Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character

Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- April 01, 2024 | VOL. 181, NO. 4 CURRENT ISSUE pp.255-346
- March 01, 2024 | VOL. 181, NO. 3 pp.171-254
- February 01, 2024 | VOL. 181, NO. 2 pp.83-170
- January 01, 2024 | VOL. 181, NO. 1 pp.1-82
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Personalizing the Treatment of Substance Use Disorders
- Nora D. Volkow , M.D.
Search for more papers by this author
The opioid crisis in the United States has brought drug addiction to the forefront of the public mind and to the attention of health care personnel, organizations, and agencies. The epidemic of overdoses, beginning with those caused by prescription opioid analgesics and then broadening to include heroin and fentanyl and its analogs, has prompted major initiatives in local communities, states, and at the federal level to treat addiction and pain more effectively. The crisis has highlighted an insulated addiction treatment system that for decades was segregated from the rest of health care because of stigma associated with addiction and, by extension, the medications used to treat it. Stigmatizing attitudes have been slow to erode, but the moralizing and punitive viewpoints of the past are gradually giving way to a medical and even a cultural consensus that addiction is a chronic disorder of the brain, one that is strongly influenced by social factors, and one that is also treatable.
Parallel research in animal models and brain-imaging studies in individuals with substance use disorders has given us an increasingly precise picture of their neurobiology, including molecular and synaptic changes and the neuronal circuits involved, along with the consequences of their disruption. Most people are exposed to addictive substances at some point in their lives, including alcohol and nicotine, and many use these substances recreationally without developing addiction. Similarly, many patients who use opioids to treat their pain don’t develop addiction. But in a subset of individuals who are vulnerable because of genetics, age, and other variables, repeated exposure to addictive drugs diminishes the capacity of basal ganglia circuits to respond to natural reward and to motivate the behaviors needed for survival and well-being, while enhancing the sensitivity of stress and emotional circuits, including those from the extended amygdala, triggering anxiety and dysphoria when not taking the drug and weakening prefrontal executive-control circuitry necessary for self-regulation ( 1 ).
These changes, along with learning mechanisms that tie expectation of reward to drug cues, intensify each other in a kind of perfect storm: Inability to feel reward from non-drug activities, including social interactions, takes away the enjoyment of life and increases social isolation. Intense symptoms of withdrawal drive a search for temporary relief, and constant reminders of the drug in the environment contribute to persistent craving and preoccupation with obtaining the drug. Weakened capacity to resist the urge to take the drug or follow through on resolutions to quit leads, very often, to relapse and the accompanying regret or shame at having failed. Further increasing relapse risk are the frequently associated symptoms of depression, anxiety, and impaired sleep.
Until recently, the development of treatments for addiction was aimed at bringing about cessation of drug consumption (abstinence), which was the outcome required for U.S. Food and Drug Administration (FDA) approval of medications for substance use disorders. However, our current understanding of the mechanistic processes underlying addiction identifies a much broader set of clinically beneficial outcomes. For example, reduction of use in a person who uses heroin could decrease his or her risk of overdose, and improvements in sleep, depression, or executive function could also reduce relapse risk. In addition, technological advances and our growing understanding of the underlying neurobiology have given us the opportunity to target discrete neurobiological processes and personalize interventions to the unique deficits in a given individual and across the course of an individual’s disorder. A dimensional, personalized, and dynamic approach to treating substance use disorders could draw from medication use, neuromodulation techniques, behavioral approaches, and their combinations as the individual moves toward recovery.
Alternative Endpoints
To achieve a dimensional approach to treatment requires thinking anew about how we develop new treatments and what we expect in a treatment.
The existing pharmacopoeia for substance use disorders is severely limited. The FDA has approved medications only for alcohol, nicotine, and opioid use disorders ( Table 1 ), and currently there are no approved medications for cannabis, cocaine, methamphetamine, or inhalant use disorders. The absence of medications to treat most substance use disorders and the limited number of existing medications for alcohol, nicotine, and opioid use disorders make development of new therapeutics a high priority. Yet drug development for substance use disorders faces great hurdles.
a AMPA=α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; FDA=U.S. Food and Drug Administration; GABA=γ-aminobutyric acid; NMDA= N -methyl- d -aspartate.
TABLE 1. Drugs approved by the FDA for treatment of substance use disorders a
To obtain FDA approval for most substance use disorders, medications until recently had to demonstrate that they produce abstinence in a significant subset of patients, as measured by negative urine tests. However, the abstinence endpoint is a high bar to achieve, equivalent to requiring remission of pain from an analgesic or remission of depression from an antidepressant. Yet, the FDA granted approval of analgesics and antidepressants on the basis of reduction of symptom severity, not remission ( 2 ). The high bar for addiction medications has discouraged investment by the pharmaceutical industry, and significant public sector help was required to bring many of the currently available medications for substance use disorders to market, including buprenorphine, extended-release naltrexone, lofexidine, and naloxone nasal spray.
Treatment programs for substance use disorders inherited a dichotomous working definition of recovery from the 12-step world of past generations, where being completely “drug free” was not merely the gold standard but the only standard, short of which an addicted individual was regarded as having failed or would not be considered to be “recovering.” Yet evidence indicates that abstinence is not the only clinically relevant outcome for every individual and that alternative endpoints can contribute to recovery even when abstinence is not completely achieved.
Reduced alcohol use (measured as percentage of heavy drinking days) is now being used as an endpoint in clinical trials for treatments for alcohol use disorder. The FDA has also recently expressed its openness to considering endpoints other than abstinence as targets in medication development for other substance use disorders ( 3 , 4 ). Given the illegality of many addictive drugs, it has been argued that any reduction in use should be considered a benefit to the individual’s health and safety ( 5 ). Every time a person addicted to heroin must obtain the drug, he or she faces the risks associated with the drug trade as well as with exposure to fentanyl or a contaminant that could lead to overdose or poisoning.
Recently, researchers found in a pooled sample of study participants with cocaine use disorder that those who had high-frequency use at the start of the study and had reduced to low-frequency use by the end of the study showed outcomes at 1-year follow-up similar to those of participants who had quit altogether ( 6 ).
Treating the Dimensions of Substance Use Disorder
Endpoints other than abstinence may lead not only to treatments that are helpful in reducing drug use but also to the use of compounds that target specific neurobiological processes and symptoms relevant to addiction and the risk for relapse.
In April 2018, the FDA, in partnership with the Addiction Policy Forum and the National Institute on Drug Abuse (NIDA), convened a meeting to solicit input from patients with opioid use disorder as part of its Patient-Focused Drug Development initiative ( 7 ). Among other things, participants emphasized their desire for a more holistic and individualized approach to treatment, as well as their wish for medications that would address specific symptoms of withdrawal, such as cravings, depression, cognitive impairments, pain, and sleep problems. The same year, the FDA approved lofexidine for treating physical symptoms of opioid withdrawal during detoxification—the first approved drug for treating symptoms associated with opioid use disorder with a restricted purpose and not expected to lead, by itself, to continued abstinence. After detoxification, the individual would ideally be treated with naltrexone or buprenorphine as a longer-term treatment to help prevent relapse and achieve recovery. Other potential targets for medications are those that, while not addressing addiction directly, target major risk factors for relapse.
One such factor is insomnia, for it is frequently interrelated with substance use disorders, with each exacerbating the risk of the other. Findings of shared targets and circuits between disrupted sleep and addiction offer unique opportunities for treatment development. For example, while studying the role of orexin in narcolepsy, researchers serendipitously discovered an unusually high number of orexin-producing neurons in the postmortem brain of a heroin-addicted individual ( 8 ). They subsequently established in preclinical models and postmortem brain studies that long-term use of heroin was associated with an increase in orexin-producing neurons. Since orexin is already targeted by suvorexant, an FDA-approved drug for insomnia, NIDA is funding research to test its efficacy, along with that of other novel orexin receptor antagonists, as therapeutic agents in opioid use disorder.
Similarly, dysphoria and depression, which are frequently associated with protracted withdrawal, are another relevant area where our growing understanding of underlying neurocircuitry could guide selection of promising new targets. For example, the habenular complex is intricately involved in dysphoria and negative emotional states and is associated with depression ( 9 ) and addiction ( 10 ). Both alpha-5 nicotinic acetylcholine receptors and mu-opioid receptors are highly expressed in the habenula, where they modulate its activity, contributing to the adverse symptoms of withdrawal that follow nicotine and heroin discontinuation, respectively, and to the relief that follows during intoxication. Targeting the habenula has already been shown to be beneficial in animal models of addiction treatment ( 11 ), and it has been a target for deep brain stimulation for the treatment of depression ( 12 ).
Because of the high comorbidity of substance use disorder with depression, psychiatrists have used antidepressants off-label to treat their addicted patients, even though randomized clinical trials of antidepressants have failed to achieve the desired outcome of abstinence. Recognizing that improving depression could still be beneficial for patients with substance use disorders, studies should revisit the possible efficacy of antidepressants as an element of addiction treatment, using endpoints other than abstinence. Bupropion, which blocks the dopamine and norepinephrine transporters and is an approved antidepressant medication, is also approved for the treatment of nicotine addiction. Given the involvement of the mu-opioid receptor system in mood, it would be expected that targeting depression might have particular value in treating opioid use disorder; an interesting feature of the opioid partial agonist buprenorphine is that it has antidepressant properties ( 13 ), and opioid-addicted patients who have depression respond particularly well to this medication ( 14 ).
Another important therapeutic target is that of addressing social isolation, and while this might be optimally achieved with behavioral interventions, including group treatment, medications could still hold promise. Addicted individuals report reduced pleasure from social contact, as well as fear of the stigma attached to their drug use, and thus they tend to isolate themselves. Isolation in turn drives drug taking ( 15 ). Here again, we could take advantage of our increased understanding of the neurobiology of social attachment to bolster social connections. For example, oxytocin, a neurochemical involved in social bonding that also modulates key processes associated with addiction, including reward and stress responses, is being evaluated as a possible addiction treatment and may enhance the efficacy of psychosocial addiction treatments ( 16 , 17 ).
A dimensional approach to the treatment of substance use disorder is also relevant to neuromodulation. Early research has shown that transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) may be useful in reducing drug cravings, and TMS is already an approved therapy for treatment-resistant depression. Research is needed to study how TMS, tDCS, or peripheral nerve stimulation could be used to improve symptoms associated with addiction, from acute symptoms of withdrawal to the more protracted symptoms of dysphoria and sleep problems. As we understand better how to use neuromodulation technologies to modify brain circuits, it may create opportunities to strengthen specific circuits that can buffer or compensate for others that have been impaired by drug use or constitute a predisposing vulnerability.
Behavioral therapies are also suited to dimensional approaches to substance use disorder treatment. Considerable research already shows the benefits of cognitive-behavioral treatments in improving self-regulation and of contingency management in strengthening the degraded motivation to engage in non-drug-related activities, so clearly these modalities are effective for addressing specific dimensions of the addiction process. Similarly, behavioral treatments to improve executive function could help build resilience against relapse, as shown by methylphenidate’s reported ability to reduce impulsivity in individuals with cocaine use disorder ( 18 ).
Making Addiction Treatment More Dynamic and Personalized
Trajectories of use vary among people who use drugs, ranging from persistent use or declining use to cessation and relapse or sustained cessation. Studies of people who inject opioids, for example, have identified factors that, to some extent, are predictive of these trajectories ( 19 ). Being in a stable relationship, for instance, has been associated with early cessation (highlighting the importance of social support).
Addiction is an evolving disorder that changes through time and across the lifespan of the individual and one that has an unpredictable element that springs from the unique experiences an individual is exposed to. Some widely used behavioral treatments already accommodate and address this changeability of substance use disorder. Cognitive-behavioral therapy teaches the individual to identify external triggers and respond more appropriately to internal states (e.g., mood, craving) that place them at risk for relapse. New technologies are developing algorithms to identify indicators of relapse risk and incorporating them into wearable devices and smartphones with the goal of delivering an intervention in a timely, targeted manner. In the future, as big-data analytics and machine-learning algorithms yield more insight into behavioral and biological markers of relapse risk, tools or devices to avert relapse farther in advance may be developed.
Toward the Future
Neuroscience has revealed that addiction involves a set of interconnected processes that can be targeted strategically, rather than being a disorder defined principally by a single behavior (uncontrollable excessive drug use). Addiction medicine is also increasingly recognizing that factors traditionally associated with recovery are components of treatment. For example, for any meaningful recovery to occur, the individual must be able to integrate him- or herself into a socially meaningful environment. People with substance use disorders who are professionally active or engage in meaningful activity and have a caring family face less of a challenge than those who have no social supports and whose isolation places them at high risk for relapse. The integration of peer mentors, recovery coaching, and supportive housing into addiction treatment is an example of this shift, but more research is needed to determine the most effective ways to sustain social inclusion and to achieve recovery ( 20 ).
Addiction is a complex disorder that involves brain circuits necessary for survival and one that is strongly influenced by genes, development, and social factors. We now understand the underlying mechanisms well enough that we can turn this complexity into an opportunity to include these dimensions as targets for substance use disorder treatment, as well as to personalize interventions to accommodate the unique neurobiological characteristics and social contexts of individual patients.
Dr. Volkow is Director of the National Institute on Drug Abuse.
The author thanks Eric M. Wargo and Emily B. Einstein for their valuable help in the preparation of this article.
1 Koob GF, Volkow ND : Neurocircuitry of addiction . Neuropsychopharmacology 2010 ; 35:217–238 (erratum in Neuropsychopharmacology 2010 Mar; 35:1051) Crossref , Medline , Google Scholar
2 Volkow ND, Woodcock J, Compton WM, et al. : Medication development in opioid addiction: meaningful clinical end points . Sci Transl Med 2018 ; 10: eaan2595 Crossref , Medline , Google Scholar
3 Kiluk BD, Carroll KM, Duhig A, et al. : Measures of outcome for stimulant trials: ACTTION recommendations and research agenda . Drug Alcohol Depend 2016 ; 158:1–7 Crossref , Medline , Google Scholar
4 US Food and Drug Administration Draft Guidance (FDA): Opioid Use Disorder: Endpoints for Demonstrating Effectiveness of Drugs for Medication-Assisted Treatment: Guidance for Industry. Silver Spring, Md, FDA, August 2018. https://www.fda.gov/media/114948/download Google Scholar
5 McCann DJ, Ramey T, Skolnick P : Outcome measures in medication trials for substance use disorders . Curr Treat Options Psychiatry 2015 ; 2:113–121 Crossref , Google Scholar
6 Roos CR, Nich C, Mun CJ, et al. : Clinical validation of reduction in cocaine frequency level as an endpoint in clinical trials for cocaine use disorder . Drug Alcohol Depend 2019 ; 205: 107648 Crossref , Medline , Google Scholar
7 Center for Drug Evaluation and Research, US Food and Drug Administration: The Voice of the Patient: A Series of Reports From the US Food and Drug Administration’s (FDA’s) Patient-Focused Drug Development Initiative: Opioid Use Disorder. Rockville, Md, Center for Drug Evaluation and Research, 2018. https://www.fda.gov/media/124391/download Google Scholar
8 Thannickal TC, John J, Shan L, et al. : Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy . Sci Transl Med 2018 ; 10: eaao4953 Crossref , Medline , Google Scholar
9 Lawson RP, Nord CL, Seymour B, et al. : Disrupted habenula function in major depression . Mol Psychiatry 2017 ; 22:202–208 Crossref , Medline , Google Scholar
10 Velasquez KM, Molfese DL, Salas R : The role of the habenula in drug addiction . Front Hum Neurosci 2014 ; 8:174 Crossref , Medline , Google Scholar
11 Friedman A, Lax E, Dikshtein Y, et al. : Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior . Neuropharmacology 2010 ; 59:452–459 Crossref , Medline , Google Scholar
12 Morishita T, Fayad SM, Higuchi MA, et al. : Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes . Neurotherapeutics 2014 ; 11:475–484 Crossref , Medline , Google Scholar
13 Serafini G, Adavastro G, Canepa G, et al. : The efficacy of buprenorphine in major depression, treatment-resistant depression, and suicidal behavior: a systematic review . Int J Mol Sci 2018 ; 19:2410 Crossref , Google Scholar
14 Dreifuss JA, Griffin ML, Frost K, et al. : Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: results from a multisite study . Drug Alcohol Depend 2013 ; 131:112–118 Crossref , Medline , Google Scholar
15 Venniro M, Zhang M, Caprioli D, et al. : Volitional social interaction prevents drug addiction in rat models . Nat Neurosci 2018 ; 21:1520–1529 Crossref , Medline , Google Scholar
16 Stauffer CS, Moschetto JM, McKernan SM, et al. : Oxytocin-enhanced motivational interviewing group therapy for methamphetamine use disorder in men who have sex with men: study protocol for a randomized controlled trial . Trials 2019 ; 20:145 Crossref , Medline , Google Scholar
17 Lee MR, Weerts EM : Oxytocin for the treatment of drug and alcohol use disorders . Behav Pharmacol 2016 ; 27:640–648 Crossref , Medline , Google Scholar
18 Goldstein RZ, Woicik PA, Maloney T, et al. : Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task . Proc Natl Acad Sci USA 2010 ; 107:16667–16672 Crossref , Medline , Google Scholar
19 Dong H, Hayashi K, Singer J, et al. : Trajectories of injection drug use among people who use drugs in Vancouver, Canada, 1996–2017: growth mixture modeling using data from prospective cohort studies . Addiction 2019 ; 114:2173–2186 Crossref , Medline , Google Scholar
20 Substance Abuse and Mental Health Services Administration (SAMHSA): Social Inclusion (web page). Rockville, Md, SAMHSA, 2019. https://www.samhsa.gov/homelessness-programs-resources/hpr-resources/social-inclusion Google Scholar
- Ghazaleh Soleimani , Ph.D. ,
- Juho Joutsa , M.D., Ph.D. ,
- Khaled Moussawi , M.D., Ph.D. ,
- Shan H. Siddiqi , M.D. ,
- Rayus Kuplicki , Ph.D. ,
- Marom Bikson , Ph.D. ,
- Martin P. Paulus , M.D., Ph.D. ,
- Michael D. Fox , M.D., Ph.D. ,
- Colleen A. Hanlon , Ph.D. ,
- Hamed Ekhtiari , M.D., Ph.D.
- Lara N. Coughlin , Ph.D. ,
- Paul Pfeiffer , M.D. , M.S. ,
- Dara Ganoczy , M.P.H. ,
- Lewei A. Lin , M.D. , M.S.
- Ned H. Kalin , M.D.

- Substance Use Disorder Treatment
- Opioid Use Disorder
- Alcohol Use Disorder
- Nicotine Use Disorder
- Signs of Addiction
Addiction Research
Discover the latest in addiction research, from the neuroscience of substance use disorders to evidence-based treatment practices. reports, updates, case studies and white papers are available to you at hazelden betty ford’s butler center for research..

Why do people become addicted to alcohol and other drugs? How effective is addiction treatment? What makes certain substances so addictive? The Butler Center for Research at the Hazelden Betty Ford Foundation investigates these and other questions and publishes its scientific findings in a variety of alcohol and drug addiction research papers and reports. Research topics include:
- Evidence-based treatment practices
- Addiction treatment outcomes
- Addiction, psychiatry and the brain
- Addictive substances such as prescription opioids and heroin
- Substance abuse in youth/teens, older adults and other demographic groups such as health care or legal professionals
These research queries and findings are presented in the form of updates, white papers and case studies. In addition, the Butler Center for Research collaborates with the Recovery Advocacy team to study special-focus addiction research topics, summarized in monthly Emerging Drug Trends reports. Altogether, these studies provide the latest in addiction research for anyone interested in learning more about the neuroscience of addiction and how addiction affects individuals, families and society in general. The research also helps clinicians and health care professionals further understand, diagnose and treat drug and alcohol addiction. Learn more about each of the Butler Center's addiction research studies below.
Research Updates
Written by Butler Center for Research staff, our one-page, topic-specific summaries discuss current research on topics of interest within the drug abuse and addiction treatment field.
View our most recent updates, or view the archive at the bottom of the page.
Patient Outcomes Study Results at Hazelden Betty Ford
Trends and Patterns in Cannabis Use across Different Age Groups
Alcohol and Tobacco Harm Reduction Interventions
Harm Reduction: History and Context
Racial and Ethnic Health Disparities and Addiction
Psychedelics as Therapeutic Treatment
Sexual and Gender Minority Youth and SUDs
Health Care Professionals and Mental Health
Grief and Addiction
Helping Families Cope with Addiction
Emerging Drug Trends Report and National Surveys
Shedding New Light on America’s No. 1 Health Problem
In collaboration with the University of Maryland School of Public Health and with support from the Butler Center for Research, the Recovery Advocacy team routinely issues research reports on emerging drug trends in America. Recovery Advocacy also commissions national surveys on attitudes, behaviors and perspectives related to substance use. From binge drinking and excessive alcohol use on college campuses, to marijuana potency concerns in an age of legalized marijuana, deeper analysis and understanding of emerging drug trends allows for greater opportunities to educate, inform and prevent misuse and deaths.
Each drug trends report explores the topic at hand, documenting the prevalence of the problem, relevant demographics, prevention and treatment options available, as well as providing insight and perspectives from thought leaders throughout the Hazelden Betty Ford Foundation.
View the latest Emerging Drug Trends Report:
Pediatricians First Responders for Preventing Substance Use
- Clearing Away the Confusion: Marijuana Is Not a Public Health Solution to the Opioid Crisis
- Does Socioeconomic Advantage Lessen the Risk of Adolescent Substance Use?
- The Collegiate Recovery Movement Is Gaining Strength
- Considerations for Policymakers Regarding Involuntary Commitment for Substance Use Disorders
- Widening the Lens on the Opioid Crisis
- Concerns Rising Over High-Potency Marijuana Use
- Beyond Binging: “High-Intensity Drinking”
View the latest National Surveys :
- College Administrators See Problems As More Students View Marijuana As Safe
College Parents See Serious Problems From Campus Alcohol Use
- Youth Opioid Study: Attitudes and Usage
About Recovery Advocacy
Our mission is to provide a trusted national voice on all issues related to addiction prevention, treatment and recovery, and to facilitate conversation among those in recovery, those still suffering and society at large. We are committed to smashing stigma, shaping public policy and educating people everywhere about the problems of addiction and the promise of recovery. Learn more about recovery advocacy and how you can make a difference.
Evidence-Based Treatment Series
To help get consumers and clinicians on the same page, the Butler Center for Research has created a series of informational summaries describing:
- Evidence-based addiction treatment modalities
- Distinctive levels of substance use disorder treatment
- Specialized drug and alcohol treatment programs
Each evidence-based treatment series summary includes:
- A definition of the therapeutic approach, level of care or specialized program
- A discussion of applicability, usage and practice
- A description of outcomes and efficacy
- Research citations and related resources for more information
View the latest in this series:
Motivational Interviewing
Cognitive Behavioral Therapy
Case Studies and White Papers
Written by Hazelden Betty Ford Foundation researchers and clinicians, case studies and white papers presented by the Butler Center for Research provide invaluable insight into clinical processes and complex issues related to addiction prevention, treatment and recovery. These in-depth reports examine and chronicle clinical activities, initiatives and developments as a means of informing practitioners and continually improving the quality and delivery of substance use disorder services and related resources and initiatives.
- What does it really mean to be providing medication-assisted treatment for opioid addiction?
Adolescent Motivational Interviewing
Peer Recovery Support: Walking the Path Together
Addiction and Violence During COVID-19
The Brain Disease Model of Addiction
Healthcare Professionals and Compassion Fatigue
Moving to Trauma-Responsive Care
Virtual Intensive Outpatient Outcomes: Preliminary Findings
Driving Under the Influence of Cannabis
Vaping and E-Cigarettes
Using Telehealth for Addiction Treatment
Grandparents Raising Grandchildren
Substance Use Disorders Among Military Populations
Co-Occurring Mental Health and Substance Use Disorders
Women and Alcohol
Prescription Rates of Opioid Analgesics in Medical Treatment Settings
Applications of Positive Psychology to Substance Use Disorder
Substance Use Disorders Among Legal Professionals
Factors Impacting Early Alcohol and Drug Use Among Youths
Animal-Assisted Therapy for Substance Use Disorders
Prevalence of Adolescent Substance Misuse
Problem Drinking Behaviors Among College Students
The Importance of Recovery Management
Substance Use Factors Among LGBTQ individuals
Prescription Opioids and Dependence
Alcohol Abuse Among Law Enforcement Officers
Helping Families Cope with Substance Dependence
The Social Norms Approach to Student Substance Abuse Prevention
Drug Abuse, Dopamine and the Brain's Reward System
Women and Substance Abuse
Substance Use in the Workplace
Health Care Professionals: Addiction and Treatment
Cognitive Improvement and Alcohol Recovery
Drug Use, Misuse and Dependence Among Older Adults
Emerging Drug Trends
Does Socioeconomic Advantage Lessen the Risk of Adolescent Substance Use
The Collegiate Recovery Movement is Gaining Strength
Involuntary Commitment for Substance Use Disorders
Widening the Lens of the Opioid Crisis
Beyond Binge Drinking: High Intensity Drinking
High Potency Marijuana
National Surveys
College Administrators See Problems as More Students View Marijuana as Safe
Risky Opioid Use Among College-Age Youth
Case Studies/ White Papers
What does it really mean to be providing medication-assisted treatment for opioid addiction
Are you or a loved one struggling with alcohol or other drugs? Call today to speak confidentially with a recovery expert. Most insurance accepted.
Harnessing science, love and the wisdom of lived experience, we are a force of healing and hope for individuals, families and communities affected by substance use and mental health conditions..
Research Topics
The National Institute on Drug Abuse (NIDA) is the largest supporter of the world’s research on substance use and addiction. Part of the National Institutes of Health, NIDA conducts and supports biomedical research to advance the science on substance use and addiction and improve individual and public health. Look below for more information on drug use, health, and NIDA’s research efforts.
Information provided by NIDA is not a substitute for professional medical care.
In an emergency? Need treatment?
In an emergency:.
- Are you or someone you know experiencing severe symptoms or in immediate danger? Please seek immediate medical attention by calling 9-1-1 or visiting an Emergency Department . Poison control can be reached at 1-800-222-1222 or www.poison.org .
- Are you or someone you know experiencing a substance use and/or mental health crisis or any other kind of emotional distress? Please call or text 988 or chat www.988lifeline.org to reach the 988 Suicide & Crisis Lifeline. 988 connects you with a trained crisis counselor who can help.
FIND TREATMENT:
- For referrals to substance use and mental health treatment programs, call the Substance Abuse and Mental Health Administration (SAMHSA) National Helpline at 1-800-662-HELP (4357) or visit www.FindTreatment.gov to find a qualified healthcare provider in your area.
- For other personal medical advice, please speak to a qualified health professional. Find more health resources on USA.gov .
DISCLAIMER:
The emergency and referral resources listed above are available to individuals located in the United States and are not operated by the National Institute on Drug Abuse (NIDA). NIDA is a biomedical research organization and does not provide personalized medical advice, treatment, counseling, or legal consultation. Information provided by NIDA is not a substitute for professional medical care or legal consultation.

Research by Substance
Find evidence-based information on specific drugs and substance use disorders.
- Cannabis (Marijuana)
- Commonly Used Drugs Charts
- Methamphetamine
- MDMA (Ecstasy/Molly)
- Over-the-Counter Medicines
- Prescription Medicines
- Psilocybin (Magic Mushrooms)
- Psychedelic and Dissociative Drugs
- Psychedelic and Dissociative Drugs as Medicines
- Steroids (Anabolic)
- Synthetic Cannabinoids (K2/Spice)
- Synthetic Cathinones (Bath Salts)
- Tobacco/Nicotine and Vaping

Drug Use and Addiction
Learn how science has deepened our understanding of drug use and its impact on individual and public health.
- Addiction Science
- Adolescent Brain
- Comorbidity
- Drug Checking
- Drug Testing
- Drugged Driving
- Drugs and the Brain
- Harm Reduction
- Infographics
- Mental Health
- Monitoring the Future
- National Drug Early Warning System (NDEWS)
- Overdose Death Rates
- Overdose Prevention Centers
- Overdose Reversal Medications
- Stigma and Discrimination
- Syringe Services Programs
- Trauma and Stress
- Trends and Statistics
- Words Matter: Preferred Language for Talking About Addiction

People and Places
NIDA research supports people affected by substance use and addiction throughout the lifespan and across communities.
- College-Age and Young Adults
- Criminal Justice
- Global Health
- LGBTQ Populations and Substance Use
- Military Life and Substance Use
- National Drug and Alcohol Facts Week Organizers and Participants
- Older Adults
- Parents and Educators
- Women and Drugs
Related Resources
- Learn more about Overdose Prevention from the Department of Health and Human Services.
- Learn more about substance use treatment, prevention, recovery support, and related services from the Substance Abuse and Mental Health Services Administration .
- Learn more about the health effects of alcohol and alcohol use disorder from the National Institute on Alcohol Abuse and Alcoholism .
- Learn more the approval and regulation of prescription medicines from the Food and Drug Administration .
- Learn more about efforts to measure, prevent, and address public health impacts of substance use from the Centers for Disease Control and Prevention .
- Learn more about controlled substance law and regulation enforcement from the Drug Enforcement Administration .
- Learn more about policies impacting substance use from the Office of National Drug Control Policy .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 22 February 2021
Addiction as a brain disease revised: why it still matters, and the need for consilience
- Markus Heilig 1 ,
- James MacKillop ORCID: orcid.org/0000-0003-4118-9500 2 , 3 ,
- Diana Martinez 4 ,
- Jürgen Rehm ORCID: orcid.org/0000-0001-5665-0385 5 , 6 , 7 , 8 ,
- Lorenzo Leggio ORCID: orcid.org/0000-0001-7284-8754 9 &
- Louk J. M. J. Vanderschuren ORCID: orcid.org/0000-0002-5379-0363 10
Neuropsychopharmacology volume 46 , pages 1715–1723 ( 2021 ) Cite this article
84k Accesses
94 Citations
321 Altmetric
Metrics details
The view that substance addiction is a brain disease, although widely accepted in the neuroscience community, has become subject to acerbic criticism in recent years. These criticisms state that the brain disease view is deterministic, fails to account for heterogeneity in remission and recovery, places too much emphasis on a compulsive dimension of addiction, and that a specific neural signature of addiction has not been identified. We acknowledge that some of these criticisms have merit, but assert that the foundational premise that addiction has a neurobiological basis is fundamentally sound. We also emphasize that denying that addiction is a brain disease is a harmful standpoint since it contributes to reducing access to healthcare and treatment, the consequences of which are catastrophic. Here, we therefore address these criticisms, and in doing so provide a contemporary update of the brain disease view of addiction. We provide arguments to support this view, discuss why apparently spontaneous remission does not negate it, and how seemingly compulsive behaviors can co-exist with the sensitivity to alternative reinforcement in addiction. Most importantly, we argue that the brain is the biological substrate from which both addiction and the capacity for behavior change arise, arguing for an intensified neuroscientific study of recovery. More broadly, we propose that these disagreements reveal the need for multidisciplinary research that integrates neuroscientific, behavioral, clinical, and sociocultural perspectives.
Similar content being viewed by others
Subtypes in addiction and their neurobehavioral profiles across three functional domains
Gunner Drossel, Leyla R. Brucar, … Anna Zilverstand
Drug addiction: from bench to bedside
Julian Cheron & Alban de Kerchove d’Exaerde
The neurobiology of drug addiction: cross-species insights into the dysfunction and recovery of the prefrontal cortex
Ahmet O. Ceceli, Charles W. Bradberry & Rita Z. Goldstein
Introduction
Close to a quarter of a century ago, then director of the US National Institute on Drug Abuse Alan Leshner famously asserted that “addiction is a brain disease”, articulated a set of implications of this position, and outlined an agenda for realizing its promise [ 1 ]. The paper, now cited almost 2000 times, put forward a position that has been highly influential in guiding the efforts of researchers, and resource allocation by funding agencies. A subsequent 2000 paper by McLellan et al. [ 2 ] examined whether data justify distinguishing addiction from other conditions for which a disease label is rarely questioned, such as diabetes, hypertension or asthma. It concluded that neither genetic risk, the role of personal choices, nor the influence of environmental factors differentiated addiction in a manner that would warrant viewing it differently; neither did relapse rates, nor compliance with treatment. The authors outlined an agenda closely related to that put forward by Leshner, but with a more clinical focus. Their conclusion was that addiction should be insured, treated, and evaluated like other diseases. This paper, too, has been exceptionally influential by academic standards, as witnessed by its ~3000 citations to date. What may be less appreciated among scientists is that its impact in the real world of addiction treatment has remained more limited, with large numbers of patients still not receiving evidence-based treatments.
In recent years, the conceptualization of addiction as a brain disease has come under increasing criticism. When first put forward, the brain disease view was mainly an attempt to articulate an effective response to prevailing nonscientific, moralizing, and stigmatizing attitudes to addiction. According to these attitudes, addiction was simply the result of a person’s moral failing or weakness of character, rather than a “real” disease [ 3 ]. These attitudes created barriers for people with substance use problems to access evidence-based treatments, both those available at the time, such as opioid agonist maintenance, cognitive behavioral therapy-based relapse prevention, community reinforcement or contingency management, and those that could result from research. To promote patient access to treatments, scientists needed to argue that there is a biological basis beneath the challenging behaviors of individuals suffering from addiction. This argument was particularly targeted to the public, policymakers and health care professionals, many of whom held that since addiction was a misery people brought upon themselves, it fell beyond the scope of medicine, and was neither amenable to treatment, nor warranted the use of taxpayer money.
Present-day criticism directed at the conceptualization of addiction as a brain disease is of a very different nature. It originates from within the scientific community itself, and asserts that this conceptualization is neither supported by data, nor helpful for people with substance use problems [ 4 , 5 , 6 , 7 , 8 ]. Addressing these critiques requires a very different perspective, and is the objective of our paper. We readily acknowledge that in some cases, recent critiques of the notion of addiction as a brain disease as postulated originally have merit, and that those critiques require the postulates to be re-assessed and refined. In other cases, we believe the arguments have less validity, but still provide an opportunity to update the position of addiction as a brain disease. Our overarching concern is that questionable arguments against the notion of addiction as a brain disease may harm patients, by impeding access to care, and slowing development of novel treatments.
A premise of our argument is that any useful conceptualization of addiction requires an understanding both of the brains involved, and of environmental factors that interact with those brains [ 9 ]. These environmental factors critically include availability of drugs, but also of healthy alternative rewards and opportunities. As we will show, stating that brain mechanisms are critical for understanding and treating addiction in no way negates the role of psychological, social and socioeconomic processes as both causes and consequences of substance use. To reflect this complex nature of addiction, we have assembled a team with expertise that spans from molecular neuroscience, through animal models of addiction, human brain imaging, clinical addiction medicine, to epidemiology. What brings us together is a passionate commitment to improving the lives of people with substance use problems through science and science-based treatments, with empirical evidence as the guiding principle.
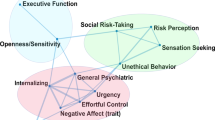
To achieve this goal, we first discuss the nature of the disease concept itself, and why we believe it is important for the science and treatment of addiction. This is followed by a discussion of the main points raised when the notion of addiction as a brain disease has come under criticism. Key among those are claims that spontaneous remission rates are high; that a specific brain pathology is lacking; and that people suffering from addiction, rather than behaving “compulsively”, in fact show a preserved ability to make informed and advantageous choices. In the process of discussing these issues, we also address the common criticism that viewing addiction as a brain disease is a fully deterministic theory of addiction. For our argument, we use the term “addiction” as originally used by Leshner [ 1 ]; in Box 1 , we map out and discuss how this construct may relate to the current diagnostic categories, such as Substance Use Disorder (SUD) and its different levels of severity (Fig. 1) .
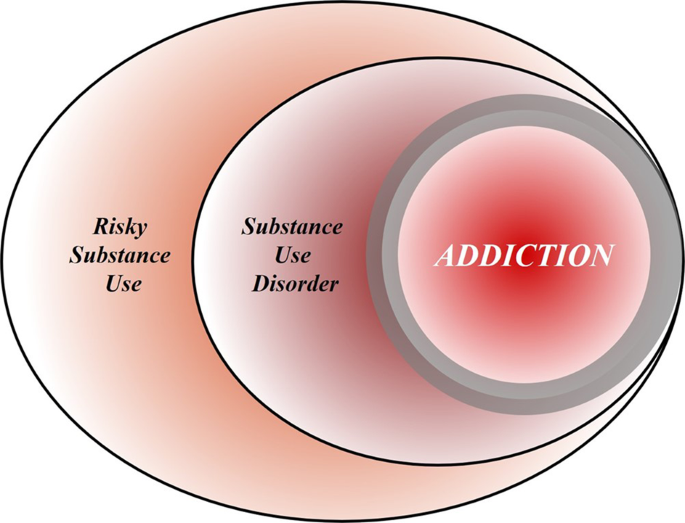
Risky (hazardous) substance use refers to quantity/frequency indicators of consumption; SUD refers to individuals who meet criteria for a DSM-5 diagnosis (mild, moderate, or severe); and addiction refers to individuals who exhibit persistent difficulties with self-regulation of drug consumption. Among high-risk individuals, a subgroup will meet criteria for SUD and, among those who have an SUD, a further subgroup would be considered to be addicted to the drug. However, the boundary for addiction is intentionally blurred to reflect that the dividing line for defining addiction within the category of SUD remains an open empirical question.
Box 1 What’s in a name? Differentiating hazardous use, substance use disorder, and addiction
Although our principal focus is on the brain disease model of addiction, the definition of addiction itself is a source of ambiguity. Here, we provide a perspective on the major forms of terminology in the field.
Hazardous Substance Use
Hazardous (risky) substance use refers to quantitative levels of consumption that increase an individual’s risk for adverse health consequences. In practice, this pertains to alcohol use [ 110 , 111 ]. Clinically, alcohol consumption that exceeds guidelines for moderate drinking has been used to prompt brief interventions or referral for specialist care [ 112 ]. More recently, a reduction in these quantitative levels has been validated as treatment endpoints [ 113 ].
Substance Use Disorder
SUD refers to the DSM-5 diagnosis category that encompasses significant impairment or distress resulting from specific categories of psychoactive drug use. The diagnosis of SUD is operationalized as 2 or more of 11 symptoms over the past year. As a result, the diagnosis is heterogenous, with more than 1100 symptom permutations possible. The diagnosis in DSM-5 is the result of combining two diagnoses from the DSM-IV, abuse and dependence, which proved to be less valid than a single dimensional approach [ 114 ]. Critically, SUD includes three levels of severity: mild (2–3 symptoms), moderate (4–5 symptoms), and severe (6+ symptoms). The International Classification of Diseases (ICD) system retains two diagnoses, harmful use (lower severity) and substance dependence (higher severity).
Addiction is a natural language concept, etymologically meaning enslavement, with the contemporary meaning traceable to the Middle and Late Roman Republic periods [ 115 ]. As a scientific construct, drug addiction can be defined as a state in which an individual exhibits an inability to self-regulate consumption of a substance, although it does not have an operational definition. Regarding clinical diagnosis, as it is typically used in scientific and clinical parlance, addiction is not synonymous with the simple presence of SUD. Nowhere in DSM-5 is it articulated that the diagnostic threshold (or any specific number/type of symptoms) should be interpreted as reflecting addiction, which inherently connotes a high degree of severity. Indeed, concerns were raised about setting the diagnostic standard too low because of the issue of potentially conflating a low-severity SUD with addiction [ 116 ]. In scientific and clinical usage, addiction typically refers to individuals at a moderate or high severity of SUD. This is consistent with the fact that moderate-to-severe SUD has the closest correspondence with the more severe diagnosis in ICD [ 117 , 118 , 119 ]. Nonetheless, akin to the undefined overlap between hazardous use and SUD, the field has not identified the exact thresholds of SUD symptoms above which addiction would be definitively present.
Integration
The ambiguous relationships among these terms contribute to misunderstandings and disagreements. Figure 1 provides a simple working model of how these terms overlap. Fundamentally, we consider that these terms represent successive dimensions of severity, clinical “nesting dolls”. Not all individuals consuming substances at hazardous levels have an SUD, but a subgroup do. Not all individuals with a SUD are addicted to the drug in question, but a subgroup are. At the severe end of the spectrum, these domains converge (heavy consumption, numerous symptoms, the unambiguous presence of addiction), but at low severity, the overlap is more modest. The exact mapping of addiction onto SUD is an open empirical question, warranting systematic study among scientists, clinicians, and patients with lived experience. No less important will be future research situating our definition of SUD using more objective indicators (e.g., [ 55 , 120 ]), brain-based and otherwise, and more precisely in relation to clinical needs [ 121 ]. Finally, such work should ultimately be codified in both the DSM and ICD systems to demarcate clearly where the attribution of addiction belongs within the clinical nosology, and to foster greater clarity and specificity in scientific discourse.
What is a disease?
In his classic 1960 book “The Disease Concept of Alcoholism”, Jellinek noted that in the alcohol field, the debate over the disease concept was plagued by too many definitions of “alcoholism” and too few definitions of “disease” [ 10 ]. He suggested that the addiction field needed to follow the rest of medicine in moving away from viewing disease as an “entity”, i.e., something that has “its own independent existence, apart from other things” [ 11 ]. To modern medicine, he pointed out, a disease is simply a label that is agreed upon to describe a cluster of substantial, deteriorating changes in the structure or function of the human body, and the accompanying deterioration in biopsychosocial functioning. Thus, he concluded that alcoholism can simply be defined as changes in structure or function of the body due to drinking that cause disability or death. A disease label is useful to identify groups of people with commonly co-occurring constellations of problems—syndromes—that significantly impair function, and that lead to clinically significant distress, harm, or both. This convention allows a systematic study of the condition, and of whether group members benefit from a specific intervention.
It is not trivial to delineate the exact category of harmful substance use for which a label such as addiction is warranted (See Box 1 ). Challenges to diagnostic categorization are not unique to addiction, however. Throughout clinical medicine, diagnostic cut-offs are set by consensus, commonly based on an evolving understanding of thresholds above which people tend to benefit from available interventions. Because assessing benefits in large patient groups over time is difficult, diagnostic thresholds are always subject to debate and adjustments. It can be debated whether diagnostic thresholds “merely” capture the extreme of a single underlying population, or actually identify a subpopulation that is at some level distinct. Resolving this issue remains challenging in addiction, but once again, this is not different from other areas of medicine [see e.g., [ 12 ] for type 2 diabetes]. Longitudinal studies that track patient trajectories over time may have a better ability to identify subpopulations than cross-sectional assessments [ 13 ].
By this pragmatic, clinical understanding of the disease concept, it is difficult to argue that “addiction” is unjustified as a disease label. Among people who use drugs or alcohol, some progress to using with a quantity and frequency that results in impaired function and often death, making substance use a major cause of global disease burden [ 14 ]. In these people, use occurs with a pattern that in milder forms may be challenging to capture by current diagnostic criteria (See Box 1 ), but is readily recognized by patients, their families and treatment providers when it reaches a severity that is clinically significant [see [ 15 ] for a classical discussion]. In some cases, such as opioid addiction, those who receive the diagnosis stand to obtain some of the greatest benefits from medical treatments in all of clinical medicine [ 16 , 17 ]. Although effect sizes of available treatments are more modest in nicotine [ 18 ] and alcohol addiction [ 19 ], the evidence supporting their efficacy is also indisputable. A view of addiction as a disease is justified, because it is beneficial: a failure to diagnose addiction drastically increases the risk of a failure to treat it [ 20 ].
Of course, establishing a diagnosis is not a requirement for interventions to be meaningful. People with hazardous or harmful substance use who have not (yet) developed addiction should also be identified, and interventions should be initiated to address their substance-related risks. This is particularly relevant for alcohol, where even in the absence of addiction, use is frequently associated with risks or harm to self, e.g., through cardiovascular disease, liver disease or cancer, and to others, e.g., through accidents or violence [ 21 ]. Interventions to reduce hazardous or harmful substance use in people who have not developed addiction are in fact particularly appealing. In these individuals, limited interventions are able to achieve robust and meaningful benefits [ 22 ], presumably because patterns of misuse have not yet become entrenched.
Thus, as originally pointed out by McLellan and colleagues, most of the criticisms of addiction as a disease could equally be applied to other medical conditions [ 2 ]. This type of criticism could also be applied to other psychiatric disorders, and that has indeed been the case historically [ 23 , 24 ]. Today, there is broad consensus that those criticisms were misguided. Few, if any healthcare professionals continue to maintain that schizophrenia, rather than being a disease, is a normal response to societal conditions. Why, then, do people continue to question if addiction is a disease, but not whether schizophrenia, major depressive disorder or post-traumatic stress disorder are diseases? This is particularly troubling given the decades of data showing high co-morbidity of addiction with these conditions [ 25 , 26 ]. We argue that it comes down to stigma. Dysregulated substance use continues to be perceived as a self-inflicted condition characterized by a lack of willpower, thus falling outside the scope of medicine and into that of morality [ 3 ].
Chronic and relapsing, developmentally-limited, or spontaneously remitting?
Much of the critique targeted at the conceptualization of addiction as a brain disease focuses on its original assertion that addiction is a chronic and relapsing condition. Epidemiological data are cited in support of the notion that large proportions of individuals achieve remission [ 27 ], frequently without any formal treatment [ 28 , 29 ] and in some cases resuming low risk substance use [ 30 ]. For instance, based on data from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) study [ 27 ], it has been pointed out that a significant proportion of people with an addictive disorder quit each year, and that most afflicted individuals ultimately remit. These spontaneous remission rates are argued to invalidate the concept of a chronic, relapsing disease [ 4 ].
Interpreting these and similar data is complicated by several methodological and conceptual issues. First, people may appear to remit spontaneously because they actually do, but also because of limited test–retest reliability of the diagnosis [ 31 ]. For instance, using a validated diagnostic interview and trained interviewers, the Collaborative Studies on Genetics of Alcoholism examined the likelihood that an individual diagnosed with a lifetime history of substance dependence would retain this classification after 5 years. This is obviously a diagnosis that, once met, by definition cannot truly remit. Lifetime alcohol dependence was indeed stable in individuals recruited from addiction treatment units, ~90% for women, and 95% for men. In contrast, in a community-based sample similar to that used in the NESARC [ 27 ], stability was only ~30% and 65% for women and men, respectively. The most important characteristic that determined diagnostic stability was severity. Diagnosis was stable in severe, treatment-seeking cases, but not in general population cases of alcohol dependence.
These data suggest that commonly used diagnostic criteria alone are simply over-inclusive for a reliable, clinically meaningful diagnosis of addiction. They do identify a core group of treatment seeking individuals with a reliable diagnosis, but, if applied to nonclinical populations, also flag as “cases” a considerable halo of individuals for whom the diagnostic categorization is unreliable. Any meaningful discussion of remission rates needs to take this into account, and specify which of these two populations that is being discussed. Unfortunately, the DSM-5 has not made this task easier. With only 2 out of 11 symptoms being sufficient for a diagnosis of SUD, it captures under a single diagnostic label individuals in a “mild” category, whose diagnosis is likely to have very low test–retest reliability, and who are unlikely to exhibit a chronic relapsing course, together with people at the severe end of the spectrum, whose diagnosis is reliable, many of whom do show a chronic relapsing course.
The NESARC data nevertheless show that close to 10% of people in the general population who are diagnosed with alcohol addiction (here equated with DSM-IV “dependence” used in the NESARC study) never remitted throughout their participation in the survey. The base life-time prevalence of alcohol dependence in NESARC was 12.5% [ 32 ]. Thus, the data cited against the concept of addiction as a chronic relapsing disease in fact indicate that over 1% of the US population develops an alcohol-related condition that is associated with high morbidity and mortality, and whose chronic and/or relapsing nature cannot be disputed, since it does not remit.
Secondly, the analysis of NESARC data [ 4 , 27 ] omits opioid addiction, which, together with alcohol and tobacco, is the largest addiction-related public health problem in the US [ 33 ]. This is probably the addictive condition where an analysis of cumulative evidence most strikingly supports the notion of a chronic disorder with frequent relapses in a large proportion of people affected [ 34 ]. Of course, a large number of people with opioid addiction are unable to express the chronic, relapsing course of their disease, because over the long term, their mortality rate is about 15 times greater than that of the general population [ 35 ]. However, even among those who remain alive, the prevalence of stable abstinence from opioid use after 10–30 years of observation is <30%. Remission may not always require abstinence, for instance in the case of alcohol addiction, but is a reasonable proxy for remission with opioids, where return to controlled use is rare. Embedded in these data is a message of literally vital importance: when opioid addiction is diagnosed and treated as a chronic relapsing disease, outcomes are markedly improved, and retention in treatment is associated with a greater likelihood of abstinence.
The fact that significant numbers of individuals exhibit a chronic relapsing course does not negate that even larger numbers of individuals with SUD according to current diagnostic criteria do not. For instance, in many countries, the highest prevalence of substance use problems is found among young adults, aged 18–25 [ 36 ], and a majority of these ‘age out’ of excessive substance use [ 37 ]. It is also well documented that many individuals with SUD achieve longstanding remission, in many cases without any formal treatment (see e.g., [ 27 , 30 , 38 ]).
Collectively, the data show that the course of SUD, as defined by current diagnostic criteria, is highly heterogeneous. Accordingly, we do not maintain that a chronic relapsing course is a defining feature of SUD. When present in a patient, however, such as course is of clinical significance, because it identifies a need for long-term disease management [ 2 ], rather than expectations of a recovery that may not be within the individual’s reach [ 39 ]. From a conceptual standpoint, however, a chronic relapsing course is neither necessary nor implied in a view that addiction is a brain disease. This view also does not mean that it is irreversible and hopeless. Human neuroscience documents restoration of functioning after abstinence [ 40 , 41 ] and reveals predictors of clinical success [ 42 ]. If anything, this evidence suggests a need to increase efforts devoted to neuroscientific research on addiction recovery [ 40 , 43 ].
Lessons from genetics
For alcohol addiction, meta-analysis of twin and adoption studies has estimated heritability at ~50%, while estimates for opioid addiction are even higher [ 44 , 45 ]. Genetic risk factors are to a large extent shared across substances [ 46 ]. It has been argued that a genetic contribution cannot support a disease view of a behavior, because most behavioral traits, including religious and political inclinations, have a genetic contribution [ 4 ]. This statement, while correct in pointing out broad heritability of behavioral traits, misses a fundamental point. Genetic architecture is much like organ structure. The fact that normal anatomy shapes healthy organ function does not negate that an altered structure can contribute to pathophysiology of disease. The structure of the genetic landscape is no different. Critics further state that a “genetic predisposition is not a recipe for compulsion”, but no neuroscientist or geneticist would claim that genetic risk is “a recipe for compulsion”. Genetic risk is probabilistic, not deterministic. However, as we will see below, in the case of addiction, it contributes to large, consistent probability shifts towards maladaptive behavior.
In dismissing the relevance of genetic risk for addiction, Hall writes that “a large number of alleles are involved in the genetic susceptibility to addiction and individually these alleles might very weakly predict a risk of addiction”. He goes on to conclude that “generally, genetic prediction of the risk of disease (even with whole-genome sequencing data) is unlikely to be informative for most people who have a so-called average risk of developing an addiction disorder” [ 7 ]. This reflects a fundamental misunderstanding of polygenic risk. It is true that a large number of risk alleles are involved, and that the explanatory power of currently available polygenic risk scores for addictive disorders lags behind those for e.g., schizophrenia or major depression [ 47 , 48 ]. The only implication of this, however, is that low average effect sizes of risk alleles in addiction necessitate larger study samples to construct polygenic scores that account for a large proportion of the known heritability.
However, a heritability of addiction of ~50% indicates that DNA sequence variation accounts for 50% of the risk for this condition. Once whole genome sequencing is readily available, it is likely that it will be possible to identify most of that DNA variation. For clinical purposes, those polygenic scores will of course not replace an understanding of the intricate web of biological and social factors that promote or prevent expression of addiction in an individual case; rather, they will add to it [ 49 ]. Meanwhile, however, genome-wide association studies in addiction have already provided important information. For instance, they have established that the genetic underpinnings of alcohol addiction only partially overlap with those for alcohol consumption, underscoring the genetic distinction between pathological and nonpathological drinking behaviors [ 50 ].
It thus seems that, rather than negating a rationale for a disease view of addiction, the important implication of the polygenic nature of addiction risk is a very different one. Genome-wide association studies of complex traits have largely confirmed the century old “infinitisemal model” in which Fisher reconciled Mendelian and polygenic traits [ 51 ]. A key implication of this model is that genetic susceptibility for a complex, polygenic trait is continuously distributed in the population. This may seem antithetical to a view of addiction as a distinct disease category, but the contradiction is only apparent, and one that has long been familiar to quantitative genetics. Viewing addiction susceptibility as a polygenic quantitative trait, and addiction as a disease category is entirely in line with Falconer’s theorem, according to which, in a given set of environmental conditions, a certain level of genetic susceptibility will determine a threshold above which disease will arise.
A brain disease? Then show me the brain lesion!
The notion of addiction as a brain disease is commonly criticized with the argument that a specific pathognomonic brain lesion has not been identified. Indeed, brain imaging findings in addiction (perhaps with the exception of extensive neurotoxic gray matter loss in advanced alcohol addiction) are nowhere near the level of specificity and sensitivity required of clinical diagnostic tests. However, this criticism neglects the fact that neuroimaging is not used to diagnose many neurologic and psychiatric disorders, including epilepsy, ALS, migraine, Huntington’s disease, bipolar disorder, or schizophrenia. Even among conditions where signs of disease can be detected using brain imaging, such as Alzheimer’s and Parkinson’s disease, a scan is best used in conjunction with clinical acumen when making the diagnosis. Thus, the requirement that addiction be detectable with a brain scan in order to be classified as a disease does not recognize the role of neuroimaging in the clinic.
For the foreseeable future, the main objective of imaging in addiction research is not to diagnose addiction, but rather to improve our understanding of mechanisms that underlie it. The hope is that mechanistic insights will help bring forward new treatments, by identifying candidate targets for them, by pointing to treatment-responsive biomarkers, or both [ 52 ]. Developing innovative treatments is essential to address unmet treatment needs, in particular in stimulant and cannabis addiction, where no approved medications are currently available. Although the task to develop novel treatments is challenging, promising candidates await evaluation [ 53 ]. A particular opportunity for imaging-based research is related to the complex and heterogeneous nature of addictive disorders. Imaging-based biomarkers hold the promise of allowing this complexity to be deconstructed into specific functional domains, as proposed by the RDoC initiative [ 54 ] and its application to addiction [ 55 , 56 ]. This can ultimately guide the development of personalized medicine strategies to addiction treatment.
Countless imaging studies have reported differences in brain structure and function between people with addictive disorders and those without them. Meta-analyses of structural data show that alcohol addiction is associated with gray matter losses in the prefrontal cortex, dorsal striatum, insula, and posterior cingulate cortex [ 57 ], and similar results have been obtained in stimulant-addicted individuals [ 58 ]. Meta-analysis of functional imaging studies has demonstrated common alterations in dorsal striatal, and frontal circuits engaged in reward and salience processing, habit formation, and executive control, across different substances and task-paradigms [ 59 ]. Molecular imaging studies have shown that large and fast increases in dopamine are associated with the reinforcing effects of drugs of abuse, but that after chronic drug use and during withdrawal, brain dopamine function is markedly decreased and that these decreases are associated with dysfunction of prefrontal regions [ 60 ]. Collectively, these findings have given rise to a widely held view of addiction as a disorder of fronto-striatal circuitry that mediates top-down regulation of behavior [ 61 ].
Critics reply that none of the brain imaging findings are sufficiently specific to distinguish between addiction and its absence, and that they are typically obtained in cross-sectional studies that can at best establish correlative rather than causal links. In this, they are largely right, and an updated version of a conceptualization of addiction as a brain disease needs to acknowledge this. Many of the structural brain findings reported are not specific for addiction, but rather shared across psychiatric disorders [ 62 ]. Also, for now, the most sophisticated tools of human brain imaging remain crude in face of complex neural circuit function. Importantly however, a vast literature from animal studies also documents functional changes in fronto-striatal circuits, as well their limbic and midbrain inputs, associated with addictive behaviors [ 63 , 64 , 65 , 66 , 67 , 68 ]. These are circuits akin to those identified by neuroimaging studies in humans, implicated in positive and negative emotions, learning processes and executive functions, altered function of which is thought to underlie addiction. These animal studies, by virtue of their cellular and molecular level resolution, and their ability to establish causality under experimental control, are therefore an important complement to human neuroimaging work.
Nevertheless, factors that seem remote from the activity of brain circuits, such as policies, substance availability and cost, as well as socioeconomic factors, also are critically important determinants of substance use. In this complex landscape, is the brain really a defensible focal point for research and treatment? The answer is “yes”. As powerfully articulated by Francis Crick [ 69 ], “You, your joys and your sorrows, your memories and your ambitions, your sense of personal identity and free will, are in fact no more than the behavior of a vast assembly of nerve cells and their associated molecules”. Social and interpersonal factors are critically important in addiction, but they can only exert their influences by impacting neural processes. They must be encoded as sensory data, represented together with memories of the past and predictions about the future, and combined with representations of interoceptive and other influences to provide inputs to the valuation machinery of the brain. Collectively, these inputs drive action selection and execution of behavior—say, to drink or not to drink, and then, within an episode, to stop drinking or keep drinking. Stating that the pathophysiology of addiction is largely about the brain does not ignore the role of other influences. It is just the opposite: it is attempting to understand how those important influences contribute to drug seeking and taking in the context of the brain, and vice versa.
But if the criticism is one of emphasis rather than of principle—i.e., too much brain, too little social and environmental factors – then neuroscientists need to acknowledge that they are in part guilty as charged. Brain-centric accounts of addiction have for a long time failed to pay enough attention to the inputs that social factors provide to neural processing behind drug seeking and taking [ 9 ]. This landscape is, however, rapidly changing. For instance, using animal models, scientists are finding that lack of social play early in life increases the motivation to take addictive substances in adulthood [ 70 ]. Others find that the opportunity to interact with a fellow rat is protective against addiction-like behaviors [ 71 ]. In humans, a relationship has been found between perceived social support, socioeconomic status, and the availability of dopamine D2 receptors [ 72 , 73 ], a biological marker of addiction vulnerability. Those findings in turn provided translation of data from nonhuman primates, which showed that D2 receptor availability can be altered by changes in social hierarchy, and that these changes are associated with the motivation to obtain cocaine [ 74 ].
Epidemiologically, it is well established that social determinants of health, including major racial and ethnic disparities, play a significant role in the risk for addiction [ 75 , 76 ]. Contemporary neuroscience is illuminating how those factors penetrate the brain [ 77 ] and, in some cases, reveals pathways of resilience [ 78 ] and how evidence-based prevention can interrupt those adverse consequences [ 79 , 80 ]. In other words, from our perspective, viewing addiction as a brain disease in no way negates the importance of social determinants of health or societal inequalities as critical influences. In fact, as shown by the studies correlating dopamine receptors with social experience, imaging is capable of capturing the impact of the social environment on brain function. This provides a platform for understanding how those influences become embedded in the biology of the brain, which provides a biological roadmap for prevention and intervention.
We therefore argue that a contemporary view of addiction as a brain disease does not deny the influence of social, environmental, developmental, or socioeconomic processes, but rather proposes that the brain is the underlying material substrate upon which those factors impinge and from which the responses originate. Because of this, neurobiology is a critical level of analysis for understanding addiction, although certainly not the only one. It is recognized throughout modern medicine that a host of biological and non-biological factors give rise to disease; understanding the biological pathophysiology is critical for understanding etiology and informing treatment.
Is a view of addiction as a brain disease deterministic?
A common criticism of the notion that addiction is a brain disease is that it is reductionist and in the end therefore deterministic [ 81 , 82 ]. This is a fundamental misrepresentation. As indicated above, viewing addiction as a brain disease simply states that neurobiology is an undeniable component of addiction. A reason for deterministic interpretations may be that modern neuroscience emphasizes an understanding of proximal causality within research designs (e.g., whether an observed link between biological processes is mediated by a specific mechanism). That does not in any way reflect a superordinate assumption that neuroscience will achieve global causality. On the contrary, since we realize that addiction involves interactions between biology, environment and society, ultimate (complete) prediction of behavior based on an understanding of neural processes alone is neither expected, nor a goal.
A fairer representation of a contemporary neuroscience view is that it believes insights from neurobiology allow useful probabilistic models to be developed of the inherently stochastic processes involved in behavior [see [ 83 ] for an elegant recent example]. Changes in brain function and structure in addiction exert a powerful probabilistic influence over a person’s behavior, but one that is highly multifactorial, variable, and thus stochastic. Philosophically, this is best understood as being aligned with indeterminism, a perspective that has a deep history in philosophy and psychology [ 84 ]. In modern neuroscience, it refers to the position that the dynamic complexity of the brain, given the probabilistic threshold-gated nature of its biology (e.g., action potential depolarization, ion channel gating), means that behavior cannot be definitively predicted in any individual instance [ 85 , 86 ].
Driven by compulsion, or free to choose?
A major criticism of the brain disease view of addiction, and one that is related to the issue of determinism vs indeterminism, centers around the term “compulsivity” [ 6 , 87 , 88 , 89 , 90 ] and the different meanings it is given. Prominent addiction theories state that addiction is characterized by a transition from controlled to “compulsive” drug seeking and taking [ 91 , 92 , 93 , 94 , 95 ], but allocate somewhat different meanings to “compulsivity”. By some accounts, compulsive substance use is habitual and insensitive to its outcomes [ 92 , 94 , 96 ]. Others refer to compulsive use as a result of increasing incentive value of drug associated cues [ 97 ], while others view it as driven by a recruitment of systems that encode negative affective states [ 95 , 98 ].
The prototype for compulsive behavior is provided by obsessive-compulsive disorder (OCD), where compulsion refers to repeatedly and stereotypically carrying out actions that in themselves may be meaningful, but lose their purpose and become harmful when performed in excess, such as persistent handwashing until skin injuries result. Crucially, this happens despite a conscious desire to do otherwise. Attempts to resist these compulsions result in increasing and ultimately intractable anxiety [ 99 ]. This is in important ways different from the meaning of compulsivity as commonly used in addiction theories. In the addiction field, compulsive drug use typically refers to inflexible, drug-centered behavior in which substance use is insensitive to adverse consequences [ 100 ]. Although this phenomenon is not necessarily present in every patient, it reflects important symptoms of clinical addiction, and is captured by several DSM-5 criteria for SUD [ 101 ]. Examples are needle-sharing despite knowledge of a risk to contract HIV or Hepatitis C, drinking despite a knowledge of having liver cirrhosis, but also the neglect of social and professional activities that previously were more important than substance use. While these behaviors do show similarities with the compulsions of OCD, there are also important differences. For example, “compulsive” substance use is not necessarily accompanied by a conscious desire to withhold the behavior, nor is addictive behavior consistently impervious to change.
Critics question the existence of compulsivity in addiction altogether [ 5 , 6 , 7 , 89 ], typically using a literal interpretation, i.e., that a person who uses alcohol or drugs simply can not do otherwise. Were that the intended meaning in theories of addiction—which it is not—it would clearly be invalidated by observations of preserved sensitivity of behavior to contingencies in addiction. Indeed, substance use is influenced both by the availability of alternative reinforcers, and the state of the organism. The roots of this insight date back to 1940, when Spragg found that chimpanzees would normally choose a banana over morphine. However, when physically dependent and in a state of withdrawal, their choice preference would reverse [ 102 ]. The critical role of alternative reinforcers was elegantly brought into modern neuroscience by Ahmed et al., who showed that rats extensively trained to self-administer cocaine would readily forego the drug if offered a sweet solution as an alternative [ 103 ]. This was later also found to be the case for heroin [ 103 ], methamphetamine [ 104 ] and alcohol [ 105 ]. Early residential laboratory studies on alcohol use disorder indeed revealed orderly operant control over alcohol consumption [ 106 ]. Furthermore, efficacy of treatment approaches such as contingency management, which provides systematic incentives for abstinence [ 107 ], supports the notion that behavioral choices in patients with addictions remain sensitive to reward contingencies.
Evidence that a capacity for choosing advantageously is preserved in addiction provides a valid argument against a narrow concept of “compulsivity” as rigid, immutable behavior that applies to all patients. It does not, however, provide an argument against addiction as a brain disease. If not from the brain, from where do the healthy and unhealthy choices people make originate? The critical question is whether addictive behaviors—for the most part—result from healthy brains responding normally to externally determined contingencies; or rather from a pathology of brain circuits that, through probabilistic shifts, promotes the likelihood of maladaptive choices even when reward contingencies are within a normal range. To resolve this question, it is critical to understand that the ability to choose advantageously is not an all-or-nothing phenomenon, but rather is about probabilities and their shifts, multiple faculties within human cognition, and their interaction. Yes, it is clear that most people whom we would consider to suffer from addiction remain able to choose advantageously much, if not most, of the time. However, it is also clear that the probability of them choosing to their own disadvantage, even when more salutary options are available and sometimes at the expense of losing their life, is systematically and quantifiably increased. There is a freedom of choice, yet there is a shift of prevailing choices that nevertheless can kill.
Synthesized, the notion of addiction as a disease of choice and addiction as a brain disease can be understood as two sides of the same coin. Both of these perspectives are informative, and they are complementary. Viewed this way, addiction is a brain disease in which a person’s choice faculties become profoundly compromised. To articulate it more specifically, embedded in and principally executed by the central nervous system, addiction can be understood as a disorder of choice preferences, preferences that overvalue immediate reinforcement (both positive and negative), preferences for drug-reinforcement in spite of costs, and preferences that are unstable ( “I’ll never drink like that again;” “this will be my last cigarette” ), prone to reversals in the form of lapses and relapse. From a contemporary neuroscience perspective, pre-existing vulnerabilities and persistent drug use lead to a vicious circle of substantive disruptions in the brain that impair and undermine choice capacities for adaptive behavior, but do not annihilate them. Evidence of generally intact decision making does not fundamentally contradict addiction as a brain disease.
Conclusions
The present paper is a response to the increasing number of criticisms of the view that addiction is a chronic relapsing brain disease. In many cases, we show that those criticisms target tenets that are neither needed nor held by a contemporary version of this view. Common themes are that viewing addiction as a brain disease is criticized for being both too narrow (addiction is only a brain disease; no other perspectives or factors are important) or too far reaching (it purports to discover the final causes of addiction). With regard to disease course, we propose that viewing addiction as a chronic relapsing disease is appropriate for some populations, and much less so for others, simply necessitating better ways of delineating the populations being discussed. We argue that when considering addiction as a disease, the lens of neurobiology is valuable to use. It is not the only lens, and it does not have supremacy over other scientific approaches. We agree that critiques of neuroscience are warranted [ 108 ] and that critical thinking is essential to avoid deterministic language and scientific overreach.
Beyond making the case for a view of addiction as a brain disease, perhaps the more important question is when a specific level of analysis is most useful. For understanding the biology of addiction and designing biological interventions, a neurobiological view is almost certainly the most appropriate level of analysis, in particular when informed by an understanding of the behavioral manifestations. In contrast, for understanding the psychology of addiction and designing psychological interventions, behavioral science is the natural realm, but one that can often benefit from an understanding of the underlying neurobiology. For designing policies, such as taxation and regulation of access, economics and public administration provide the most pertinent perspectives, but these also benefit from biological and behavioral science insights.
Finally, we argue that progress would come from integration of these scientific perspectives and traditions. E.O. Wilson has argued more broadly for greater consilience [ 109 ], unity of knowledge, in science. We believe that addiction is among the areas where consilience is most needed. A plurality of disciplines brings important and trenchant insights to bear on this condition; it is the exclusive remit of no single perspective or field. Addiction inherently and necessarily requires multidisciplinary examination. Moreover, those who suffer from addiction will benefit most from the application of the full armamentarium of scientific perspectives.
Funding and disclosures
Supported by the Swedish Research Council grants 2013-07434, 2019-01138 (MH); Netherlands Organisation for Health Research and Development (ZonMw) under project number 912.14.093 (LJMJV); NIDA and NIAAA intramural research programs (LL; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health); the Peter Boris Chair in Addictions Research, Homewood Research Institute, and the National Institute on Alcohol Abuse and Alcoholism grants AA025911, AA024930, AA025849, AA027679 (JM; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health).
MH has received consulting fees, research support or other compensation from Indivior, Camurus, BrainsWay, Aelis Farma, and Janssen Pharmaceuticals. JM is a Principal and Senior Scientist at BEAM Diagnostics, Inc. DM, JR, LL, and LJMJV declare no conflict of interest.
Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–7.
Article CAS PubMed Google Scholar
McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–95.
Schomerus G, Lucht M, Holzinger A, Matschinger H, Carta MG, Angermeyer MC. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcohol. 2011;46:105–12.
Heyman GM. Addiction and choice: theory and new data. Front Psychiatry. 2013;4:31.
Article PubMed PubMed Central Google Scholar
Heather N, Best D, Kawalek A, Field M, Lewis M, Rotgers F, et al. Challenging the brain disease model of addiction: European launch of the addiction theory network. Addict Res Theory. 2018;26:249–55.
Article Google Scholar
Pickard H, Ahmed SH, Foddy B. Alternative models of addiction. Front Psychiatr.y 2015;6:20.
Google Scholar
Hall W, Carter A, Forlini C. The brain disease model of addiction: is it supported by the evidence and has it delivered on its promises? Lancet Psychiatr. 2015;2:105–10.
Hart CL. Viewing addiction as a brain disease promotes social injustice. Nat Hum Behav. 2017;1:0055.
Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosc.i 2016;17:592–9.
Article CAS Google Scholar
Jellinek EM. The disease concept of alcoholism. Hillhouse Press on behalf of the Christopher J. Smithers Foundation: New Haven, CT; 1960.
Stevenson A. Oxford dictionary of English. 3 ed. New York, NY: Oxford University Press; 2010.
Fan J, May SJ, Zhou Y, Barrett-Connor E. Bimodality of 2-h plasma glucose distributions in whites: the Rancho Bernardo study. Diabetes Care 2005;28:1451–6.
King AC, Vena A, Hasin D, De Wit D, O’Connor CJ, Cao D. Subjective responses to alcohol in the development and maintenance of alcohol use disorder (AUD). Am J Psychiatry. 2021. https://doi.org/10.1176/appi.ajp.2020.20030247 .
GBD. 2016 Alcohol and Drug Use Collaborators. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5:987–1012.
Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br Med J. 1976;1:1058–61.
Article CAS PubMed PubMed Central Google Scholar
Epstein DH, Heilig M, Shaham Y. Science-based actions can help address the opioid crisis. Trends Pharm Sci. 2018;39:911–16.
Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abus Treat. 2005;28:321–9.
Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Datab System Rev. 2013;5:CD009329.
Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings a systematic review and meta-analysis. JAMA. 2014;311:1889–900.
Article PubMed CAS Google Scholar
Mark TL, Kranzler HR, Song X. Understanding US addiction physicians’ low rate of naltrexone prescription. Drug Alcohol Depend. 2003;71:219–28.
Article PubMed Google Scholar
Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–65.
Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med. 1997;12:274–83.
Laing RD. The divided self; a study of sanity and madness. London: Tavistock Publications; 1960.
Foucault M, Khalfa J. History of madness. New York: Routledge; 2006.
Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL. et al.Comorbidity of mental disorders with alcohol and other drug abuse. Results Epidemiologic Catchment Area (ECA) study.JAMA. 1990;264:2511–8.
Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–16.
Lopez-Quintero C, Hasin DS, de Los Cobos JP, Pines A, Wang S, Grant BF, et al. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction. 2011;106:657–69.
Humphreys K. Addiction treatment professionals are not the gatekeepers of recovery. Subst Use Misuse. 2015;50:1024–7.
Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;86:214–21.
Sobell LC, Cunningham JA, Sobell MB. Recovery from alcohol problems with and without treatment: prevalence in two population surveys. Am J Public Health. 1996;86:966–72.
Culverhouse R, Bucholz KK, Crowe RR, Hesselbrock V, Nurnberger JI Jr, Porjesz B, et al. Long-term stability of alcohol and other substance dependence diagnoses and habitual smoking: an evaluation after 5 years. Arch Gen Psychiatry. 2005;62:753–60.
Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42.
Skolnick P. The opioid epidemic: crisis and solutions. Annu Rev Pharm Toxicol. 2018;58:143–59.
Hser YI, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry. 2015;23:76–89.
Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:102–23.
Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66.
Article PubMed PubMed Central CAS Google Scholar
Lee MR, Sher KJ. “Maturing Out” of binge and problem drinking. Alcohol Res: Curr Rev. 2018;39:31–42.
Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction. 2005;100:281–92.
Berridge V. The rise, fall, and revival of recovery in drug policy. Lancet. 2012;379:22–23.
Parvaz MA, Moeller SJ, d’Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, et al. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. 2017;22:1391–401.
Korponay C, Kosson DS, Decety J, Kiehl KA, Koenigs M. Brain volume correlates with duration of abstinence from substance abuse in a region-specific and substance-specific manner. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:626–35.
PubMed PubMed Central Google Scholar
Janes AC, Datko M, Roy A, Barton B, Druker S, Neal C, et al. Quitting starts in the brain: a randomized controlled trial of app-based mindfulness shows decreases in neural responses to smoking cues that predict reductions in smoking. Neuropsychopharmacology. 2019;44:1631–38.
Humphreys K, Bickel WK. Toward a neuroscience of long-term recovery from addiction. JAMA Psychiatry. 2018;75:875–76.
Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015;45:1061–72.
Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–32.
Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. AJ Psychiatry. 2003;160:687–95.
Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Wray NR, Lin T, Austin J, McGrath JJ, Hickie IB, Murray GK, et al. From basic science to clinical application of polygenic risk scores: a primer. JAMA Psychiatry. 2021;78:101–9.
Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21:1656–69.
Visscher PM, Wray NR. Concepts and misconceptions about the polygenic additive model applied to disease. Hum Hered. 2015;80:165–70.
Heilig M, Leggio L. What the alcohol doctor ordered from the neuroscientist: theragnostic biomarkers for personalized treatments. Prog Brain Res. 2016;224:401–18.
Rasmussen K, White DA, Acri JB. NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology. 2019;44:657–59.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. AJ Psychiatry. 2010;167:748–51.
Kwako LE, Schwandt ML, Ramchandani VA, Diazgranados N, Koob GF, Volkow ND, et al. Neurofunctional domains derived from deep behavioral phenotyping in alcohol use disorder. AJ Psychiatry. 2019;176:744–53.
Kwako LE, Bickel WK, Goldman D. Addiction biomarkers: dimensional approaches to understanding addiction. Trends Mol Med. 2018;24:121–28.
Xiao P, Dai Z, Zhong J, Zhu Y, Shi H, Pan P. Regional gray matter deficits in alcohol dependence: a meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend. 2015;153:22–8.
Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23:615–24.
Klugah-Brown B, Di X, Zweerings J, Mathiak K, Becker B, Biswal B. Common and separable neural alterations in substance use disorders: a coordinate-based meta-analyses of functional neuroimaging studies in humans. Hum Brain Mapp. 2020;41:4459–77.
Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Investig. 2003;111:1444–51.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69.
Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15.
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharm Rev. 2016;68:816–71.
Korpi ER, den Hollander B, Farooq U, Vashchinkina E, Rajkumar R, Nutt DJ, et al. Mechanisms of action and persistent neuroplasticity by drugs of abuse. Pharm Rev. 2015;67:872–1004.
Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63.
Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories–indications for novel treatments of addiction. Eur J Neurosci. 2014;40:2163–82.
Lesscher HM, Vanderschuren LJ. Compulsive drug use and its neural substrates. Rev Neurosci. 2012;23:731–45.
Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–54.
Crick F. The astonishing hypothesis: the scientific search for the soul. Scribner; Maxwell Macmillan International: New York, NY; 1994.
Vanderschuren LJ, Achterberg EJ, Trezza V. The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev. 2016;70:86–105.
Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21:1520–29.
Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, et al. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67:275–8.
Wiers CE, Shokri-Kojori E, Cabrera E, Cunningham S, Wong C, Tomasi D, et al. Socioeconomic status is associated with striatal dopamine D2/D3 receptors in healthy volunteers but not in cocaine abusers. Neurosci Lett. 2016;617:27–31.
Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74.
Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e66.
Gilbert PA, Zemore SE. Discrimination and drinking: a systematic review of the evidence. Soc Sci Med 2016;161:178–94.
Oshri A, Gray JC, Owens MM, Liu S, Duprey EB, Sweet LH, et al. Adverse childhood experiences and amygdalar reduction: high-resolution segmentation reveals associations with subnuclei and psychiatric outcomes. Child Maltreat. 2019;24:400–10.
Holmes CJ, Barton AW, MacKillop J, Galván A, Owens MM, McCormick MJ, et al. Parenting and salience network connectivity among African Americans: a protective pathway for health-risk behaviors. Biol Psychiatry. 2018;84:365–71.
Brody GH, Gray JC, Yu T, Barton AW, Beach SR, Galván A, et al. Protective prevention effects on the association of poverty with brain development. JAMA Pediatr. 2017;171:46–52.
Hanson JL, Gillmore AD, Yu T, Holmes CJ, Hallowell ES, Barton AW, et al. A family focused intervention influences hippocampal-prefrontal connectivity through gains in self-regulation. Child Dev. 2019;90:1389–401.
Borsboom D, Cramer A, Kalis A. Brain disorders? Not really… why network structures block reductionism in psychopathology research. Behav Brain Sci. 2018;42:1–54.
Field M, Heather N, Wiers RW. Indeed, not really a brain disorder: Implications for reductionist accounts of addiction. Behav Brain Sci. 2019;42:e9.
Pascoli V, Hiver A, Van Zessen R, Loureiro M, Achargui R, Harada M, et al. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature. 2018;564:366–71.
James W. The dilemma of determinism. Whitefish, MT: Kessinger Publishing; 2005.
Gessell B. Indeterminism in the brain. Biol Philos. 2017;32:1205–23.
Jedlicka P. Revisiting the quantum brain hypothesis: toward quantum (neuro)biology? Front Mol Neurosci. 2017;10:366.
Heyman GM. Addiction: a disorder of choice. Cambridge, MA: Harvard University Press; 2010.
Heather NQ. Is addiction a brain disease or a moral failing? A: Neither. Neuroethics. 2017;10:115–24.
Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–7.
Hogarth L, Lam-Cassettari C, Pacitti H, Currah T, Mahlberg J, Hartley L, et al. Intact goal-directed control in treatment-seeking drug users indexed by outcome-devaluation and Pavlovian to instrumental transfer: critique of habit theory. Eur J Neurosci. 2019;50:2513–25.
Mathis V, Kenny PJ. From controlled to compulsive drug-taking: the role of the habenula in addiction. Neurosci Biobehav Rev. 2019;106:102–11.
Luscher C, Robbins TW, Everitt BJ. The transition to compulsion in addiction. Nat Rev Neurosci. 2020;21:247–63.
Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–89.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–68.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91.
Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–4.
Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive-compulsive disorder. Nat Rev Dis Prim. 2019;5:52.
Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 2004;305:1017–9.
American_Psychiatric_Association. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th ed. Arlington, VA, US: American Psychiatric Publishing, Inc; 2013.
Book Google Scholar
Spragg SDS. Morphine addiction in chimpanzees. Comp Psychol Monogr. 1940;15:132–32.
Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20.
Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015;78:463–73.
Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, et al. A molecular mechanism for choosing alcohol over an alternative reward. Science. 2018;360:1321–26.
Bigelow GE. An operant behavioral perspective on alcohol abuse and dependence. In: Heather N, Peters TJ, Stockwell T, editors. International handbook of alcohol dependence and problems. John Wiley & Sons Ltd; 2001. p. 299–315.
Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu Rev Psychol. 2004;55:431–61.
Satel S, Lilienfeld SO. Brainwashed: the seductive appeal of mindless neuroscience. New York, NY: Basic Books; 2015.
Wilson EO. Consilience: the unity of knowledge. New York, NY: Vintage Books; 1999.
Saunders JB, Degenhardt L, Reed GM, Poznyak V. Alcohol use disorders in ICD-11: past, present, and future. Alcohol Clin Exp Res 2019;43:1617–31.
Organization. WH. ICD-11 for mortality and morbidity statistics. 2018. https://icd.who.int/browse11/l-m/en . Accessed 21 Oct 2020.
Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, brief intervention, and referral to treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus. 2007;28:7–30.
Witkiewitz K, Hallgren KA, Kranzler HR, Mann KF, Hasin DS, Falk DE, et al. Clinical validation of reduced alcohol consumption after treatment for alcohol dependence using the World Health Organization risk drinking levels. Alcohol Clin Exp Res 2017;41:179–86.
Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. AJ Psychiatry. 2013;170:834–51.
Rosenthal RJ, Faris SB. The etymology and early history of ‘addiction’. Addict Res Theory. 2019;27:437–49.
Martin CS, Steinley DL, Verges A, Sher KJ. The proposed 2/11 symptom algorithm for DSM-5 substance-use disorders is too lenient. Psychol Med. 2011;41:2008–10.
Degenhardt L, Bharat C, Bruno R, Glantz MD, Sampson NA, Lago L, et al. Concordance between the diagnostic guidelines for alcohol and cannabis use disorders in the draft ICD-11 and other classification systems: analysis of data from the WHO’s World Mental Health Surveys. Addiction. 2019;114:534–52.
PubMed Google Scholar
Lago L, Bruno R, Degenhardt L. Concordance of ICD-11 and DSM-5 definitions of alcohol and cannabis use disorders: a population survey. Lancet Psychiatry. 2016;3:673–84.
Lundin A, Hallgren M, Forsman M, Forsell Y. Comparison of DSM-5 classifications of alcohol use disorders with those of DSM-IV, DSM-III-R, and ICD-10 in a general population sample in Sweden. J Stud Alcohol Drugs. 2015;76:773–80.
Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions neuroclinical assessment: a neuroscience-based framework for addictive disorders. Biol Psychiatry. 2016;80:179–89.
Rehm J, Heilig M, Gual A. ICD-11 for alcohol use disorders: not a convincing answer to the challenges. Alcohol Clin Exp Res. 2019;43:2296–300.
Download references
Acknowledgements
The authors want to acknowledge comments by Drs. David Epstein, Kenneth Kendler and Naomi Wray.
Author information
Authors and affiliations.
Center for Social and Affective Neuroscience, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden
Markus Heilig
Peter Boris Centre for Addictions Research, McMaster University and St. Joseph’s Healthcare Hamilton, Hamilton, ON, Canada
- James MacKillop
Homewood Research Institute, Guelph, ON, Canada
New York State Psychiatric Institute and Columbia University Irving Medical Center, New York, NY, USA
Diana Martinez
Institute for Mental Health Policy Research & Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health (CAMH), Toronto, ON, Canada
Jürgen Rehm
Dalla Lana School of Public Health and Department of Psychiatry, University of Toronto (UofT), Toronto, ON, Canada
Klinische Psychologie & Psychotherapie, Technische Universität Dresden, Dresden, Germany
Department of International Health Projects, Institute for Leadership and Health Management, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, Translational Addiction Medicine Branch, National Institute on Drug Abuse Intramural Research Program and National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research, National Institutes of Health, Baltimore and Bethesda, MD, USA
Lorenzo Leggio
Department of Population Health Sciences, Unit Animals in Science and Society, Faculty of Veterinary Medicine, Utrecht University, Utrecht, the Netherlands
Louk J. M. J. Vanderschuren
You can also search for this author in PubMed Google Scholar
Contributions
All authors jointly drafted the paper.
Corresponding author
Correspondence to Markus Heilig .
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions

About this article
Cite this article.
Heilig, M., MacKillop, J., Martinez, D. et al. Addiction as a brain disease revised: why it still matters, and the need for consilience. Neuropsychopharmacol. 46 , 1715–1723 (2021). https://doi.org/10.1038/s41386-020-00950-y
Download citation
Received : 10 November 2020
Revised : 11 December 2020
Accepted : 14 December 2020
Published : 22 February 2021
Issue Date : September 2021
DOI : https://doi.org/10.1038/s41386-020-00950-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Persistent impacts of smoking on resting-state eeg in male chronic smokers and past-smokers with 20 years of abstinence.
- Yoonji Jeon
- Dongil Chung
Scientific Reports (2023)
A contextualized reinforcer pathology approach to addiction
- Samuel F. Acuff
- James G. Murphy
Nature Reviews Psychology (2023)
Functional genomic mechanisms of opioid action and opioid use disorder: a systematic review of animal models and human studies
- Camille Falconnier
- Alba Caparros-Roissard
- Pierre-Eric Lutz
Molecular Psychiatry (2023)
A neuromarker for drug and food craving distinguishes drug users from non-users
- Leonie Koban
- Tor D. Wager
Nature Neuroscience (2023)
Does a Survivorship Model of Opioid Use Disorder Improve Public Stigma or Policy Support? A General Population Randomized Experiment
- Jarratt D. Pytell
- Geetanjali Chander
- Emma E. McGinty
Journal of General Internal Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

See All Patient & Visitor Information
- All Specialties & Services
- Gastroenterology (GI)
- Heart & Vascular
- LGBTQ+ Care & Services
- Neurology & Neurosurgery
- Orthopaedics
- Patient Information
- Billing & Financial Assistance
- Classes & Events
- Insurance Information
- International Patients
- Visiting Us From Out of State?
- Online Second Opinions
- Price Transparency
- Request Medical Records
- Video Visits
- Visitor Information
- Dining Options
- Locations & Directions
- Our Neighborhood & City
- Visitor's Guide
- Magnet Site Visit: Public Notice
Schedule an Appointment
We offer online appointment scheduling for adult and pediatric primary care and many specialties.
Patient Portal
Communicate with your doctor, view test results, schedule appointments and more.
See All Healthcare Professionals Information
- Referring Physicians
- About our Physicians
- Physician Relations Team
- Refer a Patient
- Molecular and Genomic Diagnostic Laboratories
- Nurse Residency Program
- Nursing Careers at UChicago Medicine
- Nursing Careers at Ingalls Memorial
- Nursing Programs
- Nursing Research
- Nursing Student Opportunities
- Employee Resources
- Continuing Medical Education
- Continuing Nursing Education
- Employee Login
- Graduate Medical Education
- Password Reset Self-Service
UChicago Medicine and Ingalls Memorial offer a broad range of challenging clinical and non-clinical career opportunities doing work that really matters.
Research & Clinical Trials
- Clinical Trials
Find a Clinical Trial
- Clinical Trial FAQs
- Clinical Trial Resources
- Office of Clinical Research
- Clinical Trials at Ingalls
- Our Research
- Firsts at the Forefront
- Research & Innovation
- Our Nobel Laureates
- The Forefront: Health & Science News
- Research & Discoveries News
- Biological Science News
Learn more about clinical trials and find a trial that might be right for you.
- Research and Discoveries
JAMA study examines facilities’ low use of monthly injections for treating opioid addiction
March 26, 2024
Written By Jamie Bartosch
- Vineet Arora MD
- Opioid Use Disorders
- Call Us At 1-888-824-0200
Compared to taking a daily pill, a monthly dose of long-acting injectable (LAI) buprenorphine can be a simpler and more effective treatment for people with opioid use disorder. But do substance use treatment facilities in the United States take advantage of this highly effective medication?
To answer that question, researchers from the University of Chicago spent nearly a year analyzing data from the National Substance Use and Mental Health Services Survey . They found that only 32.6% of substance use treatment facilities that offered medications for opioid use disorder offered LAI buprenorphine to their patients. The researchers suggested that administrative obstacles make it more difficult — and often more expensive — to obtain LAI buprenorphine compared to the oral version of the medication.
The study, published in the Journal of the American Medical Association in January, also revealed that facilities offering primary care were more likely to offer LAI buprenorphine. The researchers hypothesized that the difference may be due to those facilities facing fewer regulatory and administrative hurdles to prescribe the medication as a monthly injection.
“This paper highlights gaps that exist in the system,” said Nitin Vidyasagar, the study’s lead author and a second-year student at the University of Chicago Pritzker School of Medicine. “We can now use the information to help treat people who need it the most.”
Samuel R. Bunting, MD, MSHA , a UChicago Medicine adult psychiatry resident and the study’s second author, hopes the study will serve as “a guidepost in the sand” to measure future progress with this underused and potentially lifesaving medicine.
“The takeaway is, we still have a lot of work to do to make the full complement of opioid treatment options available to patients,” he said.
Vidyasagar and Bunting came up with the idea for this paper while studying how resources for HIV prevention were distributed in healthcare facilities. Bunting is also studying the use of LAI medications for psychiatric conditions, research prompted by frustration with difficulties getting these medicines for his own patients.
Vidyasagar and Bunting collaborated with two UChicago Medicine staff members for this JAMA study: addiction medicine specialist and primary care doctor Mim Ari, MD , and Pritzker Dean for Medical Education Vineet Arora, MD, MAPP .
Given the epidemic levels of opioid addiction, Ari said she hopes the paper sparks conversations on how to design better health policies, remove barriers and build partnerships that will support sustainable recovery for people with opioid use disorder.
“I hope it’s seen as a call to action, to say there’s more that we can be doing as clinicians with this treatment,” she said.
Pritzker School of Medicine has been nationally recognized for its work training medical students to address patients with opioid use disorder in the emergency room.
Students developed an O.P.I.A.T.E. initiative — Outpatient Principles in Addiction Training and Education — that was implemented in UChicago Medicine’s Emergency Department in 2019 and scaled up to all incoming students as part of the new Pritzker Phoenix curriculum.
The initiative involves screening patients who may be at risk for opioid overdose and supplying them and their close contacts with a life-saving naloxone kit to take home.

Mim Ari, MD
Mim Ari, MD, is an Assistant Professor of Medicine at UChicago Medicine.

Vineet Arora, MD
Vineet Arora, MD, MAPP, specializes in improving the learning environment for medical trainees and the quality, safety and experience of care delivered to hospitalized adults. She serves as Dean for Medical Education at the Pritzker School of Medicine.
Addiction treatment
Our addiction and substance use programs at UChicago Medicine and Ingalls Memorial offer a wide range of treatment services for substance use and compulsive and impulse control disorders.
Research Articles
I'd Like to
- Make an Appointment
- Find Classes & Events
- Make a Donation
- Apply for a Job
- Patients & Visitors
- Healthcare Professionals
- Comer Children's Hospital
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Psychiatry
- v.52(Suppl1); 2010 Jan
Substance use and addiction research in India
Pratima murthy.
Department of Psychiatry, De-Addiction Centre, National Institute of Mental Health and Neuro Sciences, Bangalore - 560 029, India
N. Manjunatha
B. n. subodh, prabhat kumar chand, vivek benegal.
Substance use patterns are notorious for their ability to change over time. Both licit and illicit substance use cause serious public health problems and evidence for the same is now available in our country. National level prevalence has been calculated for many substances of abuse, but regional variations are quite evident. Rapid assessment surveys have facilitated the understanding of changing patterns of use. Substance use among women and children are increasing causes of concern. Preliminary neurobiological research has focused on identifying individuals at high risk for alcohol dependence. Clinical research in the area has focused primarily on alcohol and substance related comorbidity. There is disappointingly little research on pharmacological and psychosocial interventions. Course and outcome studies emphasize the need for better follow-up in this group. While lack of a comprehensive policy has been repeatedly highlighted and various suggestions made to address the range of problems caused by substance use, much remains to be done on the ground to prevent and address these problems. It is anticipated that substance related research publications in the Indian Journal of Psychiatry will increase following the journal having acquired an ‘indexed’ status.
INTRODUCTION
Substance use has been a topic of interest to many professionals in the area of health, particularly mental health. An area with enormous implications for public health, it has generated a substantial amount of research. In this paper we examine research in India in substance use and related disorders. Substance use includes the use of licit substances such as alcohol, tobacco, diversion of prescription drugs, as well as illicit substances.
METHODOLOGY
For this review, we have carried out a systematic web-based review of the Indian Journal of Psychiatry (IJP). The IJP search included search of both the current and archives section and an issue-to-issue search of articles with any title pertaining to substance use. This has included original articles, reviews, case series and reports with significant implications. Letters to editor and abstracts of annual conference presentations have not been included.
Publications in other journals were accessed through a Medlar search (1992-2009) and a Pubmed search (1950-2009). Other publications related to substance use available on the websites of international and national agencies have also been reviewed. In this review, we focus mainly on publications in the IJP and have selectively reviewed the literature from other sources.
For the sake of convenience, we discuss the publications under the following areas: Epidemiology, clinical issues (diagnosis, psychopathology, comorbidity), biological studies (genetics, imaging, electrophysiology, and vulnerability), interventions and outcomes as well as community interventions and policies. There is a vast amount of literature on tobacco use and consequences in international and national journals, but this is outside the scope of this review. Tobacco is mentioned in this review of substance use to highlight that it should be remembered as the primary licit substance of abuse in our country.
The number of articles (area wise) available from IJP, other Indian journals and international journals are indicated in Figures Figures1 1 and and2. 2 . A majority of the publications in international journals relate to tobacco, substance use co-morbidity and miscellaneous areas like animal studies.

Publications in the area of substance use and related disorders

Break up of areas of publication
EPIDEMIOLOGY
Much of the earlier epidemiological research has been regional and it has been very difficult to draw inferences of national prevalence from these studies.
Regional studies
Studies between 1968 until 2000 have been primarily on alcohol use [ Table 1 ]. They have varied in terms of populations surveyed (ranged from 115 to 16,725), sampling procedures (convenient, purposive and representative), focus of enquiry (alcohol use, habitual excessive use, alcohol abuse, alcoholism, chronic alcoholism, alcohol and drug abuse and alcohol dependence), location (urban, rural or both, Slums), in the screening instruments used (survey questionnaires and schedules, semi-structured interviews, quantity frequency index, Michigan Alcohol Screening Test (MAST) etc). Alcohol ‘use/abuse’ prevalence in different regions has thus varied from 167/1000 to 370/1000; ‘alcohol addiction’ or ‘alcoholism’ or ‘chronic alcoholism’ from 2.36/1000 to 34.5/1000; alcohol and drug use/abuse from 21.4 to 28.8/1000. A meta-analysis by Reddy and Chandrashekhar[ 26 ] (1998) revealed an overall substance use prevalence of 6.9/1000 for India with urban and rural rates of 5.8 and 7.3/1000 population. The rates among men and women were 11.9 and 1.7% respectively.
Regional epidemiological studies in substance use: A summary
U - Urban; R - Rural; Sl - Slum; SR - Semi-rural; NM - Not mentioned
Regional studies between 2001 and 2007 continue to reflect this variability. Currently, the interest is to look at hazardous alcohol use. A study in southern rural India[ 27 ] showed that 14.2% of the population surveyed had hazardous alcohol use on the AUDIT. A similar study in the tertiary hospital[ 28 ] showed that 17.6% admitted patients had hazardous alcohol use.
The only incidence study on alcohol use from Delhi[ 17 ] found that annual incidence of nondependent alcohol use and dependent alcohol use among men was 3 and 2 per 1000 persons in a total cohort of 2,937 households.
National Studies
The National Household Survey of Drug Use in the country[ 29 ] is the first systematic effort to document the nation-wide prevalence of drug use [ Table 2 ]. Alcohol (21.4%) was the primary substance used (apart from tobacco) followed by cannabis (3.0%) and opioids (0.7%). Seventeen to 26% of alcohol users qualified for ICD 10 diagnosis of dependence, translating to an average prevalence of about 4%. There was a marked variation in alcohol use prevalence in different states of India (current use ranged from a low of 7% in the western state of Gujarat (officially under Prohibition) to 75% in the North-eastern state of Arunachal Pradesh. Tobacco use prevalence was high at 55.8% among males, with maximum use in the age group 41-50 years.
Nationwide studies on substance use prevalence
H-H - House to house survey; M - Male; F - Female; A - Alcohol, C - Cannabis; O - Opioids; T - Tobacco
The National Family Health Survey (NFHS)[ 30 ] provides some insights into tobacco and alcohol use. The changing trends between NFHS 2 and NFHS 3 reflect an increase in alcohol use among males since the NFHS 2, and an increase in tobacco use among women.
The Drug Abuse Monitoring System,[ 29 ] which evaluated the primary substance of abuse in inpatient treatment centres found that the major substances were alcohol (43.9%), opioids (26%) and cannabis (11.6%).
Patterns of substance use
Rapid situation assessments (RSA) are useful to study patterns of substance use. An RSA by the UNODC in 2002[ 31 ] of 4648 drug users showed that cannabis (40%), alcohol (33%) and opioids (15%) were the major substances used. A Rapid Situation and Response Assessment (RSRA) among 5800 male drug users[ 32 ] revealed that 76% of the opioid users currently injected buprenorphine, 76% injected heroin, 70% chasing and 64% using propoxyphene. Most drug users concomitantly used alcohol (80%). According to the World Drug Report,[ 33 ] of 81,802 treatment seekers in India in 2004-2005, 61.3% reported use of opioids, 15.5% cannabis, 4.1% sedatives, 1.5% cocaine, 0.2% amphetamines and 0.9% solvents.
Special populations
In the last decade, there has been a shift in viewing substance use and abuse as an exclusive adult male phenomenon to focusing on the problem in other populations. In the GENACIS study[ 34 ] covering a population of 2981 respondents [1517 males; 1464 females], across five districts of Karnataka, 5.9% of all female respondents (N =87) reported drinking alcohol at least once in the last 12 months, compared to 32.7% among male respondents (N = 496). Special concerns with women’s drinking include the fetal alcohol spectrum effects described with alcohol use during pregnancy.[ 35 ]
Abuse of other substances among women has largely been studied through Rapid Assessment Surveys. A survey of 1865 women drug users by 110 NGOs across the country[ 36 ] revealed that 25% currently were heroin users, 18% used dextropropoxyphene, 11% opioid containing cough syrups and 7% buprenorphine. Eighty seven per cent concomitantly used alcohol and 83% used tobacco. Twenty five per cent of respondents had lifetime history of injecting drug use and 24% had been injecting in the previous month. There are serious sexually transmitted disease risks, including HIV that women partners and drug users face.[ 36 , 37 ]
Substance use in medical fraternity
As early as 1977, a drug abuse survey in Lucknow among medical students revealed that 25.1% abused a drug at least once in a month. Commonly abused drugs included minor tranquilizers, alcohol, amphetamines, bhang and non barbiturate sedatives. In a study of internees on the basis of a youth survey developed by the WHO in 1982,[ 38 ] 22.7% of males ‘indulged in alcohol abuse’ at least once in a month, 9.3% abused cannabis, followed by tranquilizers. Common reasons cited were social reasons, enjoyment, curiosity and relief from psychological stress. Most reported that it was easy to obtain drugs like marijuana and amphetamines. Substance use among medical professionals has become the subject of recent editorials.[ 39 , 40 ]
Substance use among children
The Global Youth Tobacco Survey[ 41 ] in 2006 showed that 3.8% of students smoke and 11.9% currently used smokeless tobacco. Tobacco as a gateway to other drugs of abuse has been the topic of a symposium.[ 42 ]
A study of 300 street child laborers in slums of Surat in 1993[ 43 ] showed that 135 (45%) used substances. The substances used were smoking tobacco, followed by chewable tobacco, snuff, cannabis and opioids. Injecting drug use[ 44 ] is also becoming apparent among street children as are inhalants.[ 45 ]
A study in the Andamans[ 46 ] shows that onset of regular use of alcohol in late childhood and early adolescence is associated with the highest rates of consumption in adult life, compared to later onset of drinking.
Studies in other populations
A majority of 250 rickshaw pullers interviewed in New Delhi[ 47 ] in 1986 reported using tobacco (79.2%), alcohol (54.4%), cannabis (8.0%) and opioids (0.8%). The substances reportedly helped them to be awake at night while working. In a study of prevalence of psychiatric illness in an industrial population[ 48 ] in 2007, harmful use/dependence on substances (42.83%) was the most common psychiatric condition. A study among industrial workers from Goa on hazardous alcohol use using the AUDIT and GHQ 12 estimated a prevalence of 211/1000 with hazardous drinking.[ 19 ]
Hospital-based studies
These studies have basically described profiles of substance use among patients and include patterns of alcohol use,[ 49 – 53 ] opioid use,[ 54 – 56 ] pediatric substance use,[ 57 ] female substance use,[ 58 ] children of alcoholics[ 59 ] and geriatric substance use.[ 60 ]
Alcohol misuse has been implicated in 20% of brain injuries[ 61 ] and 60% of all injuries in the emergency room setting.[ 62 ] In a retrospective study of emergency treatment seeking in Sikkim between 2000 and 2005,[ 63 ] substance use emergencies constituted 1.16% of total psychiatric emergencies. Alcohol withdrawal was the commonest cause for reporting to the emergency (57.4%).
Effects of substance use disorders
Mortality and morbidity due to alcohol and tobacco have been extensively reviewed elsewhere[ 35 , 64 – 66 ] and are beyond the scope of this review. The effects of cannabis have also been reviewed.[ 67 ] Mortality with injecting drug use is a serious concern with increase in crude mortality rates to 4.25 among injecting drug users compared to the general population.[ 68 ] Increased susceptibility to HIV/AIDS and other sexually transmitted diseases has been reported with alcohol[ 69 ] as well as injecting drug use.[ 70 ]
Clinical issues
Harmful alcohol use patterns among admitted patients in general hospital has highlighted the importance of routine screening and intervention in health care settings.[ 71 ]
Peer influence is a significant factor for heroin initiation.[ 72 ] Precipitants of relapse (dysfunction, stress and life events) differ among alcohol and opioid dependents.[ 73 ] Chronologies in the development of dependence have been evaluated in alcohol dependence.[ 74 , 75 ]
Craving a common determinant of relapse has been shown to reduce with increase in length of period of abstinence.[ 76 ]
Alcohol dependence constitutes a significant group among the psychiatric population in the Armed Forces.[ 77 ] A study of personality factors[ 78 ] among 100 alcohol dependent persons showed significantly high neuroticism, extroversion, anxiety, depression, psychopathic deviation, stressful life events and significantly low self-esteem as compared with normal control subjects. Alcohol dependence causes impairment in set shifting, visual scanning and response inhibition abilities and relative abstinence has been found to improve this deficit.[ 79 , 80 ] Alcohol use has had a significant association with head injury and cognitive deficits.[ 81 , 82 ] Persistent drinking is associated with persisting memory deficits in head injured alcohol dependent patients.[ 82 ] Mild intellectual impairment has been demonstrated in patients with bhang and ganja dependence.[ 83 – 86 ]
Kumar and Dhawan[ 87 ] found that health related reasons like death/physical complications due to drug use in peers and patients themselves, knowledge of HIV and difficulties in accessing veins were the main reason for reverse transition (shift from parenteral to inhalation route).
Evaluation and assessment
Diagnostic issues have focused on cross-system agreement[ 88 ] between ICD-10 and DSM IV, variability in diagnostic criteria across MAST, RDC, DSM and ICD[ 89 ] and suitability of MAST as a tool for detecting alcoholism.[ 90 ] The CIWA-A was found useful in monitoring alcohol withdrawal syndrome.[ 91 ]
The utility of liver functions for diagnosis of alcoholism and monitoring recovery has been demonstrated in clinical settings.[ 92 – 94 ] A range of hepatic dysfunction has been demonstrated through liver biopsies.[ 95 ]
A few studies have focused on scale development for motivation[ 96 , 97 ] and addiction related dysfunction[ 98 ] (Brief Addiction Rating Scale). An evaluation of two psychomotor tests comparing smokers and non-smokers found no differences across the two groups.[ 99 ]
Typology research has included validation of Babor’s[ 100 ] cluster A and B typologies, age of onset typology,[ 101 ] and a review on typology of alcoholism.[ 102 ]
Craving plays an important role in persistence of substance use and relapse. Frequency of craving has been shown to decrease with increase in length of abstinence among heroin dependent patients. Socio-cultural factors did not influence the subjective experience of craving.[ 76 ]
In a study of heroin dependent patients, their self-report moderately agreed with urinalysis using thin layer chromatography (TLC), gas liquid chromatography (GLC) and high performance liquid chromatography (HPLC).[ 103 ] The authors, however, recommend that all drug dependence treatment centers have facilities for drug testing in order to validate self-report.
Comorbidity/dual diagnosis
Cannabis related psychopathology has been a favorite topic of enquiry in both retrospective[ 104 , 105 ] and prospective studies[ 106 ] and vulnerability to affective psychosis has been highlighted. The controversial status of a specific cannabis withdrawal syndrome and cannabis psychosis has been reviewed.[ 67 ]
High life time prevalence of co-morbidity (60%) has been demonstrated among both opioid and alcohol dependent patients.[ 107 ] In alcohol dependence, high rates of depression and cluster B personality disorders[ 54 , 108 ] and phobia[ 109 ] have been demonstrated, but the need to revaluate for depressive symptoms after detoxification has been highlighted.[ 110 ] It is necessary to evaluate for ADHD, particularly in early onset alcohol dependent patients.[ 111 ] Seizures are overrepresented in subjects with alcohol and merit detailed evaluation.[ 112 ] Delirium and convulsions can also complicate opioid withdrawal states.[ 113 , 114 ] Skin disease,[ 115 ] and sexual dysfunction[ 116 ] have also been the foci of enquiry. Phenomenological similarities between alcoholic hallucinosis and paranoid schizophrenia have been discussed.[ 117 ] Opioid users with psychopathology[ 118 ] have diverse types of psychopathology as do users of other drugs.[ 119 ]
In a study of 22 dual diagnosed schizophrenia patients, substance use disorder preceded the onset of schizophrenic illness in the majority.[ 120 ] While one study found high rates of comorbid substance use (54%) in patients with schizophrenia with comorbid substance users showing more positive symptoms[ 121 ] which remitted more rapidly in the former group,[ 122 ] other studies suggest that substance use comorbidity in schizophrenia is low, and is an important contributor to better outcome in schizophrenia in developing countries like India.[ 123 , 124 ]
The diagnosis and management of dual diagnosis has been reviewed in detail.[ 125 ]
Social factors
Co-dependency has been described in spouses of alcoholics and found to correlate with the Addiction Severity scores of their husbands.[ 126 ] Coping behavior described among wives of alcoholics include avoidance, indulgence and fearful withdrawal.[ 127 ] These authors did not find any differences in personality between wives of alcoholics compared to controls.[ 128 ] Delusional jealousy and fighting behavior of substance abusers/dependents are important determinants of suicidal attempts among their spouses.[ 129 ] Parents of narcotic dependent patients, particularly mothers also show significant distress.[ 130 ]
BIOLOGY OF ADDICTION
An understanding of the cellular and molecular mechanisms of drug dependence has led to a reformulation of the etiology of this complex disorder.[ 131 ] An understanding of specific neurotransmitter systems has led to the development of specific pharmacotherapies for these disorders.
Cellular and molecular mechanisms
Altered alcohol metabolism due to polymorphisms in the alcohol metabolizing enzymes may influence clinical and behavioral toxicity due to alcohol. Erythrocyte aldehyde dehydrogenase was demonstrated to be suitable as a peripheral trait marker for alcohol dependence.[ 132 ] Single nucleotide polymorphism of the ALDH 2 gene has been studied in six Indian populations and provides the baseline for future studies in alcoholism.[ 133 ] An evaluation of ADH 1B and ALDH 2 gene polymorphism in alcohol dependence showed a high frequency of the ALDH2*2/*2 genotype among alcohol-dependent subjects.[ 134 ] DRD2 polymorphisms have been studied in patients with alcohol dependence, but a study in an Indian population failed to show a positive association. Genetic polymorphisms of the opioid receptor µ1 has been associated with alcohol and heroin addiction in a population from Eastern India.[ 135 ]
Neuro-imaging and electrophysiological studies
Certain individuals may develop early and severe problems due to alcohol misuse and be poorly responsive to treatment. Such vulnerability has been related to individual differences in brain functioning [ Figure 3 ]. Individuals with a high family history of alcoholism (specifically of the early-onset type, developing before 25 years of age) display a cluster of disinhibited behavioral traits, usually evident in childhood and persisting into adulthood.[ 136 ]

Brain volume differences between children and adolescents at high risk and low risk for alcohol dependence
Early onset drinking may be influenced by delayed brain maturation. Alcohol-naïve male offspring of alcohol-dependent fathers have smaller (or slowly maturing) brain volumes compared to controls in brain areas responsible for attention, motivation, judgment and learning.[ 137 , 138 ] The lag is hypothesized to work through a critical function of brain maturation-perhaps delayed myelination (insulation of brain pathways).
Functionally, this is thought to create a state of central nervous system hyperexcitability or disinhibition.[ 139 ] Individuals at risk have also been shown to have specific electro-physiological characteristics such as reduced amplitude of the P300 component of the event related potential.[ 140 , 141 ] Auditory P300 abnormalities have also been demonstrated among opiate dependent men and their male siblings.[ 142 ]
Such brain disinhibition is manifest by a spectrum of behavioral abnormalities such as inattention (low boredom thresholds), hyperactivity, impulsivity, oppositional behaviors and conduct problems, which are apparent from childhood and persist into adulthood. These brain processes not only promote impulsive risk-taking behaviors like early experimentation with alcohol and other substances but also appear to increase the reinforcement from alcohol while reducing the subjective appreciation of the level of intoxication, thus making it more likely that these individuals are likely not only to start experimenting with alcohol use at an early age but are more likely to have repeated episodes of bingeing.[ 143 ]
INTERVENTIONS, COURSE AND OUTCOME
Although there are a few review articles on pharmacological treatment of alcoholism,[ 144 , 145 ] there is a dearth of randomized studies on relapse prevention treatment in our setting.
Treatment of complications of substance use has been confined to case reports. A case report of thiamine resistant Wernicke Korsakoff Syndrome[ 146 ] successfully treated with a combination of magnesium sulphate and thiamine. Another case of subclinical psychological deterioration[ 147 ] (alcoholic dementia) improved with thiamine and vitamin B supplementation.
Pharmacological intervention
A randomized double blind study compared the effectiveness of detoxification with either lorazepam or chlordiazepoxide among hundred alcohol dependent inpatients with simple withdrawal. Lorazepam was found to be as effective as the more traditional drug chlordiazepoxide in attenuating alcohol withdrawal symptoms as assessed using the revised Clinical Institute Withdrawal Assessment for Alcohol scale.[ 148 ] This has implications for treatment in peripheral settings where liver function tests may not be available. However, benzodiazepines must be used carefully and monitored as dependence is very common.[ 149 ]
In a study closer to the real-world situation from Mumbai, 100 patients with alcohol dependence with stable families were randomized to receive disulfiram or topiramate. At the end of nine months, though patients on topiramate had less craving, a greater proportion of patients on disulfiram were abstinent (90% vs. 56%). Patients in the disulfiram group also had a longer time to their first drink and relapse.[ 150 ] Similar studies by the same authors and with similar methodology had earlier found that disulfiram was superior to acamprosate and Naltrexone. Though the study lacked blinding, it had an impressively low (8%) dropout rate.[ 151 , 152 ] A chart based review has shown there was no significant difference with regard to abstinence among the patients prescribed acamprosate, naltrexone or no drugs. Although patients on acamprosate had significantly better functioning, lack of randomization and variations in base line selection parameters may have influenced these findings.[ 153 ] Short term use of disulfiram among alcohol dependence patients with smoking was not associated with decrease pulmonary function test (FEV 1 ) and airway reactivity.[ 154 ]
Usefulness of clonidine for opioid detoxification has been described by various authors. These studies date back to 1980 when there was no alternative treatment for opioid dependence and clonidine emerged as the treatment of choice for detoxification in view of its anti adrenergic activity.[ 155 – 157 ] Sublingual buprenorphine for detoxification among these patients was reported as early as 1992. At that time the dose used was much lower, i.e. 0.6 -1.2 mg/ day which is in contrast to the current recommended dose of 6-16 mg/day. Comparison of buprenorphine (0.6-1.2 mg/ day) and clonidine (0.3-0.9 mg/day) for detoxification found no difference among treatment non completers. Maximum drop out occurred on the fifth day when withdrawal symptoms were very high.[ 158 ] A 24- week outcome study of buprenorphine maintenance in opiate users showed high retention rates of 81.5%, reduction in Addiction Severity Index scores and injecting drug use. Use of slow release oral morphine for opioid maintenance has also been reported.[ 159 ] Effectiveness of baclofen in reducing withdrawal symptoms among three patients with solvent dependence is reported.[ 160 ]
Psychosocial
Psychoeducational groups have been found to facilitate recovery in alcohol and drug dependence.[ 161 ] Family intervention therapy in addition to pharmacotherapy was shown to reduce the severity of alcohol intake and improve the motivation to stop alcohol in a case-control design study.[ 162 ] Several community based models of care have been developed with encouraging results.[ 163 ]
Course and outcome
An evaluation after five years, of 800 patients with alcohol dependence treated at a de-addiction center, found that 63% had not utilized treatment services beyond one month emphasizing the need to retain patients in follow-up.[ 164 ]
In a follow-up study on patients with alcohol dependence, higher income and longer duration of in-patient treatment were found to positively correlate with improved outcome at three month follow up. Outcome data was available for 52% patients; 81% of those maintained abstinence.[ 165 ] Maximum attrition was between three to six months. In a similar study among in-patients, 46% were abstinent. The drop out rate was 10% at the end of one year.[ 101 ] Studies done in the community setting have shown the effectiveness of continued care in predicting better outcome in alcohol dependence. In one study the patient group from a low socio-economic status who received weekly follow up or home visit at a clinic located within the slum showed improvement at the end of month 3, 6 and 9, and one year, in comparison with a control group that received no active follow-up intervention.[ 166 ] In a one-year prospective study of outcome following de-addiction treatment, poor outcome was associated with higher psychosocial problems, family history of alcoholism and more follow-up with mental health services.[ 167 ]
COMMUNITY INTERVENTIONS AND POLICIES
The camp approach for treatment of alcohol dependence was popularized by the TTK hospital camp approach at Manjakkudi in Tamil Nadu.[ 168 ] Treatment of alcohol and drug abuse in a camp setting as a model of drug de-addiction in the community through a 10 day camp treatment was found to have good retention rates and favorable outcome at six months.
Community perceptions of substance related problems are useful to understand for policy development. In a 1981 study in urban and rural Punjab of 1031 respondents, 45% felt people could not drink without producing bad effects on their health, 26.2% felt they could have one or two drinks per month without affecting their health. About one third felt it was alright to have one or two drinks on an occasion. 16.9% felt it was normal to drink ‘none at all’. Alcoholics were identified by behavior such as being dead drunk, drinking too much, having arguments and fights and creating public nuisance. Current users gave the most permissive responses and non-users the most restrictive responses regarding the norms for drinking.[ 169 ] The influence of cultural norms[ 170 ] has led the tendency to view drugs as ‘good’ and ‘bad’.
Simulations done in India have demonstrated that implementing a nationwide legal drinking age of 21 years in India, can achieve about 50-60 % of the alcohol consumption reducing effects compared to prohibition.[ 171 ] However, recently there are attempts to increase the permissible legal alcohol limit. This kind of contrarian approach does not make for coherent policy.
It has been argued that the 1970s saw an overzealous implementation of a simplistic model of supply and demand.[ 171 ] A presidential address[ 172 ] in 1991 emphasized the need for a multipronged approach to addressing alcohol-related problems. Existing programs have been identified as being patchy, poorly co-ordinated and poorly funded. Primary, secondary and tertiary approaches were discussed. The address highlighted the need for supply and demand side measures to address this significant public health problem. It highlighted the political and financial power of the alcohol industry and the social ambivalence to drinking. More recently, the need to have interventions for harmful and hazardous use, the need to develop evidence based combinations of pharmacotherapy and psychosocial interventions and stepped care solutions have been highlighted.[ 173 ] Standard treatment guidelines for alcohol and other drug use disorders have suggested specific measures at the primary, secondary and tertiary health care level, including at the solo physician level.[ 174 ] An earlier report in 1988 on training general practitioners on management of alcohol related problems[ 175 ] suggests that their involvement in alcohol and health education was modest, involvement in control and regulatory activities minimal, and they perceived no role in the development of a health and alcohol policy.
There have been reviews of the National Master Plan 1994, which envisaged different responsibilities for the Ministries of Health and the Ministry of Welfare (presently Social Justice and Empowerment) and the Drug Dependence Program 1996.[ 176 , 177 ] A proposal for adoption of a specialty section on addiction medicine[ 178 ] includes the development of a dedicated webpage, co-ordinated CMEs, commissioning of position papers, promoting demand reduction strategies and developing a national registry.
SUMMARY AND CONCLUSIONS
While epidemiological research has now provided us with figures for national-level prevalence, it would be prudent to recognize that there are regional differences in substance use prevalence and patterns. It is also prudent to recognize the dynamic nature of substance use. There is thus a need for periodic national surveys to determine changing prevalence and incidence of substance use. Substance use is associated with significant mortality and morbidity. Substance use among women and children is increasingly becoming the focus of attention and merits further research. Pharmaceutical drug abuse and inhalant use are serious concerns. For illicit drug use, rapid assessment surveys have provided insights into patterns and required responses. Drug related emergencies have not been adequately studied in the Indian context.
Biological research has focused on two broad areas, neurobiology of vulnerability and a few studies on molecular genetics. There is a great need for translation research based on the wider body of basic and animal research in the area.
Clinical research has primarily focused on alcohol. An area which has received relatively more attention in substance related comorbidity. There is very little research on development and adaptation of standardized tools for assessment and monitoring, and a few family studies. Ironically, though several evidence based treatments have now become available in the country, there are very few studies examining the utilization and effectiveness of these treatments, given that most treatment is presently unsubsidized and dependent on out of pocket expenditure. Both pharmacological and psychosocial interventions have disappointingly attracted little research. Course and outcome studies emphasize the need for better follow-up in this group.
While a considerable number of publications have lamented the lack of a coherent policy, the need for human resource enhancement and professional training and recommended a stepped-care multipronged approach, much remains to be done on the ground.
Finally, publication interest in the Indian Journal of Psychiatry in the area of substance use will undoubtedly increase, with the journal having become indexed.
Source of Support: Nil
Conflict of Interest: None declared

IMAGES
VIDEO
COMMENTS
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol.When considering other substances, the report estimated that 4.4 million individuals ...
Abstract and Figures. The development of addiction involves a transition from casual to compulsive patterns of drug use. This transition to addiction is accompanied by many drug-induced changes in ...
Introduction. Close to a quarter of a century ago, then director of the US National Institute on Drug Abuse Alan Leshner famously asserted that "addiction is a brain disease", articulated a set of implications of this position, and outlined an agenda for realizing its promise [].The paper, now cited almost 2000 times, put forward a position that has been highly influential in guiding the ...
ABSTRACT. Addiction and recovery from addiction are described by synthesising fifty-two. qualitative studies from thirty-two years of research. Based on the lived experience. of individuals this ...
The findings from neurobiologic research show that addiction is a disease that emerges gradually and that has its onset predominantly during a particular risk period: adolescence. Adolescence is a ...
Addiction involves loss of control over use of a substance, often in the presence of physiological and psychological dependence on a substance and compulsion to continue seeking and using the ...
JAM Impact Factor Reaches 5.5. The Journal of Addiction Medicine's (JAM) Impact Factor (IF), released in June 2023 by Clarivate's Journal Citation Reports, jumped by nearly a full point to 5.5 in 2022 from 4.6 in 2021. JAM's 2020 IF was 3.9 and has been steadily increasing since 2014. The IF metric is a ratio of the number of citations ...
Submissions to Addiction Research and Theory are peer reviewed and published if they are both good of their kind and are within the journal's focus. Articles include theoretical, philosophical and political essays, research papers, state-of-the-science reviews, and descriptions of how to apply research on addictive behaviours to evidence ...
Read the latest research, submit your paper, and more. Peer-reviewed, original research for mental health professionals who treat and study addictive behaviors. ... APA journal Psychology of Addictive Behaviors, Vol. 34, No. 1, February 2020. The articles span the multiple areas of addiction research to which Dr. Nancy Petry made key ...
Addiction publishes peer-reviewed research reports on pharmalogical and behavioural addictions, bringing together research conducted within many different disciplines. The publication is an official journal of the Society for the Study of Addiction, and has been in publication since 1884. ... Call for Papers: Special Issue on disposable e ...
Abstract. Alcohol is a major contributor to global disease and a leading cause of preventable death, causing approximately 88,000 deaths annually in the United States alone. Alcohol use disorder is one of the most common psychiatric disorders, with nearly one-third of U.S. adults experiencing alcohol use disorder at some point during their lives.
Considerable research already shows the benefits of cognitive-behavioral treatments in improving self-regulation and of contingency management in strengthening the degraded motivation to engage in non-drug-related activities, so clearly these modalities are effective for addressing specific dimensions of the addiction process.
II. DRUG REWARD. Dopamine (DA) lies at the center of drug reward (85, 182).Every drug with addiction potential increases DA, either through direct or indirect effects on DA neurons in the ventral tegmental area (VTA) with the consequent release of DA in the nucleus accumbens (NAc) ().Drugs of abuse increase DA through their initial action on different molecular targets and, depending on their ...
For much of the past century, scientists studying drugs and drug use labored in the shadows of powerful myths and misconceptions about the nature of addiction. When scientists began to study addictive behavior in the 1930s, people with an addiction were thought to be morally flawed and lacking in willpower. Those views shaped society's ...
How does science provide solutions for drug abuse and addiction? Scientists study the effects that drugs have on the brain and on people's behavior. They use this information to develop programs for preventing drug abuse and for helping people recover from addiction. Further research helps transfer these ideas into practice in our communities. 3
These research queries and findings are presented in the form of updates, white papers and case studies. In addition, the Butler Center for Research collaborates with the Recovery Advocacy team to study special-focus addiction research topics, summarized in monthly Emerging Drug Trends reports. Altogether, these studies provide the latest in ...
Abstract. Background: Smartphones play a critical role in increasing human-machine interactions, with many advantages. However, the growing popularity of smartphone use has led to smartphone overuse and addiction. This review aims to systematically investigate the impact of smartphone addiction on health outcomes.
Research Topics. En español. The National Institute on Drug Abuse (NIDA) is the largest supporter of the world's research on substance use and addiction. Part of the National Institutes of Health, NIDA conducts and supports biomedical research to advance the science on substance use and addiction and improve individual and public health.
The view that substance addiction is a brain disease, although widely accepted in the neuroscience community, has become subject to acerbic criticism in recent years. These criticisms state that ...
Alcohol and drug addiction have been found to have significant effects ... Research suggests having a family member with a substance abuse problem has negative effects on both physical ... K. 2014. The drug trade and governance in Cape Town. Institute for Security Studies, the University of Cape Town. ISS Paper 263. Google Scholar.
The aim of this paper is to give a preferably brief overview of research on IAD and theoretical considerations from a practical perspective based on years of daily work with clients suffering from Internet addiction. Furthermore, with this paper we intend to bring in practical experience in the debate about the eventual inclusion of IAD in the ...
Research suggests that addiction recovery is quite similar to, and strongly linked with, a prolonged or pathological grief. How grief and healing matter in these meetings is an important area for research. This paper explores these interlinked processes in CA meetings to understand how they contribute to resilience. Shares on grief-related ...
Bunting is also studying the use of LAI medications for psychiatric conditions, research prompted by frustration with difficulties getting these medicines for his own patients. ... Given the epidemic levels of opioid addiction, Ari said she hopes the paper sparks conversations on how to design better health policies, remove barriers and build ...
In this paper we examine research in India in substance use and related disorders. Substance use includes the use of licit substances such as alcohol, tobacco, diversion of prescription drugs, as well as illicit substances. ... 'alcohol addiction' or 'alcoholism' or 'chronic alcoholism' from 2.36/1000 to 34.5/1000; alcohol and drug ...