6:635–351
Article CAS Google Scholar
Wu H, Ong ZY, Liu S et al (2015) Synthetic β-sheet forming peptide amphiphiles for treatment of fungal keratitis. Biomaterials 43:44–49. https://doi.org/10.1016/j.biomaterials.2014.11.052
Dogan C, Aygun G, Bahar-Tokman H et al (2019) In Vitro Antifungal Effect of Acrylic Corneal Glue (N-Butyl-2-Cyanoacrylate). Cornea 38:1563–1567
Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45:e54–e54. https://doi.org/10.1038/emm.2013.94
Zhou Y, Chen Y, Wang S et al (2019) MSCs helped reduce scarring in the cornea after fungal infection when combined with anti-fungal treatment. BMC Ophthalmol 19:226. https://doi.org/10.1186/s12886-019-1235-6
Behrens-Baumann WJ, Hofmüller W, Tammer I, Tintelnot K (2019) Keratomycosis due to Tintelnotia destructans refractory to common therapy treated successfully with systemic and local terbinafine in combination with polyhexamethylene biguanide. Int Ophthalmol 39:1379–1385. https://doi.org/10.1007/s10792-018-0930-2
Hassan HA, Geniady MM, Abdelwahab SF et al (2018) Topical eugenol successfully treats experimental Candida albicans -induced keratitis. Ophthalmic Res 60:69–79. https://doi.org/10.1159/000488907
Li Q, Gao X-R, Cui H-P et al (2016) Time-dependent matrix metalloproteinases and tissue inhibitor of metalloproteinases expression change in Fusarium solani keratitis. Int J Ophthalmol 9:512–518. https://doi.org/10.18240/ijo.2016.04.06
Gelfuso GM, Ferreira-Nunes R, Dalmolin LF et al (2020) Iontophoresis enhances voriconazole antifungal potency and corneal penetration. Int J Pharm 576:118991. https://doi.org/10.1016/j.ijpharm.2019.118991
Sueoka K, Chikama T, Pertiwi YD et al (2019) Antifungal efficacy of photodynamic therapy with TONS 504 for pathogenic filamentous fungi. Lasers Med Sci 34:743–747. https://doi.org/10.1007/s10103-018-2654-y
Rees CA, Bao R, Zegans ME, Cramer RA (2019) Natamycin and voriconazole exhibit synergistic interactions with nonantifungal ophthalmic agents against Fusarium species ocular isolates. Antimicrob Agents Chemother 63:e02505-e2518. https://doi.org/10.1128/AAC.02505-18
Austin A, Lietman T, Rose-Nussbaumer J (2017) Update on the management of infectious keratitis. Ophthalmology 124:1678–1689. https://doi.org/10.1016/j.ophtha.2017.05.012
Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE (2008) Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg 34:796–801
Erdem E, Harbiyeli II, Boral H et al (2018) Corneal collagen cross-linking for the management of mycotic keratitis. Mycopathologia 183:521–527. https://doi.org/10.1007/s11046-018-0247-8
Wei A, Wang K, Wang Y et al (2019) Evaluation of corneal cross-linking as adjuvant therapy for the management of fungal keratitis. Graefe’s Arch Clin Exp Ophthalmol 257:1443–1452. https://doi.org/10.1007/s00417-019-04314-1
Mikropoulos DG, Kymionis GD, Voulgari N et al (2019) Intraoperative photoactivated chromophore for infectious keratitis-corneal cross-linking (PACK-CXL) during penetrating keratoplasty for the management of fungal keratitis in an immunocompromised patient. Ophthalmol Ther 8:491–495. https://doi.org/10.1007/s40123-019-0196-4
Zhong J, Wang B, Li S et al (2018) Full-thickness conjunctival flap covering surgery combined with amniotic membrane transplantation for severe fungal keratitis. Exp Ther Med 15:2711–2718. https://doi.org/10.3892/etm.2018.5765
Kitazawa K, Fukuoka H, Inatomi T et al (2020) Safety of retrocorneal plaque aspiration for managing fungal keratitis. Jpn J Ophthalmol 64:228–233. https://doi.org/10.1007/s10384-020-00718-3
Florcruz N, Evans, J. R (2015) Medical interventions for fungal keratitis (Review) SUMMARY OF FINDINGS FOR THE MAIN COMPARISON. Cochrane Database Syst Rev 1469–493
Mundra J, Dhakal R, Mohamed A et al (2019) Outcomes of therapeutic penetrating keratoplasty in 198 eyes with fungal keratitis. Indian J Ophthalmol 67:1599–1605. https://doi.org/10.4103/ijo.IJO_1952_18
Fan F, Huang X, Yuan K et al (2020) Glucocorticoids may exacerbate fungal keratitis by increasing fungal aggressivity and inhibiting the formation of neutrophil extracellular traps. Curr Eye Res 45:124–133. https://doi.org/10.1080/02713683.2019.1657464
Selver OB, Egrilmez S, Palamar M et al (2015) Therapeutic corneal transplant for fungal keratitisrefractory to medical therapy. Exp Clin Transplant 13:355–359. https://doi.org/10.6002/ect.2014.0108
Uchio E, Saeki Y, Tsukahara-Kawamura T et al (2019) Clinical outcome after air-assisted manual deep anterior lamellar keratoplasty for fungal keratitis poorly responsive to medical treatment. Clin Ophthalmol 13:1913–1919. https://doi.org/10.2147/OPTH.S211099
Download references
Acknowledgements
We are grateful to our colleagues for their helpful suggestions during the planning and editing of this work.
Supported by the National Natural Science Foundation of China (No. 81970806); Medical Scientific Research Foundation of Guangdong Province of China (No. A2019098).
Author information
Authors and affiliations.
Department of Ophthalmology, First Affiliated Hospital of Jinan University, Guangzhou, China
Xiao-Yuan Sha, Qi Shi, Lian Liu & Jing-Xiang Zhong
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Lian Liu .
Ethics declarations
Conflict of interest.
The authors declare that there is no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Sha, XY., Shi, Q., Liu, L. et al. Update on the management of fungal keratitis. Int Ophthalmol 41 , 3249–3256 (2021). https://doi.org/10.1007/s10792-021-01873-3
Download citation
Received : 25 September 2020
Accepted : 19 April 2021
Published : 30 April 2021
Issue Date : September 2021
DOI : https://doi.org/10.1007/s10792-021-01873-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fungal keratitis
- Drug delivery systems
- Immune mechanism
- Therapeutic keratoplasty
- Find a journal
- Publish with us
- Track your research
Server Error
A server error has occured.

Copyright © 2024 Jobson Medical Information LLC unless otherwise noted.
All rights reserved. Reproduction in whole or in part without permission is prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Nepal J Epidemiol
- v.7(2); 2017 Jun
Fungal keratitis: study of increasing trend and common determinants
Yogesh acharya.
1 Assistant professor, Avalon University School of Medicine, Willemstad , Curacao, Netherland Antilles.
Bhawana Acharya
2 Registered nurse, VHA home health care, Toronto , Ontario, Canada.
Priyanka Karki
3 Medical officer, Nobel Medical College and Hospital, Biratnagar , Morang, Nepal.
Author’s Contribution: YA and BA conceived the idea. YA, BA, and PK reviewed the literature and drafted the manuscript. All authors reviewed, edited and agreed on the final version of this manuscript. .
Fungal keratitis is one of the leading cause of ocular morbidity. Fungal keratitis possesses a clinical challenge due to its slow pathologic process, overlapping features, diagnostic difficulty, and potential complications. Its increasing trend can be attributed to the use of contact lens, non-judiciary corticosteroid, and vegetative trauma. Early diagnosis and treatment is the cornerstone for its effective control. Knowledge of pathological course and clinical characteristics of fungal keratitis will definitely add in early diagnosis and treatment, with reduction in ocular morbidity. This review article explores the risk factor of fungal keratitis, its clinical course and management strategy.
Introduction
Eye is the most beautiful thing in the world. Pure appreciation of beauty is only possible with the intact functioning eye. It is well said that vision controls the mind. We believe in what we see and we prefer to see what we believe in. Eye is a delicate organ and is kept free of pathogens and harmful microorganism by its natural protective mechanism. This natural check and balance is absolutely necessary for a healthy eye. Breach of this delicate balance of protective environment can lead to ocular diseases with visual morbidity.
Cornea is the major refractive and protective outer layer of eye. Inflammation of the cornea is known as keratitis. There are several causes of keratitis: infectious, physical or chemical. Infectious or microbial keratitis is a predictor of general health due to its higher incidence in community and associated complications. Microbial keratitis has long been a challenge for the physicians ' due to its varied presentation, overlapping symptoms, and rapid progression. Bacterial keratitis is the most prevalent amongst microbial keratitis. But there has been a constant surge in fungal keratitis in the recent times due to multiple overlaying factors. Despite being a slow process in comparison to bacterial counterpart fungal keratitis possesses considerable ocular morbidity. Fungal keratitis carries a significant risk in developing countries and is one of the leading causes of vision loss [ 1 ]. Vegetative trauma in agriculturist and sand particles are the most common causes of mycotic keratitis in developing countries [ 2 ]. Middle age workingmen are more susceptible and constitutes majority of cases. Whereas, contact lens (CL) use is the leading cause in developed countries.
Fungi are distinct group of microbial organism. They are ubiquitous microbial eukaryotic pathogens. Fungal keratitis, also known as keratomycosis, is an important cause of microbial keratitis in the general population. Fungi are classified as yeasts or moulds. Yeasts ore oval or round bodies, with blastoconidium. Moulds are filamentous structures, known as hyphae. Hyphae can form a large mass of filaments known as mycelium. These filaments can be septate or non-septate. Fungi are capable of reproducing sexually as well as asexually. Sexual reproduction takes place through formation of spores and asexual by conidia or sporangiospores. Fungi infecting cornea are generally in asexual phase of life cycle, when cultured. Yeast like fungi are associated with the past history of ocular diseases, surgery and steroid use with poor clinical outcome. Filamentous fungi are usually found in patients with a history of ocular trauma [ 3 ].
Epidemiology:
There are more than 900,000 physician visit and 58,000 emergency visits related to keratitis and contact lens use in US, accounting an estimated expenditure of around $175 million dollar in health care [ 4 ]. Contact lens provides a direct threat for microbial keratitis and around 26 million contact lens users have significant ocular health risk. Fungal keratitis is also an important predictor of ocular health in developing countries and is a major cause of unilateral blindness
Risk factors:
Eye is susceptible to microbial infection due to local and systemic factors that invade the protective mechanism. These local factors like trauma to the eye ball, introduces and inoculates various fungal members into the eye with or without bacterial associates. Risk factors associated with fungal keratitis are Male, Trauma, Contact lens use, Topical corticosteroid use, Diabetes mellitus, and Low socioeconomic status.
Vegetative ocular trauma is undoubtedly the most common risk factor for fungal keratitis. Ocular trauma is essential to breach an intact corneal epithelium for introduction of microbial organism. Vegetative trauma predisposes to fungal infections being an important identifiable cause. It is extremely rare to encounter a case of fungal keratitis in an otherwise healthy eye, without any associated risk factors. This is because intact cornea is fairly resistant to microbial infections. Trauma helps to introduce and inoculate fungi directly into the cornea. Male are particularly more prone to fungal keratitis, as outdoor activities and farming practices predisposes to vegetative trauma.
CL use has become widespread in recent times. With an increase in CL use in the general community, the overall cases of fungal keratitis are also increasing. Although CL is implicated for major proportion of fungal keratitis, the overall prognosis is better in contact lens induced keratitis [ 3 ]. Factors associated with CL use and chances of fungal keratitis include nocturnal use during sleep, male gender, smoking history and socioeconomic status, relating to unhygienic contact lens behavior [ 5 ]. Microbes have higher chance of adherence to cornea with CL. Hypoxia and hypercapnia are pathogenic changes associated with CL induced microbial keratitis [ 6 ].
Antibiotics and corticosteroids use also render the eye susceptible to infections. Steroid has been the cornerstone of medical management for inflammatory disease process in modern medicine. Excessive steroid use leads to decrease in host defense mechanism and creates a favorable environment for fungal inoculation. Systemic disease like diabetes mellitus has emerged as a major risk factor in the recent years. Diabetes is becoming a global public health problem. Host defense is severely impaired in diabetes and high glucose provides a suitable growth media for microbial organism.
Fungal species:
Apergillus and Fusarium are two major cause of fungal keratitis. Aspergillus is associated with higher incidences of complications but shows better response with antifungal medications. Fungal keratitis due to Candida species has the worst clinical outcome [ 7 ]. Common fungal isolates from corneal scrapping of patients with clinically suspected keratitis are listed in Table-2 .
Commonly isolated fungi in microbial keratitis.
Fusarium is mainly associated with contact lens induced keratitis. It is necessary to recognize Fusarium keratitis early in the period of its progression and adequate measures should be taken to minimize the ocular morbidity [ 8 ]. There have been multiple reported incidences of Fusarium keratitis with CL and ReNu CL solution (Bausch & Lomb) for lens care. This can be attributed to poor contact lens hygiene and improper use habits. Some of these patients required emergency penetrating keratoplasty for severe complicating keratitis despite the medical treatment [ 9 ]. There are different Fusarium species and complexes responsible for keratitis; Fusarium solani species complex, Fusarium oxysporum species complex and Gibberella fujikuroi species complex. Fusarium solani species complex is the most common [ 10 ].
There are many other fungal species implicated in fungal keratitis. These fungal species are responsible for sporadic cases of fungal keratitis and include: Lophotrichus spp. [ 11 ], Alternaria spp. [ 12 ], Acremonium spp. [ 13 ], Cryptococcus albidus [ 14 ]), Pythium insidiosum [ 15 ], Beauveria bassiana [ 16 ], Paecilomyces [ 17 ], Cunninghamella spinosum [ 18 ], Scedosporium apiospermum [ 19 ]), Rhodotorula mucilaginosa [ 20 ], Cylindrocarpon lichenicola [ 21 ], Cladorrhinum bulbillosum [ 22 ]. Lophotrichus species were isolated from the necrotic corneal sample complicating fungal keratitis after dog paw traumatic injury [ 11 ].
Presentation:
Keratitis usually presents with ocular pain, foreign body sensation and blurred vision. Affected eye will be red and the patient can have injection of the conjunctiva. Intense inflammatory process after infection usually results in copious amount of ocular secretions but secretions in fugal keratitis are usually scanty in contrast to other microbial infections.
Diagnosis of fungal keratitis starts with strong clinical suspicion with concurrent presence of risk factors. Diagnosis is strongly supported by suggestive clinical presentations and fungal isolation from corneal sample. Antibiotic unresponsiveness provides a clinical clue for diagnosis. Direct microscopic examination of cornea with subsequent of corneal sample culture, still remains the gold standard for fungal diagnosis.
Keratitis is best examined under slit lamp microscopy. Slit lamp microscopy will show dry, thick and raised corneal surface. Majority of cases will have stromal infiltrates with feathery margins and endothelial plaques ( Fig-1 ).Satellite lesion is typically seen in fungal keratitis ( Fig-2 ). Hypopyon is detected in most of the cases, which can lead to ocular hypertension ( Fig-3 ). The identified risk factor for hypopyon includes infection with Fusarium and Aspergillus , in particular; and long duration of symptoms with larger lesion size [ 23 ]. It is not uncommon to see deep stromal infiltration, corneal abscess and dissemination of infection.

Peripheral corneal fungal keratitis in slit-lamp microscopy.

Fungal keratitis with satellite lesions in slit-lamp microscopy.

Corneal fungal ulcer with Hypopyon in slit-lamp microscopy.
Once the presumptive diagnosis is made, corneal scrapping and corneal biopsy is taken as required for inoculation and isolation of organism. There are different ways to illustrate the presence of fungal keratitis. These methods include Gram’s stain, Potassium hydroxide (KOH) mount, and Calcofluor white fluorescent staining and finally culture. Common culture media for fungus is Sabourauds Dextrose Agar. Direct visualization with KOH wet mount is commonly implicated followed by culture, being the most conclusive. (2) KOH is applied in corneal scrapping to dissolve epithelial strands. Use of 10% KOH better aids in fungal recognition and diagnosis. Definitive diagnosis lays on the foundation of culture and isolation. Culture facilitates the direct visualisation of fungi under microscopy. Culture and sensitivity will also guide the use of appropriate anti-fungal therapy for superior clinical recovery.
Polymerase Chain Reaction [ 24 ] and dot hybridization [ 25 ] are newer rapid detection technique for sensitive and specific diagnosis of fungal species. They have higher sensitivity than KOH wet mount and Gram smear [ 26 ] and are able to detect the fungus successfully in culture negative cases [ 27 ]. PCR high-resolution melting analysis is a variant PCR technique that is effective in differentiating between filamentous fungi and yeast form [ 28 ].
Management:
Management of fungal keratitis is directed by the objectives: a) distinctive diagnostic procedure to correctly identify the disease process, b) concurrent use of effective treatment modalities, c) eradication of the disease process, d) minimizing the complication, and e) prevention of future recurrences. This is achieved by the use of topical antifungal medication with or without surgical interventions. It is absolutely necessary to halt the disease progression early in the pathogenic process to reduce the overall complications and associated ocular morbidity.
Pharmacological management:
Pharmacological treatment of fungal keratitis rest on topical anti-fungal medications. There are currently no available antifungal recommendations in accordance with specific fungal isolation. Many of these anti-fugal differ in their corneal penetration activity and effectiveness. Topical instillation of the active antimicrobial pharmacological agents is still remains the gold standard treatment protocol. There are conflicting reports of intra-stromal injections but it has not shown proven added benefit over topical instillation [ 29 ].
Natamycin remains the cornerstone of anti-fungal therapy. Natamycin (5%) is the treatment of choice for filamentous fungi. Poor response to natamycin is directly related to increase infiltrate, larger scar size and perforation probability [ 30 ]. For candida species topical amphotericin B (0.1-0.3%) is frequently implicated with superior response [ 31 ]. There has been increasing evidence of topical voriconazole use in fungal keratitis with favourable clinical outcome. Topical voriconazole is especially useful in fungal keratitis not responding to natamycin [ 29 ]. Topical voriconazole is also effective against Cladosporium species [ 32 ]. There are different patterns of drug susceptibility in different groups of fungi. Therefore it is warranted that fungal specimen is taken and culture grown, for identification and antimicrobial sensitivity, if no response is visible after initiation of treatment [ 33 ].
Some fungal infection responds to topical fluoroquinolones, namely moxifloxacin [ 34 ]. This initial response leads to assumption of bacterial infection and eventually accounts for higher chances of complications with delay in diagnosis. It is necessary to understand that fluoroquinolone monotherapy will not be able to control most of the fungal infection and in turn can lead to prolongation of the disease course with chances of relapse.
Surgical management:
Surgery is definitely a choice for fungal keratitis, when response to pharmacological agent is poor and there is imminent threat of perforation. Surgery will eliminate the necrotic, infectious and antigenic source of ocular insult with creation of favourable environment for pharmacological agents to act and fastens healing [ 35 ].
Periodic debridement is an excellent procedure to remove dead and necrotic tissues from cornea. Debridement helps to improve the blood circulation, increase topical drug effectiveness and finally decrease the overall microbial load for speedy recovery. Conjunctival flap and lamellar or penetrating keratoplasty is applied in severe keratitis where a pharmacological agent fails. Patch graft and transplant can be used as final resort to restore the cornea and normal vision, whenever possible.
Corneal crosslinking:
Corneal crosslinking is an effective approach to control microbial keratitis. It is proven to have an excellent ulcer healing properties and induce overall reduction in hypopyon formation. There are few incidences of opacification of lens after crosslinking procedure [ 36 ]. Its efficacy is limited in viral keratitis due to incidences of corneal melting and tectonic keratoplasty [ 37 ]. Corneal collagen cross-linking with photoactivated riboflavin (CXL-PACK) has shown a promising outcome in-patient with advanced microbial keratitis with corneal melting. They decrease corneal perforation and recurrences in majority of patient [ 38 ]. CXL-PACK is safer procedure but it can increase the likelihood of bacterial keratitis in the patient because of epithelial removal necessary for the procedure. In addition to to it there are other potential factors responsible for causing keratitis, which can range from use of contact lens after local corticosteroid after the procedure. It is necessary for the physician to properly counsel the patient about this complication and caution should be taken to avoid them [ 39 , 40 ].
Complications:
Fungal keratitis can result in several complications leading to visual disability. These complications can range from formation of abscess and mild to severe corneal scarring with loss of vision due to dissemination ( Fig-4 ). Severe long-term disease process can lead to corneal perforation and dissemination of infection. Fungal infection also facilitates other superadded microbial keratitis. Patient can have an anterior segment disruption with increased intraocular pressure leading to glaucoma. Similarly it can result in endophthalmitis resulting in evisceration making the patient visually handicap.

Corneal fungal abscess at periphery with infiltration in slit-lamp microscopy.
Recent developments:
There are newer approaches for diagnosis of fungal corneal infection and treatment methods. Dot hybridization assay has been used successfully to diagnose Cryptococcus albidus , a rare fungi which and treated with intra-stromal injection of Amphotericin B [ 14 ]. MicroRNAs (MiRNAs) are RNA molecules in humans that do not code. They are responsible for regulation of functions in the human cell and show very high tissue specificity. They can be identified by PCR technique. They are evidences of increased MiRNA expression in fungal keratitis, indicating its role in pathologic process and potential target for modifications in the future [ 41 ].
Calprotectin is a neutrophil derived protein with potent antimicrobial property. Calprotectin uses Zn and Mn chelation to inhibit fungal growth. It has proven beneficial effect in Aspergillus fumigatus growth inhibition in experimental mice model and awaits further work in humans [ 42 ]. Lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1) [ 43 ], spleen-tyrosine kinase (Syk) [ 44 , 45 ] and intracellular nucleotide-binding oligomerization domain-containing protein (NOD)-like receptors [ 46 ] have also been isolated from Aspergillus infected corneal epithelial cells. LOX-1, Syk and NOD have basic role in modification of signalling pathways and its inhibitors can be used to regulate fungal growth in the future.
Photodynamic therapy with rose Bengal has been successful in restriction of certain fungal growth [ 47 ]. Riboflavin/UV-A has also been effective in Fusarium, Aspergillus and Candida after pre treatment with amphotericin B [ 48 ]. Combination therapy of antifungal medication with UV-A can prove safe and alternative to single therapy [ 49 ].
There has been progressive incline in microbial keratitis in the recent time. Fungal keratitis, in particular, has the highest risk and possesses a significant threat for increased ocular morbidity owing to its slower course and diagnostic difficulty. This rapid surge can be attributed to increased contact lens use, and non judiciary antimicrobial and corticosteroid use, complicated by systemic disease interfering with immune status. Most of the cases in western world can be attributed to unhygienic contact lens use, whereas vegetative trauma in working class is the major cause in developing countries. Male has higher incidences of fungal keratitis but corneal re-epithelialization time is higher in female in comparison with males, accounting higher recovery period [ 50 ].
Fusarium and Aspergillus are the common isolates from corneal scrapping, Fusarium associated with CL use. Healthy eye is relatively immune to fungal infection and ocular trauma provides an excellent opportunity for pathologic fungal inoculation into eye. Fungal keratitis has a relatively slow progression with dreadful complication that can range from corneal scarring to perforation and eventually loss of vision. Presence of satellite lesion is a strong indicator of fungal origin and strong clue can be provided by the fact that fungal corneal ulcers are not responsive to traditional antibiotics.
There is a major reduction in ocular morbidity with early diagnosis and treatment [ 1 ]. The traditional approach consists of slit lamp microscopic examination and corneal scrapping for identification and culture. There are newer rapid diagnostic technique with higher sensitivity and specificity like PCR. The prompt diagnosis is to be followed by effective management. Management is guided by topical antifungal therapy and surgery, if needed.
Fungal keratitis is one of the leading causes of ocular morbidity. Recognized common risk factors include vegetative trauma, widespread contact lens use, prolonged non-judiciary corticosteroid prescription, and systemic disease like diabetes mellitus. Fungal keratitis possesses a clinical challenge due to its slow pathologic disease process, overlapping features with other microbial keratitis and potential complications. The knowledge of clinical characteristics of fungal keratitis with its determinants will certainly help in early diagnosis and overall reduction in visual morbidity associated with it.
List of abbreviations:
- Contact Lens (CL)
- Potassium Hydroxide(KOH)
- Polymerase Chain Reaction(PCR)
- Collagen cross-linking with photoactivated riboflavin (CXL-PACK)
- MicroRNAs (MiRNAs)
- Lectin-like oxidized receptor-1 (LOX -1)
- Spleen-tyrosine kinase (Syk)
- Nucleotide-binding oligomerization domain (NOD)

BENEFITS OF PALLIATIVE CARE IN ADULTS WITH A DIAGNOSIS OF HEART FAILURE: AN EXPLORATORY LITERATURE REVIEW
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Simon Andres Giraldo Oliveros Sr.
- For correspondence: [email protected]
- ORCID record for Aura Cristina Herrera Obando Jr.
- ORCID record for Jhaina Isabella Larranaga Guerrero Jr.
- ORCID record for William Antonio Mejia Montehermoso Jr.
- Info/History
- Preview PDF
Introduction: Heart Failure is a clinical syndrome characterized by a series of symptoms such as dyspnea, orthopnea and edema in the lower limbs. This pathology continues to have a high prevalence despite advances in pharmacotherapy and device therapy and given that it is a pathology that significantly impairs the quality of life of patients, the implementation of care is of vital importance. However, these are underused due to lack of knowledge on the part of health personnel and also due to poor implementation in the different health providers. Objective: An exploratory review of the literature was carried out regarding the benefits of palliative care in patients with advanced heart failure, in order to synthesize the available and updated evidence. Methodology: Searched for articles published from 2017 to 2022 related to palliative care in patients with heart failure and using the PRISMA 2020 methodology for this study. This inquiry of articles was carried out in the following databases: UpToDate, PubMed, MESH, PMC (US National Library of Medicine National Institutes of health). Results: A total of 5 articles were obtained, from which they concluded that palliative care has a positive impact on the quality of life of patients with heart failure, there was a lower rate of hospital readmissions, improvements in physical, psychological and existential.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This study did not receive any funding
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
All data produced in the present work are contained in the manuscript
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Epidemiology
- Addiction Medicine (315)
- Allergy and Immunology (617)
- Anesthesia (159)
- Cardiovascular Medicine (2269)
- Dentistry and Oral Medicine (279)
- Dermatology (201)
- Emergency Medicine (369)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (798)
- Epidemiology (11559)
- Forensic Medicine (10)
- Gastroenterology (676)
- Genetic and Genomic Medicine (3560)
- Geriatric Medicine (336)
- Health Economics (615)
- Health Informatics (2297)
- Health Policy (912)
- Health Systems and Quality Improvement (860)
- Hematology (334)
- HIV/AIDS (748)
- Infectious Diseases (except HIV/AIDS) (13133)
- Intensive Care and Critical Care Medicine (755)
- Medical Education (358)
- Medical Ethics (100)
- Nephrology (388)
- Neurology (3341)
- Nursing (191)
- Nutrition (506)
- Obstetrics and Gynecology (650)
- Occupational and Environmental Health (643)
- Oncology (1753)
- Ophthalmology (524)
- Orthopedics (208)
- Otolaryngology (284)
- Pain Medicine (223)
- Palliative Medicine (66)
- Pathology (437)
- Pediatrics (999)
- Pharmacology and Therapeutics (419)
- Primary Care Research (403)
- Psychiatry and Clinical Psychology (3050)
- Public and Global Health (5980)
- Radiology and Imaging (1217)
- Rehabilitation Medicine and Physical Therapy (712)
- Respiratory Medicine (809)
- Rheumatology (367)
- Sexual and Reproductive Health (348)
- Sports Medicine (315)
- Surgery (386)
- Toxicology (50)
- Transplantation (170)
- Urology (142)
- Election 2024
- Entertainment
- Newsletters
- Photography
- Personal Finance
- AP Buyline Personal Finance
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election Results
- Delegate Tracker
- AP & Elections
- March Madness
- AP Top 25 Poll
- Movie reviews
- Book reviews
- Personal finance
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
Book Review: Short story anthology ‘The Black Girl Survives in This One’ challenges the horror canon
This cover image released by Flatiron shows “The Black Girl Survives in This One” horror stories edited by Desiree S. Evans and Saraciea J. Fennell. (Flatiron via AP)
- Copy Link copied

Ahh, the Final Girl — a point of pride, a point of contention. Too often, the white, virginal, Western ideal. But not this time.
“The Black Girl Survives in This One,” a short story anthology edited by Saraciea J. Fennell and Desiree S. Evans, is changing the literary horror canon. As self-proclaimed fans of “Scary Stories to Tell in the Dark” and “Goosebumps,” the editors have upped the ante with a new collection spotlighting Black women and girls, defying the old tropes that would box Black people in as support characters or victims.
The 15 stories are introduced with an excellent forward by Tananarive Due laying out the groundwork with a brief history of Black women in horror films and literature, and of her own experiences. She argues with an infallible persuasiveness that survival is the thread that connects Black women and the genre that has largely shunned them for so long.
These are the kind of stories that stick with you long after you’ve read them.
“Queeniums for Greenium!” by Brittney Morris features a cult-ish smoothie MLM with a deadly level of blind faith that had my heart pounding and my eyes watering with laughter at intervals. And “The Skittering Thing” by Monica Brashears captures the sheer panic of being hunted in the dark, with some quirky twists.
Many of the stories are set in the most terrifying real-life place there is: high school. As such, there are teen crushes and romance aplenty, as well as timely slang that’s probably already outdated.
Honestly, this was one of the best parts: seeing 15 different authors’ takes on a late-teens Black girl. How does she wear her hair, who are her friends, is she religious, where does she live, does she like boys or girls or no one at all? Is she a bratty teen or a goody-two-shoes or a bookworm or just doing her best to get through it? Each protagonist is totally unique and the overall cast of both characters and writers diverse.
And even though we know the Black girl survives, the end is still a shock, because the real question is how.
The anthology has something for everyone, from a classic zombie horror in “Cemetery Dance Party” by Saraciea J. Fennell to a spooky twist on Afrofuturism in “Welcome Back to The Cosmos” by Kortney Nash. Two of the stories have major “Get Out” vibes that fans of Jordan Peele will appreciate (“Black Girl Nature Group” by Maika Moulite and Maritza Moulite and “Foxhunt” by Charlotte Nicole Davies). If your flavor is throwbacks and cryptids, Justina Ireland’s “Black Pride” has you covered. Or if you like slow-burn psychological thrillers and smart protagonists, “TMI” by Zakiya Delila Harris.
Overall, it’s a bit long and the anthology could stand to drop a couple of the weaker stories. But it’s well worth adding to any scary book collection, and horror fans are sure to find some new favorites.
AP book reviews: https://apnews.com/hub/book-reviews

Acanthamoeba keratitis. A review of the literature
- PMID: 3556011
Acanthamoeba is a free-living ubiquitous ameba that is responsible for a small but increasing number of cases of keratitis. The infection is associated with minimal corneal trauma and soft contact lens wear. It typically presents as a unilateral central or paracentral corneal infiltrate, often with a ring-shaped peripheral infiltrate. The lesion is often confused with fungal, bacterial, or herpetic keratitis. Successful therapy hinges on early recognition and aggressive therapy with appropriate topical antiamebic medication often in conjunction with penetrating keratoplasty. Thirty-five cases from the world literature are reviewed.
Publication types
- Adrenal Cortex Hormones / therapeutic use
- Amoeba / drug effects
- Amoeba / growth & development
- Amoeba / immunology
- Antigens, Protozoan / immunology
- Antiprotozoal Agents / pharmacology
- Antiprotozoal Agents / therapeutic use
- Combined Modality Therapy
- Conjunctiva / parasitology
- Cornea / pathology
- Corneal Transplantation
- Keratitis / diagnosis
- Keratitis / etiology*
- Keratitis / pathology
- Keratitis / therapy
- Middle Aged
- Adrenal Cortex Hormones
- Antigens, Protozoan
- Antiprotozoal Agents

IMAGES
VIDEO
COMMENTS
Keratitis is the inflammation of the cornea and is characterized by corneal edema, infiltration of inflammatory cells, and ciliary congestion. It is associated with both infectious and non-infectious diseases, which may be systemic or localized to the ocular surface. Amongst the types of keratitis discussed above, "microbial keratitis" accounts for the majority and is primarily a cause of ...
Here, we discuss the fungal keratitis diagnostic literature and estimate the global burden through a complete systematic literature review from January, 1946 to July, 2019. An adapted GRADE score was used to evaluate incidence papers—116 studies provided the incidence of fungal keratitis as a proportion of microbial keratitis and 18 provided ...
Table 2 Summary of risk factors and associated organisms of infectious keratitis in the literature published between 2010 and 2020, categorised into six distinct regions. ... a Rare Case Report ...
1. Introduction. Mycotic keratitis is a leading cause of ocular morbidity throughout the world, particularly in tropical and subtropical countries [1, 2].According to World Health Organization; 2001 survey, corneal blindness is the second major cause of blindness after cataract [3].Furthermore, ocular trauma and corneal ulceration are amongst the most important causes of corneal blindness ...
This review explores the epidemiology of infectious keratitis, specifically the differences in disease incidence and risk factors, causative organism profile and virulence characteristics and host ...
Purpose This focused review aims to explore pediatric non-viral keratitis and to compare associated risk factors, etiologies, antibiotic susceptibilities, empiric treatments and outcomes. Methods The authors performed a literature research for articles, published on PubMed, Google Scholar, Scopus and Embase online library, relevant to pediatric keratitis etiology, risk factors, antibiotic ...
Purpose The aim of this article is to introduce the recent advance on the studies of fungal keratitis published over past 5 years. Methods We performed literature review of articles published on PubMed, Google Scholar, CNKI and Web of Science relevant to the diagnosis, pathogenesis and novel treatment of fungal keratitis. Results Excessive inflammation can lead to stromal damage and corneal ...
Acanthamoeba keratitis (AK) is a severe cause of infectious keratitis and represents a significant clinical challenge. Recent literature regarding AK epidemiology, diagnosis, treatment modalities, and prognosis is reviewed and synthesized to propose an algorithmic protocol for AK management. Globall …
Introduction. Infectious keratitis (IK) is an important cause of visual loss worldwide, particularly in developing countries, and may be the result of minor ocular trauma combined with poor hygiene. 1, 2 Although the incidence rate in industrialised countries is significantly lower, IK can arise from contact lens wear, dry eye, recent ocular surgery or systemic immunosuppression. 1, 2 The use ...
diagnostic literature and estimate the global burden through a complete systematic literature review from January, 1946 to July, 2019. An adapted GRADE score was used to evaluate incidence papers—116 studies provided the incidence of fungal keratitis as a proportion of microbial keratitis and 18 provided the incidence in a defined population. We
The English-language literature on PubMed was reviewed using the search terms "Acanthamoeba keratitis" and "diagnosis" or "treatment" and focused on studies published between 2018 and ...
Here, we discuss the fungal keratitis diagnostic literature and estimate the global burden through a complete systematic literature review from January, 1946 to July, 2019. An adapted GRADE score was used to evaluate incidence papers—116 studies provided the incidence of fungal keratitis as a proportion of microbial keratitis and 18 provided ...
Post-LRS keratitis is a rare complication that can be divided into infectious and noninfectious keratitis. The occurrence of noninfectious keratitis (2.34%) is reportedly 7.5 times higher than that of infectious keratitis (0.31%). 8 The statistics regarding the rate of keratitis after refractive surgery seem to possess some degree of inaccuracy because affected patients may present to another ...
Background and Objectives: Herpes simplex keratitis (HSK) is the leading infectious cause of corneal damage and associated loss of visual acuity. Because of its frequent recurrence, it represents a major health problem; thus, timely and accurate diagnosis is the key to successful treatment. To enable this, we aimed to determine HSK patients' demographic and clinical features. Materials and ...
A new study evaluated the in vitro efficacy of the novel combination of polymyxin B/trimethoprim (PT) + rifampin for bacterial keratitis and found it successfully eliminated all 43 isolates tested, even those which conferred multidrug-resistance (MDR). Among the 43 isolates were 20 S. aureus, 10 P. aeruginosa, three Pseudomonas stutzeri and one ...
Acanthamoeba keratitis (AK) is a severe cause of infectious keratitis and represents a significant clinical challenge. Recent literature regarding AK epidemiology, diagnosis, treatment modalities, and prognosis is reviewed and synthesized to propose an algorithmic protocol for AK management.
The methodology for this article was a narrative literature review, which consisted of reviewing empirical and conceptual peer-reviewed journal articles using a combination of the keywords African American, Black, male college students, help seeking, and counseling. This review was designed to consider the contextual factors that affect AAMCS ...
Literature Review / Research Gap 2024. By Shing Fu Kuo. Synthesizes literature and Provides research gaps [1] start uploading [2] say "done" (10 PDFs a time)
Fungal keratitis is one of the leading cause of ocular morbidity. Fungal keratitis possesses a clinical challenge due to its slow pathologic process, overlapping features, diagnostic difficulty, and potential complications. ... Early diagnosis and successful treatment of Cryptococcus albidus keratitis: a case report and literature review ...
Ocular disease, such as scleritis and peripheral ulcerative keratitis (PUK), may be a serious ocular complication. We present a patient with severe and refractory PUK treated with baricitinib. A review of the literature on Janus kinase inhibitors (JAKINIB) in refractory ocular surface pathology was …
Objective: An exploratory review of the literature was carried out regarding the benefits of palliative care in patients with advanced heart failure, in order to synthesize the available and updated evidence. Methodology: Searched for articles published from 2017 to 2022 related to palliative care in patients with heart failure and using the ...
The present PRISMA-guided article systematically reviews the current state of research on the autonomous sensory meridian response (ASMR). A systematic literature search was conducted in Pubmed, SCOPUS, and Web of Science (last search: March 2022) selecting all studies that conducted quantitative scientific research on the ASMR phenomenon. Fifty-four studies focusing on ASMR were retrieved ...
Ahh, the Final Girl — a point of pride, a point of contention. Too often, the white, virginal, Western ideal. But not this time. "The Black Girl Survives in This One," a short story anthology edited by Saraciea J. Fennell and Desiree S. Evans, is changing the literary horror canon.
Acanthamoeba is a free-living ubiquitous ameba that is responsible for a small but increasing number of cases of keratitis. The infection is associated with minimal corneal trauma and soft contact lens wear. It typically presents as a unilateral central or paracentral corneal infiltrate, often with a ring-shaped peripheral infiltrate.