Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 25 March 2024

Electrochemical hydrogenation and oxidation of organic species involving water
- Cuibo Liu ORCID: orcid.org/0000-0002-9578-8173 1 ,
- Fanpeng Chen 1 ,
- Bo-Hang Zhao 1 ,
- Yongmeng Wu 1 &
- Bin Zhang ORCID: orcid.org/0000-0003-0542-1819 1 , 2
Nature Reviews Chemistry ( 2024 ) Cite this article
1165 Accesses
11 Altmetric
Metrics details
- Electrocatalysis
- Structural properties
- Sustainability
Fossil fuel-driven thermochemical hydrogenation and oxidation using high-pressure H 2 and O 2 are still popular but energy-intensive CO 2 -emitting processes. At present, developing renewable energy-powered electrochemical technologies, especially those using clean, safe and easy-to-handle reducing agents and oxidants for organic hydrogenation and oxidation reactions, is urgently needed. Water is an ideal carrier of hydrogen and oxygen. Electrochemistry provides a powerful route to drive water splitting under ambient conditions. Thus, electrochemical hydrogenation and oxidation transformations involving water as the hydrogen source and oxidant, respectively, have been developed to be mild and efficient tools to synthesize organic hydrogenated and oxidized products. In this Review, we highlight the advances in water-participating electrochemical hydrogenation and oxidation reactions of representative organic molecules. Typical electrode materials, performance metrics and key characterization techniques are firstly introduced. General electrocatalyst design principles and controlling the microenvironment for promoting hydrogenation and oxygenation reactions involving water are summarized. Furthermore, paired hydrogenation and oxidation reactions are briefly introduced before finally discussing the challenges and future opportunities of this research field.
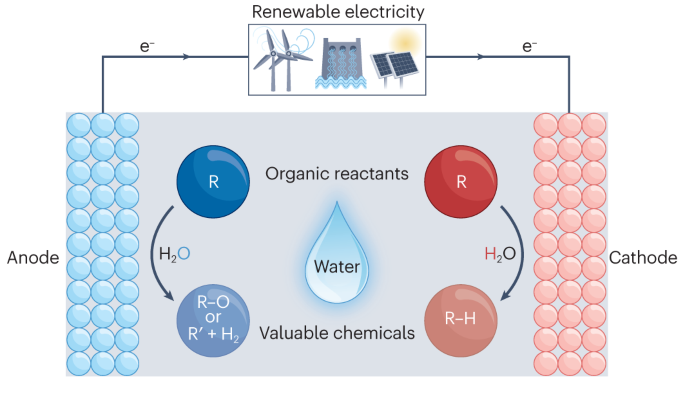
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Oxygen induced promotion of electrochemical reduction of CO2 via co-electrolysis
Ming He, Chunsong Li, … Qi Lu
Hydrogen production via microwave-induced water splitting at low temperature
J. M. Serra, J. F. Borrás-Morell, … J. M. Catalá-Civera
Integrating hydrogen utilization in CO2 electrolysis with reduced energy loss
Xiaoyi Jiang, Le Ke, … Ning Yan
Zhang, L., Zhou, M., Wang, A. & Zhang, T. Selective hydrogenation over supported metal catalysts: from nanoparticles to single atoms. Chem. Rev. 120 , 683–733 (2020).
Article CAS PubMed Google Scholar
Caron, S., Dugger, R. W., Ruggeri, S. G., Ragan, J. A. & Ripin, D. H. B. Large-scale oxidations in the pharmaceutical industry. Chem. Rev. 106 , 2943–2989 (2006).
Studt, F. et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 320 , 1320–1322 (2008).
Wang, D., Weinstein, A. B., White, P. B. & Stahl, S. S. Ligand-promoted palladium-catalyzed aerobic oxidation reactions. Chem. Rev. 118 , 2636–2679 (2018).
Zhang, H., Sun, Z. & Hu, Y. H. Steam reforming of methane: current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 149 , 111330 (2021).
Article CAS Google Scholar
Hashim, S. S., Mohamed, A. R. & Bhatia, S. Oxygen separation from air using ceramic-based membrane technology for sustainable fuel production and power generation. Renew. Sustain. Energy Rev. 15 , 1284–1293 (2011).
Pang, M. et al. Controlled partial transfer hydrogenation of quinolines by cobalt-amido cooperative catalysis. Nat. Commun. 11 , 1249 (2020).
Article CAS PubMed PubMed Central Google Scholar
Gunanathan, C. & Milstein, D. Applications of acceptorless dehydrogenation and related transformations in chemical synthesis. Science 341 , 1229712 (2013).
Article PubMed Google Scholar
Bryliakov, K. P. Catalytic asymmetric oxygenations with the environmentally benign oxidants H 2 O 2 and O 2 . Chem. Rev. 117 , 11406–11459 (2017).
Ghavtadze, N., Melkonyan, F. S., Gulevich, A. V., Huang, C. & Gevorgyan, V. Conversion of 1-alkenes into 1,4-diols through an auxiliary-mediated formal homoallylic C–H oxidation. Nat. Chem. 6 , 122–125 (2014).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117 , 13230–13319 (2017).
Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 57 , 5594–5619 (2018).
Münchow, T., Dana, S., Xu, Y., Yuan, B. & Ackermann, L. Enantioselective electrochemical cobalt-catalyzed aryl C–H activation reactions. Science 379 , 1036–1042 (2023).
Article Google Scholar
Li, J., Zhang, Y., Kuruvinashetti, K. & Kornienko, N. Construction of C–N bonds from small-molecule precursors through heterogeneous electrocatalysis. Nat. Rev. Chem. 6 , 303–319 (2022).
Tang, C., Zheng, Y., Jaroniec, M. & Qiao, S.-Z. Electrocatalytic refinery for sustainable production of fuels and chemicals. Angew. Chem. Int. Ed. 60 , 19572–19590 (2021).
Li, Y. et al. Recent progress in synergistic electrocatalysis for generation of valuable products based on water cycle. Nano Res. 16 , 6444–6476 (2023).
Campbell, K. N. & Young, E. E. The addition of hydrogen to multiple carbon–carbon bonds. IV. The electrolytic reduction of alkyl and aryl acetylenes. J. Am. Chem. Soc. 65 , 965–967 (1943).
Schlesinger, H. I. & Burg, A. B. Recent developments in the chemistry of the boron hydrides. Chem. Rev. 31 , 1–41 (1942).
Harnisch, F. & Morejón, M. C. Hydrogen from water is more than a fuel: hydrogenations and hydrodeoxygenations for a biobased economy. Chem. Rec. 21 , 2277–228 (2021).
Xu, Y. & Zhang, B. Recent advances in electrochemical hydrogen production from water assisted by alternative oxidation reactions. ChemElectroChem 6 , 3214–3226 (2019).
Fuchigami, T., Inagi, S. & Atobe, M. Fundamentals and Applications of Organic Electrochemistry 1st edn (Wiley, 2015).
Jiang, J., Wu, B. & Cha, C. Electrosynthesis of p -methoxybenzaldehyde on graphite/Nafion membrane composite electrodes. Electrochim. Acta 43 , 2549 (1998).
Zhang, P. & Sun, L. Electrocatalytic hydrogenation and oxidation in aqueous conditions. Chin. J. Chem. 38 , 996–1004 (2020).
Liu, C., Wu, Y., Zhao, B. & Zhang, B. Designed nanomaterials for electrocatalytic organic hydrogenation using water as the hydrogen source. Acc. Chem. Res. 56 , 1872–1883 (2023).
Sun, H., Ou, W., Sun, L., Wang, B. & Su, C. Recent advances in nature-inspired nanocatalytic reduction of organic molecules with water. Nano Res. 15 , 10292–10315 (2022).
Akhade, S. A. et al. Electrocatalytic hydrogenation of biomass-derived organics: a review. Chem. Rev. 120 , 11370–11419 (2020).
Chen, G., Li, X. & Feng, X. Upgrading organic compounds through the coupling of electrooxidation with hydrogen evolution. Angew. Chem. Int. Ed. 61 , e202209014 (2022).
Heard, D. M. & Lennox, A. J. J. Electrode materials in modern organic electrochemistry. Angew. Chem. Int. Ed. 59 , 18866–18884 (2020).
Asefa, T. Metal-free and noble metal-free heteroatom-doped nanostructured carbons as prospective sustainable electrocatalysts. Acc. Chem. Res. 49 , 1873–1883 (2016).
Zhao, B.-H. et al. Economically viable electrocatalytic ethylene production with high yield and selectivity. Nat. Sustain. 6 , 827–837 (2023).
Leow, W. R. et al. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 368 , 1228–1233 (2020).
Inoue, H., Abe, T. & Iwakura, C. Successive hydrogenation of styrene at a palladium sheet electrode combined with electrochemical supply of hydrogen. Chem. Commun . https://doi.org/10.1039/CC9960000055 (1996).
Sherbo, R. S., Delima, R. S., Chiykowski, V. A., MacLeod, B. P. & Berlinguette, C. P. Complete electron economy by pairing electrolysis with hydrogenation. Nat. Catal. 1 , 501–507 (2018).
Sherbo, R. S., Kurimoto, A., Brown, C. M. & Berlinguette, C. P. Efficient electrocatalytic hydrogenation with a palladium membrane reactor. J. Am. Chem. Soc. 141 , 7815–7821 (2019).
Sato, T., Sato, S. & Itoh, N. Using a hydrogen-permeable palladium membrane electrode to produce hydrogen from water and hydrogenate toluene. Int. J. Hydrog. Energy 41 , 5419e5427 (2016).
Han, G., Li, G. & Sun, Y. Electrocatalytic dual hydrogenation of organic substrates with a faradaic efficiency approaching 200%. Nat. Catal. 6 , 224–233 (2023).
Yan, Y.-Q. et al. Electrochemistry-assisted selective butadiene hydrogenation with water. Nat. Commun. 14 , 2106 (2023).
Xu, Y. et al. Electrochemical hydrogenation of oxidized contaminants for water purification without supporting electrolyte. Nat. Water 1 , 95–103 (2023).
Zhang, Y. & Kornienko, N. C≡N triple bond cleavage via trans-membrane hydrogenation. Chem. Catal. 2 , 499–507 (2022).
Kurimoto, A., Sherbo, R. S., Cao, Y., Loo, N. W. X. & Berlinguette, C. P. Electrolytic deuteration of unsaturated bonds without using D 2 . Nat. Catal. 3 , 719–726 (2020).
Kurimoto, A. et al. Bioelectrocatalysis with a palladium membrane reactor. Nat. Commun. 14 , 1814 (2023).
Kurimoto, A. et al. Physical separation of H 2 activation from hydrogenation chemistry reveals the specific role of secondary metal catalysts. Angew. Chem. Int. Ed. 60 , 11937–11942 (2021).
Conde, J. J., Maroño, M. & Sánchez-Hervás, J. M. Pd-based membranes for hydrogen separation: review of alloying elements and their influence on membrane properties. Sep. Purif. Rev. 46 , 152–177 (2017).
Leech, M. C. & Lam, K. A practical guide to electrosynthesis. Nat. Rev. Chem. 6 , 275–286 (2022).
Pastor, E. et al. Complementary probes for the electrochemical interface. Nat. Rev. Chem . 8 , 159–178 (2024).
Bard, A. J. Inner-sphere heterogeneous electrode reactions. Electrocatalysis and photocatalysis: the challenge. J. Am. Chem. Soc. 132 , 7559–7567 (2010).
Shi, Y. & Zhang, B. Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 45 , 1529–1541 (2016).
Zheng, Y., Jiao, Y., Vasileff, A. & Qiao, S.-Z. The hydrogen evolution reaction in alkaline solution: from theory, single crystal models, to practical electrocatalysts. Angew. Chem. Int. Ed. 57 , 7568–7579 (2018).
Gnaim, S. et al. Cobalt-electrocatalytic HAT for functionalization of unsaturated C–C bonds. Nature 605 , 687–695 (2022).
Zhang, S. & Findlater, M. Electrochemically driven hydrogen atom transfer catalysis: a tool for C(sp 3 )/Si–H functionalization and hydrofunctionalization of alkenes. ACS Catal. 13 , 8731–8751 (2023).
Shevick, S. L. et al. Catalytic hydrogen atom transfer to alkenes: a roadmap for metal hydrides and radicals. Chem. Sci. 11 , 12401–12422 (2020).
Chadderdon, X. H. et al. Mechanisms of furfural reduction on metal electrodes: distinguishing pathways for selective hydrogenation of bioderived oxygenates. J. Am. Chem. Soc. 139 , 14120–14128 (2017).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355 , eaad4998 (2017).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152 , J23–J26 (2005).
Gao, W., Liu, S., Sun, G., Zhang, C. & Pan, Y. Single-atom catalysts for hydrogen activation. Small 19 , 2300956 (2023).
Elgrishi, N. et al. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95 , 197–206 (2018).
Hunter, B. M., Gray, H. B. & Müller, A. M. Earth-abundant heterogeneous water oxidation catalysts. Chem. Rev. 116 , 14120–14136 (2016).
Gao, L., Cui, X., Sewell, C. D., Li, J. & Lin, Z. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 50 , 8428–8469 (2021).
Huang, Z.-F. et al. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 4 , 329–338 (2019).
Xiao, K., Wang, Y., Wu, P., Hou, L. & Liu, Z.-Q. Activating lattice oxygen in spinel ZnCo 2 O 4 through filling oxygen vacancies with fluorine for electrocatalytic oxygen evolution. Angew. Chem. Int. Ed. 62 , e202301408 (2023).
Mallat, T. & Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 104 , 3037–3058 (2004).
Ryu, J. et al. Thermochemical aerobic oxidation catalysis in water can be analysed as two coupled electrochemical half-reactions. Nat. Catal. 4 , 742–752 (2021).
Ding, H., Liu, H., Chu, W., Wu, C. & Xie, Y. Structural transformation of heterogeneous materials for electrocatalytic oxygen evolution reaction. Chem. Rev. 121 , 13174–13212 (2021).
Huang, Y., Chong, X., Liu, C., Liang, Y. & Zhang, B. Boosting hydrogen production by anodic oxidation of primary amines over a NiSe nanorod electrode. Angew. Chem. Int. Ed. 57 , 13163–13166 (2018).
Huang, C., Huang, Y., Liu, C., Yu, Y. & Zhang, B. Integrating hydrogen production with aqueous selective semi-dehydrogenation of tetrahydroisoquinolines over a Ni 2 P bifunctional electrode. Angew. Chem. Int. Ed. 58 , 12014–12017 (2019).
Yan, Z. et al. Anion insertion enhanced electrodeposition of robust metal hydroxide/oxide electrodes for oxygen evolution. Nat. Commun. 9 , 2373 (2018).
Article PubMed PubMed Central Google Scholar
Yang, G. et al. Unraveling the mechanism for paired electrocatalysis of organics with water as a feedstock. Nat. Commun. 13 , 3125 (2022).
Wu, Z.-Y. et al. Non-iridium-based electrocatalyst for durable acidic oxygen evolution reaction in proton exchange membrane water electrolysis. Nat. Mater. 22 , 100–108 (2023).
Han, S. et al. Preferential adsorption of ethylene oxide on Fe and chlorine on Ni enabled scalable electrosynthesis of ethylene chlorohydrin. Angew. Chem. Int. Ed. 62 , e202216581 (2023).
Liu, C., Han, S., Li, M., Chong, X. & Zhang, B. Electrocatalytic deuteration of halides with D 2 O as the deuterium source over a copper nanowire arrays cathode. Angew. Chem. Int. Ed. 59 , 18527–18531 (2020).
Lu, L., Li, H., Zheng, Y., Bu, F. & Lei, A. Facile and economical electrochemical dehalogenative deuteration of (hetero)aryl halides. CCS Chem. 3 , 2669–2675 (2021).
Li, P. et al. Facile and general electrochemical deuteration of unactivated alkyl halides. Nat. Commun. 13 , 3774 (2022).
Davitt, H. J. & Albright, L. F. Electrochemical hydrogenation of ethylene, acetylene, and ethylene-acetylene mixtures. J. Electrochem. Soc. 118 , 236–242 (1971).
Rubinson, J. F., Behymer, T. D. & Mark, H. B. Direct reduction of acetylene at molybdenum modified polymeric sulfur nitride, (SN) x , electrodes. J. Am. Chem. Soc. 104 , 1224–1229 (1982).
Bu, J. et al. Selective electrocatalytic semihydrogenation of acetylene impurities for the production of polymer-grade ethylene. Nat. Catal. 4 , 557–564 (2021).
Li, B. & Ge, H. Highly selective electrochemical hydrogenation of alkynes: rapid construction of mechanochromic materials. Sci. Adv. 5 , eaaw2774 (2019).
Zhu, K. et al. Unraveling the role of interfacial water structure in electrochemical semihydrogenation of alkynes. ACS Catal. 12 , 4840–4847 (2022).
Ling, Y. et al. Selenium vacancy promotes transfer semihydrogenation of alkynes from water electrolysis. ACS Catal. 11 , 9471–9478 (2021).
Xing, C. et al. Highly selective electrocatalytic olefin hydrogenation in aqueous solution. Angew. Chem. Int. Ed. 62 , e202310722 (2023).
Liu, Z., Zhang, L., Ren, Z. & Zhang, J. Advances in selective electrocatalytic hydrogenation of alkynes to alkenes. Chem. Eur. J. 29 , e202202979 (2023).
Wu, Y., Liu, C., Wang, C., Lu, S. & Zhang, B. Selective transfer semihydrogenation of alkynes with H 2 O (D 2 O) as the H (D) source over a Pd-P cathode. Angew. Chem. Int. Ed. 59 , 21170–21175 (2020).
Wang, S. et al. Highly efficient ethylene production via electrocatalytic hydrogenation of acetylene under mild conditions. Nat. Commun. 12 , 7072 (2021).
Shi, R. et al. Room-temperature electrochemical acetylene reduction to ethylene with high conversion and selectivity. Nat. Catal. 4 , 565–574 (2021).
Blanco, D. E., Lee, B. & Modestino, M. A. Optimizing organic electrosynthesis through controlled voltage dosing and artificial intelligence. Proc. Natl Acad. Sci. USA 116 , 17683–17689 (2019).
Li, R. et al. One-pot H/D exchange and low-coordinated iron electrocatalyzed deuteration of nitriles in D 2 O to α , β -deuterio aryl ethylamines. Nat. Commun. 13 , 5951 (2022).
Xia, R. et al. Electrochemical reduction of acetonitrile to ethylamine. Nat. Commun. 12 , 1949 (2021).
Zhang, D. et al. Highly efficient electrochemical hydrogenation of acetonitrile to ethylamine for primary amine synthesis and promising hydrogen storage. Chem. Catal. 1 , 393–406 (2021).
He, M. et al. Field-induced concentration and low coordination-enhanced adsorption boost electroreductive deuteration of nitriles over copper nanotips. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-2618234/v1 (2023).
Wirtanen, T., Rodrigo, E. & Waldvogel, S. R. Recent advances in the electrochemical reduction of substrates involving N–O bonds. Adv. Synth. Catal. 362 , 2088–2101 (2020).
Zhang, P. et al. Paired electrocatalytic oxygenation and hydrogenation of organic substrates with water as the oxygen and hydrogen source. Angew. Chem. Int. Ed. 58 , 9155–9159 (2019).
Qiao, W. et al. Paired electrochemical N–N coupling employing a surface-hydroxylated Ni 3 Fe-MOF-OH bifunctional electrocatalyst with enhanced adsorption of nitroarenes and anilines. ACS Catal. 11 , 13510–13518 (2021).
Xu, C., Paone, E., Rodríguez-Padrón, D., Luque, R. & Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 49 , 4273–4306 (2020).
Fan, Z. et al. Recent developments in electrode materials for the selective upgrade of biomass-derived platform molecules into high-value-added chemicals and fuels. Green Chem. 24 , 7818–7868 (2022).
Chong, X., Liu, C., Huang, Y., Huang, C. & Zhang, B. Potential-tuned selective electrosynthesis of azoxy-, azo- and amino-aromatics over a CoP nanosheet cathode. Natl Sci. Rev. 7 , 285–295 (2020).
Anibal, J. & Xu, B. Electroreductive C−C coupling of furfural and benzaldehyde on Cu and Pb surfaces. ACS Catal. 10 , 11643–11653 (2020).
Ji, K. et al. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural promoted by a Ru 1 Cu single-atom alloy catalyst. Angew. Chem. Int. Ed. 61 , e202209849 (2022).
Wang, Y., Huang, Z. & Huang, Z. Catalyst as colour indicator for endpoint detection to enable selective alkyne trans-hydrogenation with ethanol. Nat. Catal. 2 , 529–536 (2019).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2 , 65–81 (2018).
Li, X. et al. Advances in heterogeneous single-cluster catalysis. Nat. Rev. Chem . 7 , 754–767 (2023).
He, X. et al. A versatile route to fabricate single atom catalysts with high chemoselectivity and regioselectivity in hydrogenation. Nat. Commun. 10 , 3663 (2019).
Zhu, Q. et al. Fully exposed cobalt nanoclusters anchored on nitrogen-doped carbon synthesized by a host-guest strategy for semi-hydrogenation of phenylacetylene. J. Catal. 405 , 499–507 (2022).
Li, H. et al. σ -Alkynyl adsorption enables electrocatalytic semi-hydrogenation of terminal alkynes with easy-reducible/passivated groups over amorphous PdS x nanocapsules. J. Am. Chem. Soc. 144 , 19456–19465 (2022).
Zhao, Y., Liu, C., Wang, C., Chong, X. & Zhang, B. Sulfur vacancy-promoted highly selective electrosynthesis of functionalized amino-arenes via transfer hydrogenation of nitroarenes with H 2 O over a Co 3 S 4−X nanosheet cathode. CCS Chem. 3 , 507–515 (2021).
You, B. & Sun, Y. Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 51 , 1571–1580 (2018).
Shi, Y. et al. Unveiling the promotion of surface‐adsorbed chalcogenate on the electrocatalytic oxygen evolution reaction. Angew. Chem. Int. Ed. 59 , 22470–22474 (2020).
Chong, X., Liu, C., Wang, C., Yang, R. & Zhang, B. Integrating hydrogen production and transfer hydrogenation with selenite promoted electrooxidation of α -nitrotoluenes to E -nitroethenes. Angew. Chem. Int. Ed. 60 , 22010–22016 (2021).
Zhao, G. et al. Electrochemical oxidation of 5-hydroxymethylfurfural on CeO 2 -modified Co 3 O 4 with regulated intermediate adsorption and promoted charge transfer. Adv. Funct. Mater. 33 , 2213170 (2023).
Li, S. et al. Doped Mn enhanced NiS electrooxidation performance of HMF into FDCA at industrial-level current density. Adv. Funct. Mater. 33 , 2214488 (2023).
Wang, T. et al. Combined anodic and cathodic hydrogen production from aldehyde oxidation and hydrogen evolution reaction. Nat. Catal. 5 , 66–73 (2022).
Li, G. et al. Dual hydrogen production from electrocatalytic water reduction coupled with formaldehyde oxidation via a copper-silver electrocatalyst. Nat. Commun. 14 , 525 (2023).
Tang, C. et al. Energy-saving electrolytic hydrogen generation: Ni 2 P nanoarray as a high-performance non-noble-metal electrocatalyst. Angew. Chem. Int. Ed. 56 , 842–846 (2017).
Zhang, J.-Y. et al. Anodic hydrazine oxidation assists energy-efficient hydrogen evolution over a bifunctional cobalt perselenide nanosheet electrode. Angew. Chem. Int. Ed. 57 , 7649–7653 (2018).
Sun, Y. et al. Highly selective electrocatalytic oxidation of amines to nitriles assisted by water oxidation on metal-doped α -Ni(OH) 2 . J. Am. Chem. Soc. 144 , 15185–15192 (2022).
Wang, W. et al. Vacancy-rich Ni(OH) 2 drives the electrooxidation of amino C–N bonds to nitrile C≡N bonds. Angew. Chem. Int. Ed. 59 , 16974–16981 (2020).
Wang, D. et al. Direct electrochemical oxidation of alcohols with hydrogen evolution in continuous-flow reactor. Nat. Commun. 10 , 2796 (2019).
Huang, H. et al. Ni, Co hydroxide triggers electrocatalytic production of high-purity benzoic acid over 400 mA cm −2 . Energy Environ. Sci. 13 , 4990–4999 (2020).
Goetz, M. K., Bender, M. T. & Choi, K.-S. Predictive control of selective secondary alcohol oxidation of glycerol on NiOOH. Nat. Commun. 13 , 5848 (2022).
Qi, Y. et al. Insights into the activity of nickel boride/nickel heterostructures for efficient methanol electrooxidation. Nat. Commun. 13 , 4602 (2022).
Liu, F. et al. Concerted and selective electrooxidation of PET-derived alcohol to glycolic acid at an industry-level current density over a Pd-Ni(OH) 2 catalyst. Angew. Chem. Int. Ed. 62 , e202300094 (2023).
Zhu, B. et al. Unraveling a bifunctional mechanism for methanol-to-formate electro-oxidation on nickel-based hydroxides. Nat. Commun. 14 , 1686 (2023).
Fleischmann, M., Korinek, K. & Pletcher, D. The oxidation of organic compounds at a nickel anode in alkaline solution. J. Electroanal. Chem. Interfacial Electrochem. 31 , 39–49 (1971).
Chen, W. et al. Unraveling the electrophilic oxygen-mediated mechanism for alcohol electrooxidation on NiO. Natl Sci. Rev. 10 , nwad099 (2023).
You, B., Liu, X., Jiang, N. & Sun, Y. A general strategy for decoupled hydrogen production from water splitting by integrating oxidative biomass valorization. J. Am. Chem. Soc. 138 , 13639–13646 (2016).
Yuan, Y. & Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution reactions. Acc. Chem. Res. 52 , 3309–3324 (2019).
Golden, D. L., Suh, S.-E. & Stahl, S. S. Radical C(sp 3 )–H functionalization and cross-coupling reactions. Nat. Rev. Chem. 6 , 405–427 (2022).
Wen, Q. et al. In situ chalcogen leaching manipulates reactant interface toward efficient amine electrooxidation. ACS Nano 16 , 9572–9582 (2022).
Kar, S. & Milstein, D. Oxidation of organic compounds using water as the oxidant with H 2 liberation catalyzed by molecular metal complexes. Acc. Chem. Res. 55 , 2304–2315 (2022).
Balaraman, E., Khaskin, E., Leitus, G. & Milstein, D. Catalytic transformation of alcohols to carboxylic acid salts and H 2 using water as the oxygen atom source. Nat. Chem. 5 , 122–125 (2013).
Tang, S., Ben-David, Y. & Milstein, D. Oxidation of alkenes by water with H 2 liberation. J. Am. Chem. Soc. 142 , 5980–5984 (2020).
Zhou, H. et al. Selectively upgrading lignin derivatives to carboxylates through electrochemical oxidative C(OH)–C bond cleavage by a Mn-doped cobalt oxyhydroxide catalyst. Angew. Chem. Int. Ed. 60 , 8976–8982 (2021).
Lum, Y. et al. Tuning OH binding energy enables selective electrochemical oxidation of ethylene to ethylene glycol. Nat. Catal. 3 , 14–22 (2020).
Jin, K. et al. Epoxidation of cyclooctene using water as the oxygen atom source at manganese oxide electrocatalysts. J. Am. Chem. Soc. 141 , 6413–6418 (2019).
Han, S. et al. Membrane-free selective oxidation of thioethers with water over a nickel phosphide nanocube electrode. Cell Rep. Phys. Sci. 2 , 100462 (2021).
Ogura, K. & Takamagari, K. Direct conversion of methane to methanol, chloromethane and dichloromethane at room temperature. Nature 319 , 308 (1986).
Wang, Q. et al. Electrocatalytic methane oxidation greatly promoted by chlorine intermediates. Angew. Chem. Int. Ed. 60 , 17398–17403 (2021).
Nutting, J. E., Rafiee, M. & Stahl, S. S. Tetramethylpiperidine N -oxyl (TEMPO), phthalimide N -oxyl (PINO), and pelated N -oxyl species: electrochemical properties and their use in electrocatalytic reactions. Chem. Rev. 118 , 4834–4885 (2018).
Lu, N.-N., Yoo, S. J., Li, L.-J., Zeng, C.-C. & Little, R. D. A comparative study of organic electron transfer redox mediators: electron transfer kinetics for triarylimidazole and triarylamine mediators in the oxidation of 4-methoxybenzyl alcohol. Electrochim. Acta 142 , 254–260 (2014).
Platen, M. & Sleckhan, E. Oxidative deblocking of the 4-methoxybenzyl thioether protecting group: application to the directed synthesis of poly-cystinyl peptides. Liebigs Ann. Chem . 1984 , 1563–1576 (1984).
Zhao, Y. et al. Br − /BrO − -mediated highly efficient photoelectrochemical epoxidation of alkenes on α -Fe 2 O 3 . Nat. Commun. 14 , 1943 (2023).
Liu, X. et al. Bromide-mediated photoelectrochemical epoxidation of alkenes using water as an oxygen source with conversion efficiency and selectivity up to 100%. J. Am. Chem. Soc. 144 , 19770–19777 (2022).
Green, R. A., Hill-Cousins, J. T., Brown, R. C. D., Pletcher, D. & Leach, S. G. A voltammetric study of the 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) mediated oxidation of benzyl alcohol in tert-butanol/water. Electrochim. Acta 113 , 550–556 (2013).
Andrieux, C. P., Robert, M. & Savéant, J.-M. Role of environmental factors in the dynamics of intramolecular dissociative electron transfer. Effect of solvation and ion-pairing on cleavage rates of anion radicals. J. Am. Chem. Soc. 117 , 9340–9346 (1995).
Han, C. et al. Electrocatalytic hydrogenation of alkenes with Pd/carbon nanotubes at an oil–water interface. Nat. Catal. 5 , 1110–1119 (2022).
Wu, Y. et al. Converting copper sulfide to copper with surface sulfur for electrocatalytic alkyne semi-hydrogenation with water. Nat. Commun. 12 , 3881 (2021).
Zhao, Y. et al. Dopant- and surfactant-tuned electrode-electrolyte interface enabling efficient alkynol semi-hydrogenation. J. Am. Chem. Soc. 145 , 6516–6525 (2023).
Gao, Y. et al. Field-induced reagent concentration and sulfur adsorption enable efficient electrocatalytic semihydrogenation of alkynes. Sci. Adv. 8 , eabm9477 (2022).
Li, Z. et al. Electrocatalytic synthesis of adipic acid coupled with H 2 production enhanced by a ligand modification strategy. Nat. Commun. 13 , 5009 (2022).
Guo, J. X. et al. Direct seawater electrolysis by adjusting catalyst’s local reaction environment. Nat. Energy 8 , 264–272 (2023).
CAS Google Scholar
Huang, L. S. et al. Ethylene electrooxidation to 2-chloroethanol in acidic seawater with natural chloride participation. J. Am. Chem. Soc. 145 , 15565–15571 (2023).
Marken, F., Cresswell, A. J. & Bull, S. D. Recent advances in paired electrosynthesis. Chem. Rec. 21 , 2585–2600 (2021).
Llorente, M. J., Nguyen, B. H., Kubiak, C. P. & Moeller, K. D. Paired electrolysis in the simultaneous production of synthetic intermediates and substrates. J. Am. Chem. Soc. 138 , 15110–15113 (2016).
Sheng, H. et al. Linear paired electrochemical valorization of glycerol enabled by the electro-Fenton process using a stable NiSe 2 cathode. Nat. Catal. 5 , 716–725 (2022).
Gonglach, S. et al. Molecular cobalt corrole complex for the heterogeneous electrocatalytic reduction of carbon dioxide. Nat. Commun. 10 , 3864 (2019).
Kumar, A., Phillips, K. R., Cai, J., Schröder, U. & Lienhard, J. H. Integrated valorization of desalination brine through NaOH recovery: opportunities and challenges. Angew. Chem. Int. Ed. 58 , 6502–6511 (2019).
Schäfer, H. J. Contributions of organic electrosynthesis to green chemistry. C. R. Chim. 14 , 745–765 (2011).
Guo, S. et al. Electrocatalytic hydrogenation of quinolines with water over a fluorine-modified cobalt catalyst. Nat. Commun. 13 , 5297 (2022).
Li, S. et al. Biomass valorization via paired electrosynthesis over vanadium nitride-based electrocatalysts. Adv. Funct. Mater. 29 , 1904780 (2019).
Chadderdon, X. H., Chadderdon, D. J., Pfennig, T., Shanks, B. H. & Li, W. Paired electrocatalytic hydrogenation and oxidation of 5-(hydroxymethyl)furfural for efficient production of biomass-derived monomers. Green Chem. 21 , 6210–6219 (2019).
Zhang, X. et al. Simultaneously high-rate furfural hydrogenation and oxidation upgrading on nanostructured transition metal phosphides through electrocatalytic conversion at ambient conditions. Appl. Catal. B 244 , 899–908 (2019).
Mo, Y. et al. Microfluidic electrochemistry for single-electron transfer redox-neutral reactions. Science 368 , 1352–1357 (2020).
Dong, X., Roeckl, J. L., S. Waldvogel, R. & Morandi, B. Merging shuttle reactions and paired electrolysis for reversible vicinal dihalogenations. Science 371 , 507–514 (2021).
Fagnani, D. E., Kim, D., Camarero, S. I., Alfaro, J. F. & McNeil, A. J. Using waste poly(vinyl chloride) to synthesize chloroarenes by plasticizer-mediated electro(de)chlorination. Nat. Chem. 15 , 222–229 (2023).
Yurko, Y. & Elbaz, L. Direct quinone fuel cells. J. Am. Chem. Soc. 145 , 2653–2660 (2023).
Liu, C. et al. Selectivity origin of organic electrosynthesis controlled by electrode materials: a case study on pinacols. ACS Catal. 11 , 8958–8967 (2021).
Hao, L. et al. Promoting electrocatalytic hydrogenation of oxalic acid to glycolic acid via an Al 3+ ion adsorption strategy coupled with ethylene glycol oxidation. ACS Appl. Mater. Interfaces 15 , 13176–13185 (2023).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364 , eaav3506 (2019).
Han, S. et al. Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism. Nat. Catal. 6 , 402–414 (2023).
Huang, Y., Wang, Y., Wu, Y., Yu, Y. & Zhang, B. Electrocatalytic construction of the C–N bond from the derivates of CO 2 and N 2 . Sci. China Chem. 65 , 204–206 (2022).
Wu, Y. et al. Electrosynthesis of 15 N-labeled amino acids from 15 N-nitrite and ketonic acids. Sci. China Chem. 66 , 1854–1859 (2023).
Meng, N. et al. Electrosynthesis of formamide from methanol and ammonia under ambient conditions. Nat. Commun. 13 , 5452 (2022).
Li, M. et al. Electrosynthesis of amino acids from NO and α -keto acids using two decoupled flow reactor. Nat. Catal. 6 , 906–915 (2023).
Li, J., Al-Mahayni, H., Chartrand, D., Seifitokaldani, A. & Kornienko, N. Electrochemical formation of C–S bonds from CO 2 and small molecule sulfur species. Nat. Synth. 2 , 757–765 (2023).
Zhang, Y. et al. Oxy-reductive C–N bond formation via pulsed electrolysis. Preprint at https://doi.org/10.26434/chemrxiv-2022-07rlk (2022).
Fan, L. et al. Selective production of ethylene glycol at high rate via cascade catalysis. Nat. Catal. 6 , 585–595 (2023).
Ko, M. et al. Direct propylene epoxidation with oxygen using a photo-electro-heterogeneous catalytic system. Nat. Catal. 5 , 37–44 (2022).
Sen, R. et al. Electrocatalytic water oxidation: an overview with an example of translation from lab to market. Front. Chem. 10 , 861604 (2022).
Shao, J. et al. Scalable electrosynthesis of formamide through C–N coupling at the industrially relevant current density of 120 mA cm −2 . Angew. Chem. Int. Ed. 61 , e202213009 (2022).
Zhou, H. et al. Scalable electrosynthesis of commodity chemicals from biomass by suppressing non-faradaic transformations. Nat. Commun. 14 , 5621 (2023).
Fukazawa, A. et al. A new approach to stereoselective electrocatalytic semihydrogenation of alkynes to Z -alkenes using a proton-exchange membrane reactor. ACS Sustain. Chem. Eng. 7 , 11050–11055 (2019).
Green, S. K., Tompsett, G. A., Kim, H. J., Kim, W. B. & Huber, G. W. Electrocatalytic reduction of acetone in a proton-exchange-membrane reactor: a model reaction for the electrocatalytic reduction of biomass. ChemSusChem 5 , 2410–2420 (2012).
Valiente, A., Martínez-Pardo, P., Kaur, G., Johansson, M. J. & Martín-Matute, B. Electrochemical proton reduction over nickel foam for Z -stereoselective semihydrogenation/deuteration of functionalized alkynes. ChemSusChem 15 , e202102221 (2022).
Jiang, M. et al. Promoted electrocatalytic hydrogenation of furfural in a bi-phasic system. Chem. Commun. 59 , 3101–3106 (2023).
Nilges, P. & Schröder, U. Electrochemistry for biofuel generation: production of furans by electrocatalytic hydrogenation of furfurals. Energy Environ. Sci. 6 , 2925–2931 (2013).
Liu, X., Liu, R., Qiu, J., Cheng, X. & Li, G. Chemical‐reductant‐free electrochemical deuteration reaction using deuterium oxide. Angew. Chem. Int. Ed. 59 , 13962–13967 (2020).
Liu, J., Chen, Z., Koh, M. J. & Loh, K. P. Deuterium labelling by electrochemical splitting of heavy water. Energy Mater. 1 , 100016 (2021).
Download references
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2023YFA1507400 to B.Z.) and the National Natural Science Foundation of China (22371205 to C.L.).
Author information
Authors and affiliations.
Institute of Molecular Plus, Department of Chemistry, School of Science, Tianjin University, Tianjin, China
Cuibo Liu, Fanpeng Chen, Bo-Hang Zhao, Yongmeng Wu & Bin Zhang
Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Frontiers Science Center for Synthetic Biology, Tianjin University, Tianjin, China
You can also search for this author in PubMed Google Scholar
Contributions
B.Z. conceived the Review. C.L. conducted the literature search and prepared the manuscript. C.L., F.C. and B.Z. generated the images. Y.W. and B.H.Z. participated in discussions. B.Z. supervised the project and revised the manuscript.
Corresponding author
Correspondence to Bin Zhang .
Ethics declarations
Competing interests.
The authors declare no competing interests.

Peer review
Peer review information.
Nature Reviews Chemistry thanks Haohong Duan, Soumyajit Roy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Liu, C., Chen, F., Zhao, BH. et al. Electrochemical hydrogenation and oxidation of organic species involving water. Nat Rev Chem (2024). https://doi.org/10.1038/s41570-024-00589-z
Download citation
Accepted : 20 February 2024
Published : 25 March 2024
DOI : https://doi.org/10.1038/s41570-024-00589-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Nature Chemistry

Subject Area and Category
- Chemical Engineering (miscellaneous)
- Chemistry (miscellaneous)
Nature Publishing Group
Publication type
17554330, 17554349
Information
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2022. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy

IMAGES
COMMENTS
Read the latest Research articles from Nature Chemistry. ... Nature Chemistry (Nat. Chem.) ISSN 1755-4349 (online) ISSN 1755-4330 (print) nature.com sitemap. About Nature Portfolio ...
A metal-catalysed functional group metathesis approach to the carbon isotope labelling of carboxylic acids. The preparation of 14 C-labelled compounds is a crucial step in pharmaceutical ...
Fossil fuel-driven thermochemical hydrogenation and oxidation using high-pressure H2 and O2 are still popular but energy-intensive CO2-emitting processes. At present, developing renewable energy ...
Scope. Nature Chemistry is a monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry. As well as reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry, the journal also features a broad range of chemical ...
Nature uses mechanochemical transduction processes to achieve diverse and vital functions, such as hearing, cellular adhesion and gating of ion channels. One fascinating example of biological ...