

Class 12 Chemistry Case Study Questions PDF Download
- Post author: studyrate
- Post published:
- Post category: Class 12 / 12 board
- Post comments: 0 Comments
Looking for Class 12 Chemistry Case Study Based Questions in PDF format? This comprehensive article provides expert insights, engaging content, and answers to frequently asked questions to help you excel in your studies. Download the PDF now and boost your chemistry knowledge!
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

You need to improve your preparation for the Class 12 Chemistry Case Study Questions exams if you want to achieve a 95+% on the boards. You may find case study questions from every chapter that will be covered in the CBSE Class 12 Chemistry Board Exams in this post.
Table of Contents
Case Study-Based Questions for Class 12 Chemistry
Welcome to the world of Class 12 Chemistry Case Study Questions! As a student pursuing Chemistry in the 12th grade, you have already shown dedication and commitment to the subject. However, dealing with case study-based questions can be both intriguing and challenging. In this article, we will explore the nuances of such questions and offer valuable guidance to excel in your exams.
Class 12 Physics Case Study Questions Class 12 Chemistry Case Study Questions Class 12 Biology Case Study Questions Class 12 Maths Case Study Questions
Importance of Class 12 Chemistry Case Study-Based Questions
Class 12 Chemistry case study-based questions play a vital role in your overall understanding of the subject. They enable you to:
- Apply Theoretical Knowledge : Case studies allow you to apply the concepts you have learned in real-life situations, bridging the gap between theory and practical application.
- Develop Analytical Skills : By critically analyzing case scenarios, you enhance your analytical abilities, which are essential in various professional fields.
- Enhance Problem-Solving Abilities : Tackling case study-based questions hones your problem-solving skills, preparing you to face challenges with confidence.
- Gain Deeper Insights : Exploring different case studies exposes you to a wide range of chemical reactions and phenomena, broadening your understanding of Chemistry.
Tips to Excel in Class 12 Chemistry Case Study Questions
- Thoroughly Understand the Concepts: Before attempting case study questions, ensure you have a strong grasp of the underlying concepts and theories.
- Analyze the Scenario Carefully: Take your time to read and comprehend the given case study. Pay attention to every detail to identify the key points.
- Relate to Real-Life Scenarios: Try to connect the case study with real-life situations, as this will make the problem-solving process more intuitive.
- Practice Regularly: Practice a wide variety of case study questions to familiarize yourself with different scenarios and improve your problem-solving skills.
- Collaborate with Peers: Engage in group discussions and brainstorming sessions with your peers. This will provide diverse perspectives and enhance your critical thinking.
Best Books for Class 12 Chemistry
Strictly in accordance with the new term-by-term curriculum for the Class 12 Chemistry Case Study Questions exams to be held in the academic session 2024, including the new board-introduced multiple-choice question types, Stand-Alone MCQs, and MCQs based on Assertion-Reason Case-based MCQs. Included are inquiries from the official CBSE Question Bank that was released in April 2024. What changes have been made to the book: strictly in accordance with the term-by-term syllabus for the board exams that will be held during the 2024 academic year? Chapter- and topic-specific multiple-choice questions based on the unique assessment plan for the Class 12 Chemistry Case Study Questions Board Examination.

Chemistry Syllabus for 2024
Unit II: Solutions (15 Periods)
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, Raoult’s law, colligative properties – relative lowering of vapour pressure, elevation of boiling point, depression of freezing point, osmotic pressure, determination of molecular masses using colligative properties, abnormal molecular mass, Van’t Hoff factor.
Unit III: Electrochemistry (18 Periods)
Redox reactions, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, Relation between Gibbs energy change and EMF of a cell, conductance in electrolytic solutions, specific and molar conductivity, variations of conductivity with concentration, Kohlrausch’s Law, electrolysis and law of electrolysis (elementary idea), dry cell-electrolytic cells and Galvanic cells, lead accumulator, fuel cells, corrosion.
Unit IV: Chemical Kinetics (15 Periods)
Rate of a reaction (Average and instantaneous), factors affecting rate of reaction: concentration, temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant, integrated rate equations and half-life (only for zero and first order reactions), concept of collision theory (elementary idea, no mathematical treatment), activation energy, Arrhenius equation.
Unit VIII: d and f Block Elements (18 Periods)
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first-row transition metals – metallic character, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds, alloy formation, preparation and properties of K 2 Cr 2 O 7 and KMnO 4 .
Lanthanoids – Electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction and its consequences.
Actinoids – Electronic configuration, oxidation states and comparison with lanthanoids.
Unit IX: Coordination Compounds (18 Periods)
Coordination compounds – Introduction, ligands, coordination number, colour, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds. Bonding, Werner’s theory, VBT, and CFT; structure and stereoisomerism, the importance of coordination compounds (in qualitative analysis, extraction of metals and biological system).
Unit X: Haloalkanes and Haloarenes (15 Periods)
Haloalkanes: Nomenclature, nature of C–X bond, physical and chemical properties, optical rotation mechanism of substitution reactions.
Haloarenes: Nature of C–X bond, substitution reactions (Directive influence of halogen in monosubstituted compounds only). Uses and environmental effects of – dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
Unit XI: Alcohols, Phenols and Ethers (14 Periods)
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary and tertiary alcohols, mechanism of dehydration, uses with special reference to methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Unit XII: Aldehydes, Ketones and Carboxylic Acids (15 Periods)
Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes, uses.
Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
Unit XIII: Amines (14 Periods)
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, identification of primary, secondary and tertiary amines
Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry.
Unit XIV: Biomolecules (18 Periods)
Carbohydrates – Classification (aldoses and ketoses), monosaccharides (glucose and fructose), D-L configuration oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); Importance of carbohydrates.
Proteins – Elementary idea of – amino acids, peptide bond, polypeptides, proteins, structure of proteins – primary, secondary, tertiary structure and quaternary structures (qualitative idea only), denaturation of proteins; enzymes.
Hormones – Elementary idea excluding structure.
Vitamins – Classification and functions.
Nucleic Acids: DNA and RNA.
FAQ on Class 12 Chemistry Case Study Questions
Q: can i rely solely on class 12 chemistry case study based questions exam preparation.
Yes, case study-based questions are an essential part of your preparation. However, it is advisable to supplement them with other study materials and revision of theoretical concepts for comprehensive preparation.
Q: How often should I practice Class 12 Chemistry Case Study Based Questions?
Frequent practice is crucial for mastering case study-based questions. Set aside dedicated practice sessions and gradually increase the difficulty level of the questions.
Q: Can I discuss case study questions with my teachers?
Absolutely! Engaging with your teachers regarding case study questions will provide valuable insights and clarifications.

You Might Also Like
Class 12 biology case study questions chapter 8 human health and diseases, class 12 physics case study questions chapter 7 alternating current, class 12 physics assertion reason questions chapter 14 semiconductor electronics, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

The Topper Combo Flashcards
- Contains the Latest NCERT in just 350 flashcards.
- Colourful and Interactive
- Summarised Important reactions according to the latest PYQs of NEET(UG) and JEE
No thanks, I’m not interested!
Not Able To Find Desired Paper or Worksheet SEARCH
Find papers & worksheets search, case study questions for class 12 chemistry chapter 3 electrochemistry.

- (0) Comments
- 10 Downloads
Related Papers
Click to view more related papers, display_name = "class 11" && $paper->display_name = "class 12") { // echo $paper->display_name." questions papers and worksheets"; } //else { // echo $paper->display_name." sample papers and previous year papers"; //} //>, important questions, mcq's, ncert solutions - class 12 chemistry.
Get here all the Important questions for Class 12 Chemistry chapter wise as free PDF download. Here you will get Extra Important Questions with answers, Numericals and Multiple Choice Questions (MCQ's) chapter wise in Printable format. Solving Chapter wise questions is one of the best ways to prepare for the examination. Students are advised to understand the concepts and theories of Chemistry properly before the exam. You can easily find 1 Mark, 2 marks, 3 marks, and 5 marks questions from each chapter of Class 12 Chemistry and prepare for exam more effectively. These preparation material for Class 12 Chemistry , shared by teachers, parents and students, are as per latest NCERT and CBSE Pattern syllabus and assure great success in achieving high score in Final CBSE Board Examinations.
Latest MCQ's and Important Questions for CBSE Class 12 Chemistry
class 12 chemistry chapter 1 important questions with answers class 12 chemistry chapter 2 important questions with answers class 12 chemistry chapter 3 important questions with answers class 12 chemistry chapter 4 important questions with answers class 12 chemistry chapter 5 important questions with answers class 12 chemistry chapter 6 important questions with answers class 12 chemistry chapter 7 important questions with answers class 12 chemistry chapter 8 important questions with answers class 12 chemistry chapter 9 important questions with answers class 12 chemistry chapter 10 important questions with answers class 12 chemistry chapter 11 important questions with answers class 12 chemistry chapter 12 important questions with answers class 12 chemistry chapter 13 important questions with answers class 12 chemistry chapter 14 important questions with answers class 12 chemistry chapter 15 important questions with answers class 12 chemistry chapter 16 important questions with answers mcqs of chemistry class 12 chapter 1 mcqs of chemistry class 12 chapter 2 mcqs of chemistry class 12 chapter 3 mcqs of chemistry class 12 chapter 4 mcqs of chemistry class 12 chapter 5 mcqs of chemistry class 12 chapter 6 mcqs of chemistry class 12 chapter 7 mcqs of chemistry class 12 chapter 8 mcqs of chemistry class 12 chapter 9 mcqs of chemistry class 12 chapter 10 mcqs of chemistry class 12 chapter 11 mcqs of chemistry class 12 chapter 12 mcqs of chemistry class 12 chapter 13 mcqs of chemistry class 12 chapter 14 mcqs of chemistry class 12 chapter 15 mcqs of chemistry class 12 chapter 16 The Solid State Class 12 Case Study Questions Solutions Class 12 Case Study Questions Notes Electrochemistry Class 12 Case Study Questions Chemical Kinetics Class 12 Case Study Questions Surface Notes Class 12 Case Study Questions General Principles and Processes of Isolation of Elements Class 12 Case Study Questions The p-Block Elements Class 12 Case Study Questions The d and f Block Elements Class 12 Case Study Questions Coordination Compounds Class 12 Case Study Questions Haloalkanes and Haloarenes Class 12 Case Study Questions Alcohols, Phenols and Ethers Class 12 Case Study Questions Aldehydes, Ketones and Carboxylic Acids Class 12 Case Study Questions Amines Class 12 Case Study Questions Biomolecules Class 12 Case Study Questions Polymers Class 12 Case Study Questions Chemistry in Everyday Life Class 12 Case Study Questions
Total Papers :
CBSE Class 12 Chemistry Syllabus
- Solid State
- Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- General Principles and Processes of Isolation of Elements
- p-Block Elements
- d- and f-Block Elements
- Coordination Compounds
- Haloalkanes and Haloarenes.
- Alcohols, Phenols and Ethers
- Aldehydes, Ketones and Carboxylic Acids
- Organic compounds containing Nitrogen
- Biomolecules
- Chemistry in Everyday life
Unit II: Solutions 15 Periods
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, Raoult's law, colligative properties - relative lowering of vapour pressure, elevation of boiling point, depression of freezing point, osmotic pressure, determination of molecular masses using colligative properties, abnormal molecular mass, Van't Hoff factor.
Unit III: Electrochemistry 18 Periods
Redox reactions, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, Relation between Gibbs energy change and EMF of a cell, conductance in electrolytic solutions, specific and molar conductivity, variations of conductivity with concentration, Kohlrausch's Law, electrolysis and law of electrolysis (elementary idea), dry cell-electrolytic cells and Galvanic cells, lead accumulator, fuel cells, corrosion.
Unit IV: Chemical Kinetics 15 Periods
Rate of a reaction (Average and instantaneous), factors affecting rate of reaction: concentration, temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant, integrated rate equations and half-life (only for zero and first order reactions), concept of collision theory (elementary idea, no mathematical treatment), activation energy, Arrhenius equation.
Unit VIII: d and f Block Elements 18 Periods
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first-row transition metals – metallic character, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds, alloy formation, preparation and properties of K2Cr2O7 and KMnO4.
Lanthanoids – Electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction and its consequences.
Actinoids - Electronic configuration, oxidation states and comparison with lanthanoids.
Unit IX: Coordination Compounds 18 Periods
Coordination compounds - Introduction, ligands, coordination number, colour, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds. Bonding, Werner's theory, VBT, and CFT; structure and stereoisomerism, the importance of coordination compounds (in qualitative analysis, extraction of metals and biological system).
Unit X: Haloalkanes and Haloarenes. 15 Periods Haloalkanes: Nomenclature, nature of C–X bond, physical and chemical properties, optical rotation mechanism of substitution reactions.
Haloarenes: Nature of C–X bond, substitution reactions (Directive influence of halogen in monosubstituted compounds only). Uses and environmental effects of - dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
Unit XI: Alcohols, Phenols and Ethers 14 Periods
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary and tertiary alcohols, mechanism of dehydration, uses with special reference to methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Unit XII: Aldehydes, Ketones and Carboxylic Acids 15 Periods
Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes, uses.
Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
Unit XIII: Amines 14 Periods
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, identification of primary, secondary and tertiary amines.
Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry.
Unit XIV: Biomolecules 18 Periods
Carbohydrates - Classification (aldoses and ketoses), monosaccharides (glucose and fructose), D-L configuration oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); Importance of carbohydrates.
Proteins - Elementary idea of - amino acids, peptide bond, polypeptides, proteins, structure of proteins - primary, secondary, tertiary structure and quaternary structures (qualitative idea only), denaturation of proteins; enzymes. Hormones - Elementary idea excluding structure.
Vitamins - Classification and functions. Nucleic Acids: DNA and RNA.
Structure of CBSE Chemistry Sample Paper for Class 12 Science is
For Preparation of exams students can also check out other resource material
CBSE Class 12 Chemistry Sample Papers
CBSE Class 12 Chemistry Worksheets
CBSE Class 12 Chemistry Question Papers
CBSE Class 12 Chemistry Test Papers
CBSE Class 12 Chemistry Revision Notes
Question Bank of Other Subjects of Class 12
Importance of Question Bank for Exam Preparation?
There are many ways to ascertain whether a student has understood the important points and topics of a particular chapter and is he or she well prepared for exams and tests of that particular chapter. Apart from reference books and notes, Question Banks are very effective study materials for exam preparation. When a student tries to attempt and solve all the important questions of any particular subject , it becomes very easy to gauge how much well the topics have been understood and what kind of questions are asked in exams related to that chapter.. Some of the other advantaging factors of Question Banks are as follows
- Since Important questions included in question bank are collections of questions that were asked in previous exams and tests thus when a student tries to attempt them they get a complete idea about what type of questions are usually asked and whether they have learned the topics well enough. This gives them an edge to prepare well for the exam.Students get the clear idea whether the questions framed from any particular chapter are mostly either short or long answer type questions or multiple choice based and also marks weightage of any particular chapter in final exams.
- CBSE Question Banks are great tools to help in analysis for Exams. As it has a collection of important questions that were asked previously in exams thereby it covers every question from most of the important topics. Thus solving questions from the question bank helps students in analysing their preparation levels for the exam. However the practice should be done in a way that first the set of questions on any particular chapter are solved and then solutions should be consulted to get an analysis of their strong and weak points. This ensures that they are more clear about what to answer and what can be avoided on the day of the exam.
- Solving a lot of different types of important questions gives students a clear idea of what are the main important topics of any particular chapter that needs to focussed on from examination perspective and should be emphasised on for revision before attempting the final paper. So attempting most frequently asked questions and important questions helps students to prepare well for almost everything in that subject.
- Although students cover up all the chapters included in the course syllabus by the end of the session, sometimes revision becomes a time consuming and difficult process. Thus, practicing important questions from Question Bank allows students to check the preparation status of each and every small topic in a chapter. Doing that ensures quick and easy insight into all the important questions and topics in each and every individual. Solving the important questions also acts as the revision process.
Question Bank of Other Classes
To Prepare better for CBSE paperclass; ?> " title="Download Free CBSE Papers">Ribblu.com brings to you all the previous years papers & worksheets of subject; ?//> for CBSE paperclass; ?>. This CBSE paper and worksheet can be instrumental in students achieving maximum marks in their exams. These Papers and worksheets help students gain confidence and make them ready to face their school examinations. These Papers and worksheets school wise, covers important concepts from an examination perspective. Students and parents can download all the available papers & worksheets directly in the form of PDF. One can use these papers and worksheets to get extensive practice and familiarise themselves with the format of the question paper.
You can help other users
Be the first to write comment .
Upload papers and the more your paper get downloaded the more you earn the points
You may send papers on email [email protected] along with userid
- Downloaded by: meghna baruah
- Downloaded by: pooja
Rules and regulations for uploads
Write your comment, report this paper, how to earn points.
Upload Papers / Worksheets and Earn 50 Points.
The uploaded material should be original paper or worksheet of any school. Check out some videos on how to upload papers on ribblu
Rate & Review your school and Earn 25 Points.
Review any school that you may be knowing and once your review is approved, you will be credited with 25 points.
Answer on question posted on JustAsk and earn 15 points.
JustAsk is a platform where you can help others to find answers of any question. Share your Knowledge. Answer questions and once approved you will earn 15 points
Complete your profile and earn upto 25 Points.
Edit and complete your user profile and earn points. The more details you submit, the more points you will earn.
Download Ribblu Mobile App and you will (Earn 20 Points) (one time only)
CBSE Schools
- CBSE Schools In Delhi
- CBSE Schools In Noida
- CBSE Schools In Greater Noida
- CBSE Schools In Faridabad
- CBSE Schools In Ghaziabad
- CBSE Schools In Gurgaon
- CBSE Schools In Mumbai
- CBSE Schools In Pune
- CBSE Schools In Bangalore
- CBSE Schools In Hyderabad
- CBSE Schools In Kolkata
- CBSE Schools In Chennai
- CBSE Schools In Patna
- CBSE Schools In Meerut
- CBSE Schools In Kanpur
- CBSE Schools In Indore
- CBSE Schools In Ludhiana
- CBSE Schools In Dehradun
Top Schools
- Schools In Delhi
- Schools In Noida
- Schools In Greater Noida
- Schools In Faridabad
- Schools In Ghaziabad
- Schools In Gurgaon
- Schools In Mumbai
- Schools In Pune
- Schools In Bangalore
- Schools In Hyderabad
- Schools In Kolkata
- Schools In Chennai
- Schools In Patna
- Schools In Meerut
- Schools In Kanpur
- Schools In Indore
- Schools In Ludhiana
- Schools In Dehradun
Other Schools
- Pre Nursery Schools In Noida
- Day Boarding Schools In Noida
- Pre Nursery Schools In Gurgaon
- Pre Nursery Schools In Delhi
- Play Schools In Delhi
- Day Boarding Schools In Delhi
CBSE Papers
- CBSE Class 1 Sample Papers
- CBSE Class 2 Sample Papers
- CBSE Class 3 Sample Papers
- CBSE Class 4 Sample Papers
- CBSE Class 5 Sample Papers
- CBSE Class 6 Sample Papers
- CBSE Class 7 Sample Papers
- CBSE Class 8 Sample Papers
Paper Categories
- Question Bank
- Question Papers
- Revision Notes
- Sample Papers
- Test Papers
- CBSE Class 9 Sample Papers
- CBSE Class 10 Sample Papers
- CBSE Class 11 Sample Papers
- CBSE Class 12 Sample Papers

CBSE Expert
CBSE Class 12 Chemistry Case Study Questions PDF
Case studies play a pivotal role in CBSE Class 12 Chemistry, as they enable students to apply theoretical knowledge to real-life scenarios. CBSE Class 12 Chemistry Case Study Questions PDF section introduces the significance of case studies in enhancing analytical skills and understanding complex chemical reactions.
Case studies challenge students to think critically, analyze experimental data, and devise problem-solving strategies. They provide a deeper understanding of chemical principles and their practical applications, fostering a holistic learning experience. Familiarize yourself with the structure of case study questions to streamline your preparation. Each case study presents a unique chemical problem, encouraging students to identify relevant concepts and devise accurate solutions.
Table of Contents
Class 12 Chemistry Case Study Questions
CBSE Class 12 Chemistry question paper will have case study questions too. These case-based questions will be objective type in nature. So, Class 12 Chemistry students must prepare themselves for such questions. First of all, you should study NCERT Textbooks line by line, and then you should practice as many questions as possible.

Chapter-wise Solved Case Study Questions for Class 12 Chemistry
Class 12 students should go through important Case Study problems for Chemistry before the exams. This will help them to understand the type of Case Study questions that can be asked in Grade 12 Chemistry examinations. Our expert faculty for standard 12 Chemistry have designed these questions based on the trend of questions that have been asked in last year’s exams. The solutions have been designed in a manner to help the grade 12 students understand the concepts and also easy-to-learn solutions.
Tips to Excel in CBSE Class 12 Chemistry Examinations
Excel in your Chemistry exams with these practical tips.
A. Regular Practice with Case Studies
Consistent practice with case study questions enhances your ability to tackle complex problems. Dedicate time to solving various case studies to build confidence.
B. Understanding Analytical Skills
Develop strong analytical skills to approach case studies logically. Break down complex problems into simpler components and analyze them step-by-step.
C. Time Management Strategies
Allocate sufficient time for each case study during the exam. Practice time management in mock tests to complete the paper within the stipulated time.
Best Books for Class 12 Chemistry
Strictly as per the new term-wise syllabus for Board Examinations to be held in the academic session 2024 for class 12 Multiple Choice Questions based on new typologies introduced by the board- Stand-Alone MCQs, MCQs based on Assertion-Reason Case-based MCQs. Include Questions from CBSE official Question Bank released in April 2024 Answer key with Explanations What are the updates in the book: Strictly as per the Term wise syllabus for Board Examinations to be held in the academic session 2024. Chapter-wise -Topic-wise Multiple choice questions based on the special scheme of assessment for Board Examination for Class 12th Chemistry.

Mastering CBSE Class 12 Chemistry case study questions is crucial for excelling in the exams. Embrace case studies as a valuable learning tool, and with practice, you’ll ace your Chemistry exams with confidence.
Benefits of Utilizing the CBSE Class 12 Chemistry Case Study PDF
- Enhanced Learning Experience : The case study PDF offers practical examples and scenarios, making the learning process engaging and relatable for students.
- Application of Theoretical Concepts : It enables students to apply theoretical knowledge to practical situations, honing their problem-solving and analytical skills.
- Real-World Relevance : By connecting classroom learning to real-life applications, students can grasp the practical significance of chemistry in various industries.
- Critical Thinking Development : Analyzing case studies encourages students to think critically and make informed decisions based on chemical principles.
- Exam Preparation : Exposure to case studies aids in better preparation for chemistry examinations by providing a comprehensive understanding of the subject.
The CBSE Class 12 Chemistry case study PDF brings a refreshing perspective to the world of education. By intertwining theoretical knowledge with practical applications, it equips students to face real-world challenges with confidence. The diverse case studies provide invaluable insights, encouraging students to explore chemistry beyond the classroom and make a positive impact on society.
What is the CBSE Class 12 Chemistry case study PDF?
The CBSE Class 12 Chemistry case study PDF is a curated document by CBSE, presenting real-life applications of chemistry concepts for students to understand the subject’s practical relevance.
How does the case study PDF benefit students?
The case study PDF enhances the learning experience, fosters critical thinking, promotes application-based learning, and prepares students for examinations.
Are the case studies diverse in content?
Yes, the case studies cover various branches of chemistry, including organic, inorganic, physical, environmental, and analytical chemistry.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!

- Neet Online Test Pack
12th Standard stateboard question papers & Study material
தமிழ் subjects.

கணினி பயன்பாடுகள்

கணினி அறிவியல்
வணிகக் கணிதம் மற்றும் புள்ளியியல்.

கணினி தொழில்நுட்பம்

கணக்குப்பதிவியல்

English Subjects

Computer Science

Business Maths and Statistics

Accountancy

Computer Applications

Computer Technology

11th Standard stateboard question papers & Study material

9th Standard stateboard question papers & Study material

Social Science

சமூக அறிவியல்
6th standard stateboard question papers & study material.

10th Standard stateboard question papers & Study material

7th Standard stateboard question papers & Study material

8th Standard stateboard question papers & Study material

கணிதம் - old

12th Standard CBSE Subject Question Paper & Study Material

Introductory Micro and Macroeconomics

Business Studies

Indian Society

Physical Education

Bio Technology

Engineering Graphics

Entrepreneurship

Hindi Elective

Home Science

Legal Studies

Political Science

11th Standard CBSE Subject Question Paper & Study Material

Mathematics

Enterprenership

Applied Mathematics
10th standard cbse subject question paper & study material.

9th Standard CBSE Subject Question Paper & Study Material

8th Standard CBSE Subject Question Paper & Study Material

7th Standard CBSE Subject Question Paper & Study Material

6th Standard CBSE Subject Question Paper & Study Material

School Exams

Tamil Nadu State Board Exams

Scholarship Exams

Study Materials , News and Scholarships

Stateboard Tamil Nadu

Free Online Tests

Educational News

Scholarships

Entrance Exams India

Video Materials

12th Standard CBSE
Class 12th Chemsitry - Electrochemistry Case Study Questions and Answers 2022 - 2023


Class 12th Chemsitry - Electrochemistry Case Study Questions and Answers 2022 - 2023 Study Materials Sep-08 , 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 12 Chemsitry Subject - Electrochemistry, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.

A PHP Error was encountered
Severity: Warning
Message: in_array() expects parameter 2 to be array, null given
Filename: material/details.php
Line Number: 1436
Message: Use of undefined constant EXAM - assumed 'EXAM' (this will throw an Error in a future version of PHP)
Line Number: 1438
QB365 - Question Bank Software
Electrochemistry case study questions with answer key.
Final Semester - June 2015
Case Study
Read the passage given below and answer the following questions: Molar conductivity of ions are given as product of charge on ions to their ionic mobilities and Faraday's constant. \(\lambda_{A^{n+}}=n \mu_{A^{n+}} F\) (here \(\mu\) is the ionic mobility of A n+ ). For electrolytes say A X B y , molar conductivity is given by \(\lambda_{m\left(A_{x} B_{y}\right)}=x_{n} \mu_{A^{n+}} F+y_{m} \lambda_{A^{m}-F}\)
The following questions are multiple choice questions. Choose the most appropriate answer (i) At infinite dilution, the equivalent conductance of CaSO 4 is
(ii) If the degree of dissociation of CaSO 4 solution is 10% then equivalent conductance of CaSO 4 is
(iii) What is the unit of equivalent conductivity?
(iv) If the molar conductance value of Ca 2+ and Cl - at infinite dilution are 118.88 x 10 -4 m 2 mho mol -1 and 77.33 x 10 -4 m 2 mho mol -1 respectively then the molar conductance of CaCl 2 (in m 2 mho mol -1 ) will be
Read the passage given below and answer the following questions: Standard electrode potentials are used for various processes: (i) It is used to measure relative strengths of various oxidants and reductants. (ii) It is used to calculate standard cell potential. (iii) It is used to predict possible reactions. A set of half-reactions (in acidic medium) along with their standard reduction potential, E o (in volt) values are given below \(\mathrm{I}_{2}+2 e^{-} \rightarrow 2 \mathrm{I}^{-} ; \quad E^{\circ}=0.54 \mathrm{~V}\) \(\mathrm{Cl}_{2}+2 e^{-} \rightarrow 2 \mathrm{Cl}^{-} ; \quad E^{\circ}=1.36 \mathrm{~V}\) \(\mathrm{Mn}^{3+}+e^{-} \rightarrow \mathrm{Mn}^{2+} ; \quad E^{\circ}=1.50 \mathrm{~V}\) \(\mathrm{Fe}^{3+}+e^{-} \longrightarrow \mathrm{Fe}^{2+} ; \quad E^{\circ}=0.77 \mathrm{~V}\) \(\mathrm{O}_{2}+4 \mathrm{H}^{+}+4 e^{-} \longrightarrow 2 \mathrm{H}_{2} \mathrm{O} ; E^{\circ}=1.23 \mathrm{~V}\) The following questions are multiple choice questions. Choose the most appropriate answer: (i) Which of the following statements is correct?
(ii) Mn 3+ is not stable in acidic medium, while Fe 3+ is stable because
(iii) The strongest reducing agent in the aqueous solution is
(iv) The emf for the following reaction is \(\mathrm{I}_{2}+\mathrm{KCl} \rightleftharpoons 2 \mathrm{KI}+\mathrm{Cl}_{2}\)
Read the passage given below and answer the following questions : All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCI is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept. The following questions are multiple choice questions. Choose the most appropriate answer : (i) The total number of moles of chlorine gas evolved is
(ii) If cathode is a Hg electrode, then the maximum weight of amalgam formed from this solution is
(iii) In the electrolysis, the number of moles of electrons involved are
(iv) In electrolysis of aqueous NaCl solution when Pt electrode is taken, then which gas is liberated at cathode?
Read the passage given below and answer the following questions: The concentration of potassium ions inside a biological cell is at least twenty times higher than the outside. The resulting potential difference across the cell is important in several processes such as transmission of nerve impulses and maintaining the ion balance. A simple model for such a concentration cell involving a metal M is M (s) | M + (aq.; 0.05 molar) || M + (aq; 1 molar) |M (s) . The following questions are multiple choice questions. Choose the most appropriate answer: (i) For the above cell,
(ii) The value of equilibrium constant for a feasible cell reaction is
(iii) What is the emf ofthe cell when the cell reaction attains equilibrium?
(iv) The potential of an electrode change with change in
Read the passage given below and answer the following questions: The electrochemical cell shown below is concentration cell. M|M 2+ (saturated solution of a sparingly soluble salt, MX 2 ) || M 2+ (0.001 mol dm -3 ) | M The emf of the cell depends on the difference in concentrations of M 2+ ions at the two electrodes. The emf of the cell at 298 K is 0.059 V. The following questions are multiple choice questions. Choose the most appropriate answer: (i) The solubility product (K sp, mol 3 dm -9 ) of MX 2 at 298 K based on the information available for the given concentration cell is (take 2.303 x R x 298/P = 0.059)
(ii) The value of \(\Delta G\) (in kJ mol -1 ) for the given cell is (take 1F = 96500 C mol -1 )
(iii) The equilibrium constant for the following reaction is \(\mathrm{Fe}^{2+}+\mathrm{Ce}^{4+} \rightleftharpoons \mathrm{Ce}^{3+}+\mathrm{Fe}^{3+}\) (Given, \(E^{0} \mathrm{Ce}^{4+} / \mathrm{Ce}^{3+}=1.44\) and E o \(E_{\mathrm{Fe}^{3+} / \mathrm{Fe}^{2+}}=0.68 \mathrm{~V}\) )
(iv) To calculate the emf of the cell, which of the following options is correct?
Read the passage given below and answer the following questions : The potential of each electrode is known as electrode potential. Standard electrode potential is the potential when concentration of each species taking part in electrode reaction is unity and the reaction is taking place at 298 K. By convention, the standard ectrode potential of hydrogen (SHE) is 0.0 V. The electrode potential value for eacfi electrode process is a measure of relative tendency of the active species in the process to remain in the oxidisedlreduced form. The negative electrode potential means that the redox couple is stronger reducing agent than H + /H 2 couple. A positive electrode potential means that the redox couple is a weaker reducing agent than the H + /H 2 couple. Metals which have higher positive value of standard reduction potential form the oxides of greater thermal stability. In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (i) Assertion : An electrochemical cell can be set-up only if the redox reaction is spontaneous. Reason : A reaction is spontaneous if the free energy change is negative.
(ii) Assertion : The standard electrode potential of hydrogen is 0.0 V. Reason : It is by convention.
(iii) Assertion : The negative value of standard reduction potential means that reduction takes place on this electrode with reference to hydrogen electrode. Reason : The standard electrode potential of a half cell has a fixed value.
(iv) Assertion : The absolute value of electrode potential cannot be determined experimentally. Reason : The electrode potential values are generally determined with respect to SHE.
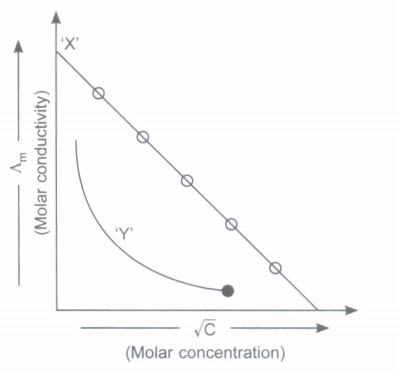
Read the passage given below and answer the following questions: Two types of conductors are generally used, metallic and electrolytic. Free electrons are the current carrier in metallic and in electrolytic conductors, free ions. Specific conductance or conductivity of an electrolytic solution is given by \(\kappa=C \times \frac{l}{A}\) where, C = l/R and l/A = G* (cell constant) Molar conductance ( \(\Lambda_{m}\) ) and equivalent conductance ( \(\Lambda_{e}\) ) of an electrolyte solution are calculated as \(\Lambda_{m}=\frac{\kappa \times 1000}{M} \text { or } \Lambda_{e}=\frac{\kappa \times 1000}{N}\) where, M = molarity of solution and Nis normality of solution. Molar conductance of strong electrolyte depends on the concentration. \(\Lambda_{m}=\Lambda_{m}^{0}-b \sqrt{C}\) \(\Lambda_{m}^{\circ}\) = molar conductance at infinite dilution, b = constant, C = conc of solution In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (i) Assertion : The molar conductivity of strong electrolyte decreases with increase in concentration. Reason : At high concentration, migration of ions is slow
(ii) Assertion : Equivalent conductance of all electrolytes increases with increasing concentration. Reason : More number of ions are available per gram equivalent at higher concentration.
(iii) Assertion : Specific conductance decreases with dilution whereas equivalent conductance increases. Reason : On dilution, number of ions per milli litre decreases but total number of ions increases considerably
(iv) Assertion : The ratio of specific conductivity to the observed conductance does not depend upon the concentration of the solution taken in the conductivity cell. Reason : Specific conductivity decreases with dilution whereas observed conductance increases with dilution.
Read the passage given below and answer the following questions: Electrical work done in unit time is equal to electrical potential multiplied by total charge passed. In order to obtain maximum work from a cell, the charge has to be passed reversibly. The reversible work done by a cell is equal to decrease in its Gibb's energy. Hence, Gibb's energy of reaction is given by \(\Delta G=-n F E_{\text {cell }}\) Hence, E is the emf of the cell and nF is the amount of energy. In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices (i) Assertion : \(\Delta G^{\circ}=-n F E^{\circ}\) Reason : E o should be positive for a spontaneous reaction
(ii) Assertion : An electrochemical cell can be set up only if the redox reaction is spontaneous. Reason : A reaction is spontaneous if free energy change is negative.
(iii) Assertion : Current stops flowing when E cell = 0. Reason : Equilibrium of the cell reaction is attained.
(iv) Assertion: E cell should have a positive value for the cell to function. Reason : E cell = E cathode - E anode
Read the passage given below and answer the following questions: Nernst equation relates the reduction potential of an electrochemical reaction to the standard potential and activities of the chemical species undergoing oxidation and reduction. Let us consider the reaction, \(M_{(a q)}^{n+} \longrightarrow n M_{(s)}\) For this reaction, the electrode potential measured with respect to standard hydrogen electrode can be given as \(E_{\left(M^{n+} / M\right)}=E_{\left(M^{n+} / M\right)}^{\circ}-\frac{R T}{n F} \ln \frac{[M]}{\left[M^{n+}\right]}\) In these questions ( i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(i) Assertion : For concentration cell, \(\begin{array}{c} \mathrm{Zn}_{(s)}\left|\mathrm{Zn}^{2+}{ }_{(a q)} \| \mathrm{Zn}^{2+}{ }_{(a q)}\right| \mathrm{Zn} \\ \mathrm{C}_{1} \quad \mathrm{C}_{2} \end{array}\) For spontaneous cell reaction, C 1 < C 2 . Reason : For concentration cell \(E_{\text {cell }}=\frac{R T}{n F} \log \frac{C_{2}}{C_{1}}\) For spontaneous reaction, \(E_{\text {cell }}=+\mathrm{ve} \Rightarrow C_{2}>C_{1}\) (ii) Assertion : For the cell reaction, \(\mathrm{Zn}_{(s)}+\mathrm{Cu}_{(a q)}^{2+} \longrightarrow \mathrm{Zn}_{(a q)}^{2+}+\mathrm{Cu}_{(s)}\) voltmeter gives zero reading at equilibrium. Reason : At the equilibrium, there is no change in concentration of Cu 2+ and Zn 2+ ions. (iii) Assertion : The Nernst equation gives the concentration dependence of emf of the cell. Reason : In a cell, current flows from cathode to anode (iv) Assertion : Increase in the concentration of copper half cell in a cell, increases the emf of the cell Reason : \(E_{\text {cell }}=E_{\text {cell }}^{\circ}+\frac{0.059}{2} \log \frac{\left[\mathrm{Cu}^{2+}\right]}{\left[\mathrm{Zn}^{2+}\right]}\)
Metallic conductance involves movement of electrons where as electrolytic conductance involves movement of ions. Specific conductance increases with increase in concentration where as A m (molar conductivity) decreases with increase in concentration. Electrochemical cell converts chemical energy of redox reaction into electricity. Mercury cell, Dry cells are primary cells where as Ni-Cd cell, lead storage battery are secondary cells. Electroehemical series is arrangement of elements in increasing order of their reduction potential. Electrolytic cell converts electrical energy into chemical energy which is used in electrolysis. Amount of products formed are decided with the help of Faraday's laws of Electrolysis. Kohlrausch law helps to determine limiting molar conductivity of weak electrolyte, their degree of ionisation ( \(\alpha\) ) and their dissociation constants. Corrosion is electrochemical phenomenon. Metal undergoing corrosion acts as anode, loses electrons to form ions which combine with substances present in atmosphere to form surface compounds. More reactive metals are coated over less reactive metals to prevent corrosions. H 2 -O 2 fuel cell was used in apollo space programme. (a) Out of 0.5 M, 0.01 M, 0.1 M and 1.0 M which solution of KCl will have highest value of specific conductance? Why? (b) Write the product of electrolysis of aq. NaCI on cathode. Why? (c) When does electrochemical cell behaves like electrolytic cell? (d) For an electrochemical cell Mg(s) + 2Ag + (aq) \(\rightarrow\) 2Ag(s) + Mg 2+ . Give the cell representation and write Nernst equation. (e) Which will have higher conductance, silver wire at 30° or at 60°C? (f) Calculate maximum work obtained from the cell Ni(s) + 2Ag + (aq) \(\rightarrow\) Ni 2+ (aq) + 2Ag(s) E o cell = 1.05V. (g) Which cell is used in hearing aids and watches?
Electrochemistry plays a very important part in our daily life. Primary cells like dry cell is used in torches, wall clock, mercury cell is used in hearing aids, watches. Secondary cells Ni-Cd cell is used in cordless phones, lithium battery is used in mobiles, lead storage battery is used in vehicle and inverter. Fuel cells like H 2 -O 2 cell was used in apollo space programme. A 38% solution of sulphuric and is used in lead storage battery. Its density is 1.30 g mL -1 . The battery holds 3.5 L of the acid. During the discharge of the battery, the density of H 2 SO 4 falls to 1.14 g mL -1 (20% solution by mass) (Molar mass of H 2 SO 4 is 98 g mol -1 ). (a) Write the chemical reaction taking place at anode when lead storage battery is in use. (b) How much electricity in Faraday is required to carry out the reduction of one mole of PbO 2 ? (c) What is molarity of sulphuric acid before discharge? (d) What is mass of sulphuric acid in solution after discharge? (e) Write the products of electrolysis when dilute sulphuric acid is electrolysed using platinum electrodes.
Observe the following table in which conductivity and molar conductivity of NaCI at 298 K at different concentration for different electrolytes is given. Answer the questions based in the table that follows: Conductivities and molar conductivities of NaCI at 298 K at different concentrations.
(a) What happens to conductivity on dilution and why? (b) Why is \(\Lambda_{\mathrm{m}}^{\mathrm{o}}\) (limiting molar conductivity) for HCI more than NaCl? (c) Calculate degree of dissociation ( \(\alpha\) ) of NaCI of 0.001 M concentration using the table. (d) Calculate \(\Lambda_{\mathrm{m}}^{\mathrm{o}}\) of CH 3 COOH using the table. (e) Calculate Ka of 0.01 M CH 3 COOH solution if \(\Lambda_{\mathrm{m}}^{\mathrm{o}}\) for CH 3 COOH is 390.07S cm 2 mol -1 , \(\Lambda_{\mathrm{m}}\) = 39.07S cm -1 .
*****************************************
- Previous Class 12th Chemsitry - Chemistry in Everyday Life Case Study Questions and Answe...
- Next Class 12th Chemsitry - Polymers Case Study Questions and Answers 2022 - 2023
Reviews & Comments about Class 12th Chemsitry - Electrochemistry Case Study Questions and Answers 2022 - 2023

Write your Comment

12th Standard CBSE Chemistry Videos
CBSE 12th Chemistry Sample Model Question Paper with Answer Key 2023
12th Standard CBSE Chemistry Usefull Links

- 10th Standard
Other 12th Standard CBSE Subjects

Other 12th Standard CBSE Chemistry Study material
Class 12th chemsitry - chemistry in everyday ... click to view, class 12th chemsitry - polymers case study questions and answers 2022 - 2023 click to view, class 12th chemsitry - biomolecules case study questions and answers 2022 - 2023 click to view, class 12th chemsitry - amines case study questions and answers 2022 - 2023 click to view, class 12th chemsitry - aldehydes , ketones and ... click to view, class 12th chemsitry - alcohols , phenols and ... click to view, class 12th chemsitry - haloalkanes and haloarenes ... click to view, class 12th chemsitry - coordination compounds case ... click to view, class 12th chemsitry - the d- and ... click to view, class 12th chemsitry - the p-block elements ... click to view, class 12th chemsitry - general principles and ... click to view, class 12th chemsitry - surface chemistry case ... click to view, class 12th chemsitry - chemical kinetics case ... click to view, class 12th chemsitry - electrochemistry case study ... click to view, class 12th chemistry - solution case study questions and answers 2022 - 2023 click to view, register & get the solution for class 12th chemsitry - electrochemistry case study questions and answers 2022 - 2023.

CBSE Class 12 Chemistry –Chapter 3 Electrochemistry- Study Materials
NCERT Solutions Class 12 All Subjects Sample Papers Past Years Papers
Solid State : Notes and Study Materials -pdf
Notes and study materials.
- Concepts of Electrochemistry
- Master File Electrochemistry
- NCERT Solutions for Electrochemistry
- NCERT Exemplar Solutions for – Electrochemistry
- Mind Map of Electrochemistry
- Concept Map of Electrochemistry
- Past Many 12th Board Years of Electrochemistry
Examples and Exercise
- Electrochemistry : Practice Paper 1
- Electrochemistry : Practice Paper 2
- Electrochemistry : Practice Paper 3
- Electrochemistry : Practice Paper 4
Chapters in CBSE Class 12 Physical Chemistry are Solid State, Solutions, Electrochemistry, Chemical Kinetics and Surface Chemistry. The total weightage of these chapters in CBSE Class 12 Chemistry board exam is of 23 Marks (out of 70). Electrochemistry is an important chapter of CBSE Class 12 Physical Chemistry. Given below is the Part I of Chapter Notes on Electrochemistry.
Introduction

Electrochemical Cell
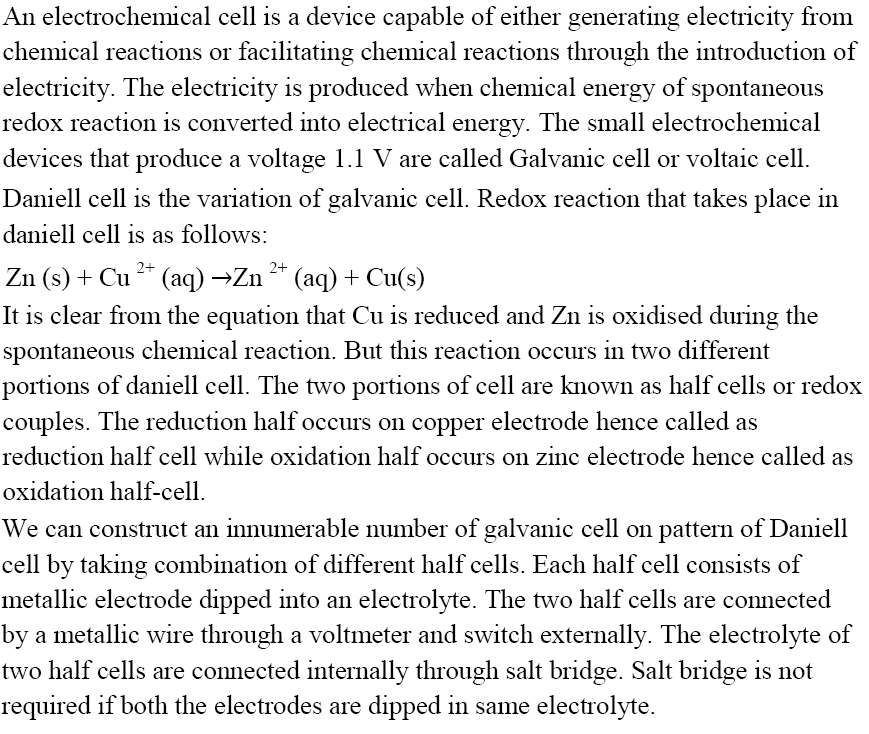
Schematic Diagram of Daniell cell
Electrolytic Cell

Electrode Potential

Standard Electrode Potential

Anode and Cathode

Cell Potential or EMF of Cell
Inert Electrode

Measurement of Electrode Potential

Values of Standard Reduction Potentials for some Important Half-Cells
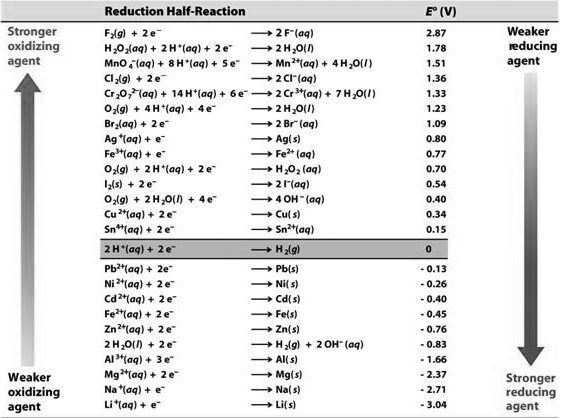
CBSE Class 12th Physics Notes: Current Electricity
Conclusions from Table

CBSE Class 12th Physics Notes: Ray Optics & Optical Instruments
Nernst Equation
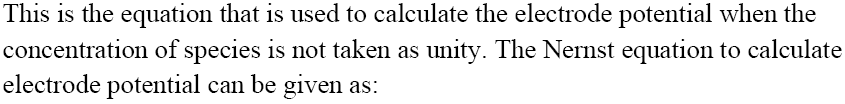
Applications of Nernst Equation

EMF of Cell

How to Solve Difficult Problems in CBSE Board and Engineering Entrance Exams
Equilibrium Constant from Nernst Equation

Intext Questions
In part I of Chapter Notes on the chapter Electrochemistry of CBSE Class 12 we have studied important concepts like Electrochemical Cell, Electrolytic Cell, Electrode Potential, Measurement of Electrode Potential, Nernst Equation etc. Now in this part, we will study some more important concepts and numericals based on them.
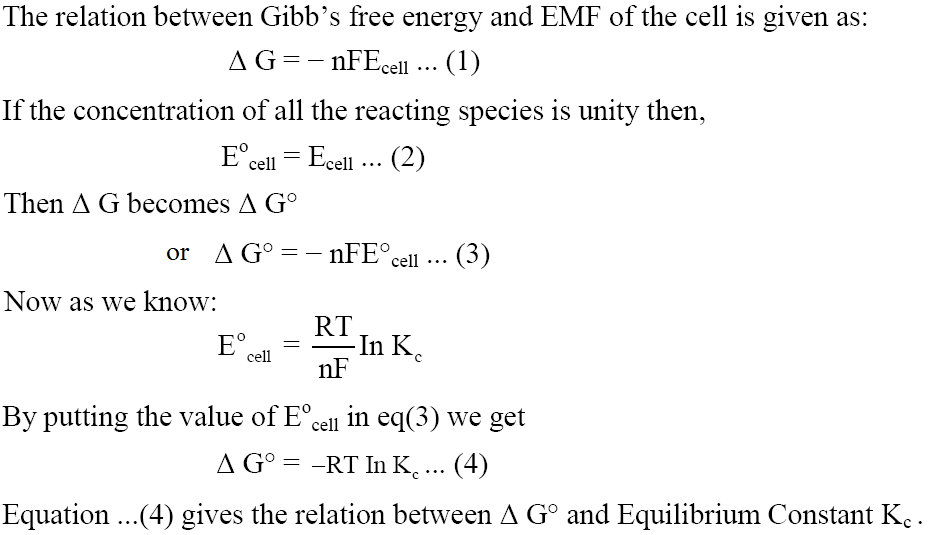
Gibbs Free Energy from Nernst Equation
Types of Materials
Metallic or Electric Conductance

Electrolytic or Ionic Conductance

Resistivity or Specific Resistance
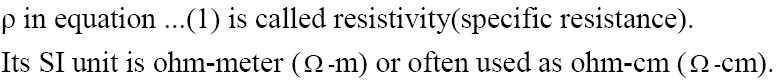
Conductance

Conductivity or Specific Conductance

Measurement of the Conductivity of Ionic Solutions
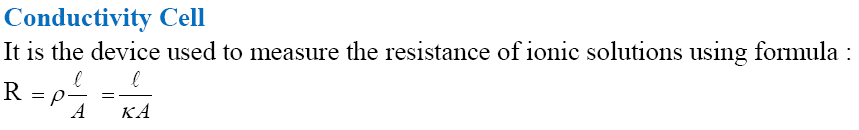
Variation of Conductivity with Concentration

Limiting Molar Conductivity
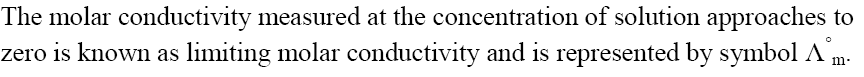
Variation of Molar Conductivity for Strong Electrolyte

Kohlrausch’s Law
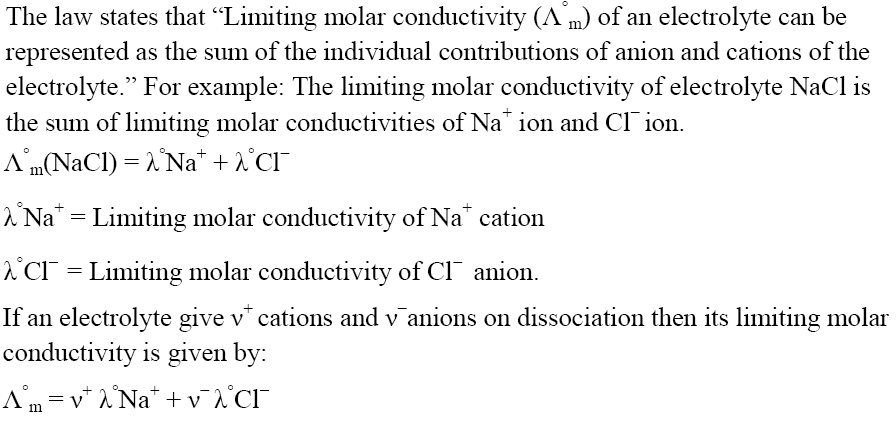
Variation of Molar Conductivity for Weak Electrolyte
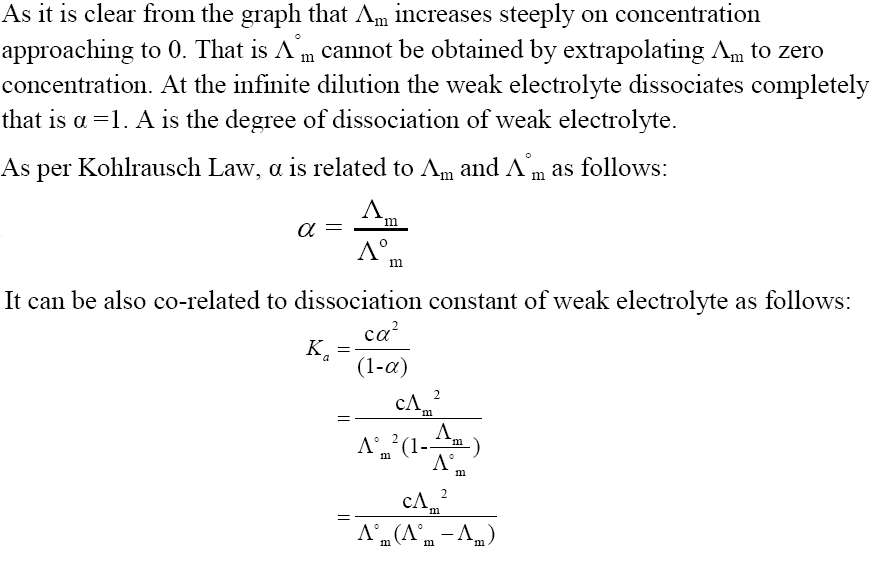
In part – I and part – II of chapter notes on Electrochemistry most of the important topics are already covered. Now in this part, we will study the following topics: Electrolytic Cell, Electrolysis, Faraday’s Law of Electrolysis, Products of Electrolysis, Battery, Fuel Cell and Corrosion. Solved examples are also included in this article for better understanding.
Electrolytic Cell and Electrolysis
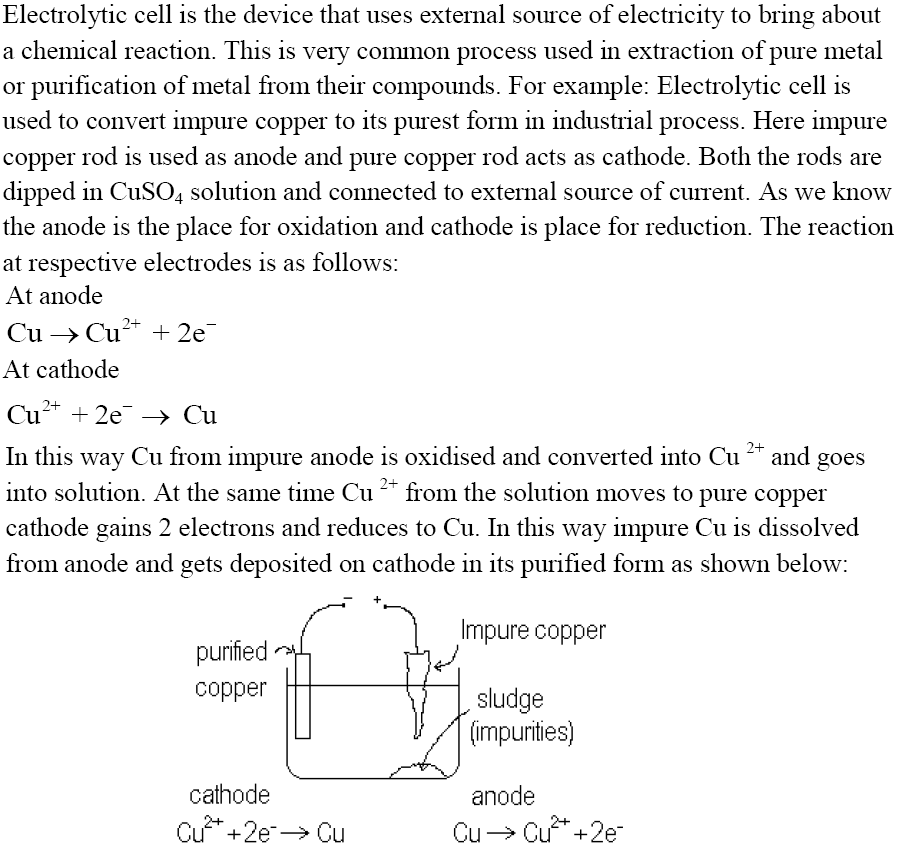
Quantitative aspects of Electrolysis

Products of Electrolysis
myCBSEguide
- Case Study Questions Class...
Case Study Questions Class 12 Chemistry
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
CBSE will ask Case Study Questions class 12 Chemistry in session 2020-21. These will be the first two questions in the board exam question paper. The first question will have 5 MCQs out of which students will attempt any 4 questions. The second question will carry 5 Assertion & Reason type questions with the choice to attempt any four.
Case Study Questions
As you know, CBSE will hold exams in May-June this year. There is already a reduction of 30% in the syllabus. Now, the case study questions have been added. So, this year the question paper is going to be a bit easier. Although it is easy yet these case study questions need special attention and regular practice.
We have added around 10 sample questions based on the latest pattern in myCBSEguide App. These all questions include two case study questions.
Class 12 Chemistry Question Bank
If you go through the previous year question papers, you will analyze that many questions are repeated word by word and many others are almost similar. So, it is always recommended to check all questions asked in previous years. This will not only help you to get an idea about the question pattern but also help you to understand the difficulty level of the questions.
myCBSEguide App has the previous year’s question bank. These questions are arranged chapter-wise. If you are preparing a particular chapter, you will get all questions asked from that chapter in the last 10 years.
Case Study Questions Examples
Here are two examples of case study questions. To get more such questions download the myCBSEguide App and browse Sample Papers there.
Read the passage given below and answer any four out of the following questions: Ammonia is present in small quantities in air and soil where it is formed by the decay of nitrogenous organic matter e.g., urea. On a large scale, ammonia is manufactured by Haber’s process. In accordance with Le Chatelier’s principle, high pressure would favour the formation of ammonia. Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states, it is associated through hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass. Ammonia gas is highly soluble in water. Its aqueous solution is weakly basic due to the formation of OH– ions. The presence of a lone pair of electrons on the nitrogen atom of the ammonia molecule makes it a Lewis base.
- caustic soda
- calcium chloride
- sodium hydroxide
- sodium chloride
- 200 10 5 Pa
- 400 10 5 Pa
- 100 10 5 Pa
- 300 10 5 Pa
- Mg 2 O 3 + K 2 O
- Al 2 O 3 + K 2 O
- NaO 3 + K 2 O
- None of these
- five bond pair and two lone pair
- four lone pair and one bond pair
- three bond pair and one lone pair
- three bond pair and two lone pair
Read the passage and answer any four out of the following questions: Colloidal particles always carry an electric charge. The nature of this charge is the same on all the particles in a given colloidal solution and may be either positive or negative. The charge on the sol particles is due to one or more reasons, viz., due to electron capture by sol particles during electrodispersion of metals. When two or more ions are present in the dispersion medium, preferential adsorption of the ion common to the colloidal particle usually takes place. When silver nitrate solution is added to the potassium iodide solution, the precipitated silver iodide adsorbs iodide ions from the dispersion medium, and negatively charged colloidal solution results. acquired a positive or a negative charge by selective adsorption on the surface of a colloidal particle The combination of the two layers of opposite charges around the colloidal particle is called Helmholtz electrical double layer. The presence of equal and similar charges on colloidal particles is largely responsible for providing stability to the colloidal solution.
In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
- Assertion and reason both are correct statements and reason is correct explanation for assertion
- Assertion and reason both are correct statements but reason is not correct explanation for assertion
- Assertion is correct statement and reason is wrong statement
- Assertion is wrong statement but reason is correct statement
- Assertion: The presence of equal and similar charges on colloidal particles is largely responsible in providing stability to the colloidal solution. Reason: The repulsive forces between charged particles having the same charge prevent them from aggregating and provide stability.
- Assertion: The first layer is mobile in Helmholtz electrical double layer. Reason: The potential difference between the fixed layer and the diffused layer of opposite charges is called zeta potential.
- Assertion: The sol particle in colloid has a charge. Reason: The charge in sol is due to electron capture by sol particles during the electrodispersion of metals.
- Assertion: Methylene blue sol is a negatively charged sol. Reason: When KI solution is added to AgNO 3 solution, positively charged sol formed.
- Assertion: If FeCl3 is added to an excess of hot water, a positively charged sol of hydrated ferric oxide is formed. Reason: When ferric chloride is added to NaOH a negatively charged sol is obtained with adsorption of OH- ions.
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
- Class 11 Biology Case Study Questions
- Class 12 Physical Education Case Study Questions
1 thought on “Case Study Questions Class 12 Chemistry”
Answer for questions
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.
Talk to our experts
1800-120-456-456
- Electrochemistry Class 12 Notes CBSE Chemistry Chapter 3 (Free PDF Download)
- Revision Notes

Electrochemistry Notes for CBSE Class 12 Chemistry Chapter 3 - Free PDF Download
Electrochemistry is a vital section of chemistry that determines the function of electrodes and reactors. Vedantu’s Electrochemistry notes class 12 tries to situate the ideas behind the chemical reactions.
An electrochemical cell is a tool that produces the difference between forms of the electrode through a chemical reaction. There are ideally two types of electron conductors that get separated by an ionic conductor. An electron conductor further links it, making it accessible.
Class 12 Electrochemistry Notes explain this function of electrons where two metallic electrodes are present. These metallic electrodes are immersed in an electrolytic solution for power generation. By thorough reading of Electrochemistry Class 12 Notes PDF Download, students will know that the ionic conductor is a vital part of cells.
Download CBSE Class 12 Chemistry Notes 2023-24 PDF
Also, check CBSE Class 12 Chemistry revision notes for other chapters:
Related Chapters
Electrochemistry Class 12 Notes Chemistry - Basic Subjective Questions
Section – a (1 mark questions).
1. The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called ___
Ans. The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called cell emf.
2. Greater the solvation of ions, ____ is the conductivity. (greater/lesser)
Ans. Conductivity depends upon solvation of ions present in solution. Greater the solvation of ions, lesser is the conductivity.
3. What is an inert electrode?
Ans. The inert electrode is an electrode that serves only as a source or sinks for electrons. It provides a surface for oxidation or reduction reaction but not for the redox reaction. It does not participate in the cell reaction.
4. Define the term specific resistance and give its SI unit.
Ans. The specific resistance of a substance is its resistance when cell is one meter long and its area of cross Section is one m 2 . Its SI unit is Ωm (ohm meter)
5. What is meant by Faraday’s constant?
Ans. Faraday’s constant is the quantity of charge carried by one mole of electrons. 1 F = 96500 C/mol
6. Conductivity of an electrolytic solution depends on _____ and _____.
Ans. Conductivity or specific conductance k (kappa). It depends on the nature of the electrolyte and concentration of the electrolyte.
7. What is Kohlrausch’s law?
Ans. Kohlrausch’s law states that the equivalent conductivity of an electrolyte at infinite dilution is equal to the sum of the conductances of the anions and cations.
8. Can absolute electrode potential of an electrode be measured?
Ans. No, only the difference in potential between two electrodes can be measured.
9. Can E cell or Δ r G for cell reaction ever be equal to zero?
Ans. At equilibrium Δ r G = 0 E cell = 0
10. What are the units of cell constant?
Ans. cm -1 or m -1
Section – B (2 Marks Questions)
11. Depict (cell representation) the galvanic cell in which the cell reaction is Cu + 2Ag + → 2Ag + Cu 2+
Ans. Cu + 2Ag + → 2Ag + Cu 2+ cell can be represented is Cu | Cu 2+ || Ag + | Ag
12. A solution is placed in two different cells having cell constant 0.1 and 0.5 cm -1 respectively. Which of the two will have greater value of specific conductance?
Ans. Both will have same value of specific conductance.
13. Why is alternating current used for measuring resistance of an electrolytic solution?
Ans. Alternating current is used for measuring the resistance of an electrolytic solution because DC current can change the composition of the solution and the concentration will not remain constant.
14. Solutions of two electrolytes 'A' and 'B' are diluted. The Λ m of 'B' increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Justify your answer.
Ans. 'B' is strong electrolyte. For strong electrolyte Λm increases slowly with dilution since the number of ions remains the same, only the interionic attraction decreases thus the molar conductivity increases slightly.
15. When acidulated water (dil H 2 SO 4 solution) is electrolysed, will the pH of the solution be affected? Justify your answer.
Ans. pH of the solution remains constant as [H + ] remains same during the whole reaction.
At anode: 2H 2 O (l) → O 2 (g) + 4H + + 4e –
At cathode: 4H + + 4e – → 2H 2 (g)
16. Can Fe 3+ oxidise Br – to Br 2 under standard conditions?
$E^{\theta }_{Fe^{3+}/Fe^{2+}}=0.77W,\;E^{\theta }_{Br_{2}/Br^{-}}=1.09W$
Ans. No, because for the reaction,
$Fe^{3+}+Br^{-}\rightarrow Fe^{2+}+\frac{1}{2}Br_{2}$
$E^{\theta }=0.771=1.09=-0.319\;V$ is negative
17. A very thin copper plate is electro-plated with gold using gold chloride in HCl. The current was passed for 20 minutes and the increase in the weight of the plate was found to be 2 gram (Au = 197). The current passed was:
Ans. w = zit
$2=\frac{197}{3}\times\frac{i\times20\times60}{96500}$
i = 2.448 amp.
19. What is the reaction taking place at the anode when an aqueous solution of copper sulphate is electrolysed using Pt-electrodes (inert)?
Ans. At anode oxidation takes place, and oxidation is defined as loss of electrons. So the reaction should be
$2H_{2}O\rightarrow O_{2}+4H^{+}+4e^{-}$
Since mobility of OH – is greater than $SO_{4}^{2-}$
$\therefore$ oxidation of $SO_{4}^{2-}$ will not occur.
20. The molar conductivities of $\Lambda_{\mathrm{NaOAc}}^{\circ}$ and $\Lambda_{\mathrm{HCl}}^{\circ}$ at infinite dilution in water at 25ºC are 91.0 and 426.2 S cm 2 /mol respectively. To calculate $\Lambda_{\mathrm{NaOAc}}^{\circ}$ , the additional value required is
Ans. To calculate molar conductance of acetic acid at infinite dilution $\Lambda_{\mathrm{NaOAc}}^{\circ}$ , molar conductance of HCl at infinite dilution $\Lambda_{\mathrm{HCl}}^{\circ}$, Sodium Acetate $\Lambda_{\mathrm{NaOAc}}^{\circ}$ and Sodium chloride $\Lambda_{\mathrm{NaCl\circ}}$ should be known.
$\Lambda_{\mathrm{mHOAc}}^{\circ}=\Lambda_{\mathrm{mHCl}}^{\circ}+\Lambda_{\mathrm{mNaOAc}}^{\circ}-\Lambda_{\mathrm{mNaCl}}^{\circ}$
PDF Summary - Class 12 Chemistry Electrochemistry Notes (Chapter 3)
Electrochemistry.
Electrochemistry is the study of generating electricity from the energy produced during a spontaneous chemical reaction, as well as the application of electrical energy to non-spontaneous chemical changes.
Electrochemical Cells
A spontaneous chemical reaction is one that can occur on its own, and in such a reaction, the system's Gibbs energy falls. This energy is then transformed into electrical energy. It is also feasible to force non-spontaneous processes to occur by providing external energy in the form of electrical energy. Electrochemical Cells are used to carry out these interconversions.
Two types of electrochemical cells are present: Galvanic cells, which converts chemical energy into electrical energy and electrolytic cells which converts electrical energy into chemical energy.
Galvanic Cells
A spontaneous chemical process or reaction is used to extract cell energy, which is then transformed to electric current.
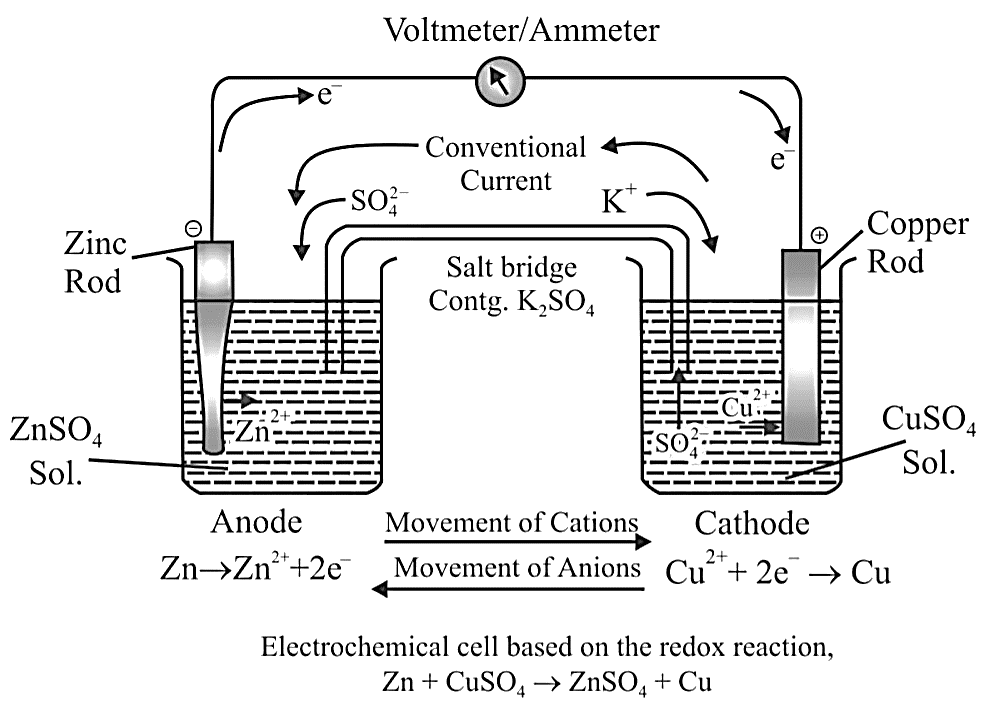
For example, a Daniell Cell is a Galvanic Cell in which the redox reaction is carried out using Zinc and Copper.
$Zn(s) + C{u^{2 + }}(aq) \to Z{n^{2 + }}(aq) + Cu(s)$
Oxidation Half: $Zn(s) \to Z{n^{2 + }}(aq) + 2{e^ - }$
Reduction Half: $C{u^{2 + }}(aq) + 2{e^ - } \to Cu(s)$
The reducing agent is $Zn$ , and the oxidising agent is $C{u^{2 + }}$ .
Electrodes are another name for half cells. The anode is the oxidation half, and Cathode is the reduction half. Cathode is a term used to describe a type of electrode. In the external circuit, electrons pass from anode to cathode. Negative polarity is assigned to the anode. Positive polarity is assigned to the cathode. Daniell Cell is a fictional character created by Daniell Cell. The anode is $Zn$ , while the cathode is $Cu$ .
Electrolytic Cell
These electrodes are submerged in an electrolytic solution that contains both cations and anions. When current is supplied, the ions migrate towards electrodes of opposite polarity, where they undergo simultaneous reduction and oxidation.
Preferential Discharge of Ions
When more than one cation or anion is present, the discharge process becomes competitive. Any ion that needs to be discharged requires energy, and if there are multiple ions present, the ion that requires the most energy will be discharged first.
Electrode Potential
It can be defined as an element's tendency to lose or gain electrons when in contact with its own ions, causing it to become positively or negatively charged. Depending on whether oxidation or reduction has occurred, the electrode potential will be referred to as oxidation or reduction potential.
$M(s)\underset{{{\text{Reduction}}}}{\overset{{{\text{Oxidation}}}}{\longleftrightarrow}}{M^{n + }}(aq) + n{e^ - }$
${M^{n + }}(aq) + n{e^ - }\underset{{{\text{Oxidation}}}}{\overset{{{\text{Reduction}}}}{\longleftrightarrow}}M(s)$
Characteristics
The magnitude and sign of the oxidation and reduction potentials are equal.
Because E is not a thermodynamic property, its values do not add up.
Standard Electrode Potential $({E^ \circ })$
It can be described as an electrode's electrode potential measured in comparison to a standard hydrogen electrode under standard conditions. The following are the standard conditions:
A 1M concentration of each ion in the solution.
A 298 K temperature.
Each gas has a pressure of one bar.
Electrochemical Series
The half-cell potential values are standard and are represented as standard reduction potential values in the table at the conclusion, commonly known as the Electrochemical Series.
Cell Potential or EMF of a Cell
Cell potential is the difference between the electrode potentials of two half cells. If no current is pulled from the cell, it is known as electromotive force (EMF).
${E_{cell}} = {E_{cathode}} + {E_{anode}}$
For this equation we take oxidation potential of anode and reduction potential of cathode.
Since anode is put on left and cathode on right, it follows therefore:
$ = {E_R} + {E_L}$
For a Daniel Cell, therefore:
$E_{cell}^ \circ = E_{C{u^{2 + }}/Cu}^ \circ - E_{Zn/Z{n^{2 + }}}^ \circ = 0.34 + (0.76) = 1.10\;V$
Cell Diagram or Representation of a Cell
In accordance with IUPAC recommendations, the following conventions or notations are used to write the cell diagram. The Daniel cell has the following representation:
$Zn(s)|Z{n^{2 + }}({C_1})||C{u^{2 + }}({C_2})|Cu(s)$
The anode half cell is written on the left, while the cathode half cell is written on the right.
The metal is separated from an aqueous solution of its own ions by a single vertical line.
Anodic Chamber: $Zn(s)|Z{n^{2 + }}(aq)$
Cathodic Chamber: $C{u^{2 + }}(aq)|Cu(s)$
A salt bridge is represented by a double vertical line.
After the formula of the corresponding ion, the molar concentration (C) is placed in brackets.
The cell's e.m.f. value is written on the cell's extreme right side. As an example:
$Zn(s)|Z{n^{2 + }}(1M)||C{u^{2 + }}(1M)|Cu$ , EMF = +1.1 V
If an inert electrode, such as platinum, is used in the cell's construction, it may be written in brackets alongside the working electrode, as when a zinc anode is coupled to a hydrogen electrode.
$Zn(s)|Z{n^{2 + }}({C_1})||{H^ + }({C_2})|{H_2}(Pt)(s)$
Salt Bridge
The salt bridge maintains charge balance and completes the circuit by allowing ions to flow freely through it. It contains a gel containing an inert electrolyte such as $N{a_2}S{O_4}$ or $KN{O_3}$ . Through the salt bridge, negative ions travel to the anode and positive ions flow to the cathode, maintaining charge balance and allowing the cell to function.
Spontaneity of a Reaction
$\Delta G = - nF{E_{cell}}$
$\Delta G$ should be negative and cell potential should be positive for a spontaneous cell reaction.
In the following equation, if we take the standard value of cell potential, we will also get the standard value of $\Delta G$ .
$\Delta {G^ \circ } = - nFE_{CELL}^ \circ $
Types of Electrodes
Metal – metal ion electrodes.
An electrolyte solution containing metal ions is dipped into a metal rod/plate. Because of the potential difference between these two phases, this electrode can function as both a cathode and an anode.
Anode: $M \to {M^{n + }} + n{e^ - }$
Cathode: ${M^{n + }} + n{e^ - } \to M$
Gas Electrodes
Electrode gases such as ${H_2}$ and $C{l_2}$ are used in conjunction with their respective ions. ${H_2}$ gas, for example, is utilised in conjunction with a dilute solution of $HCl$ (${H^ + }$ ions). To avoid reacting with the acid, the metal should be inert.
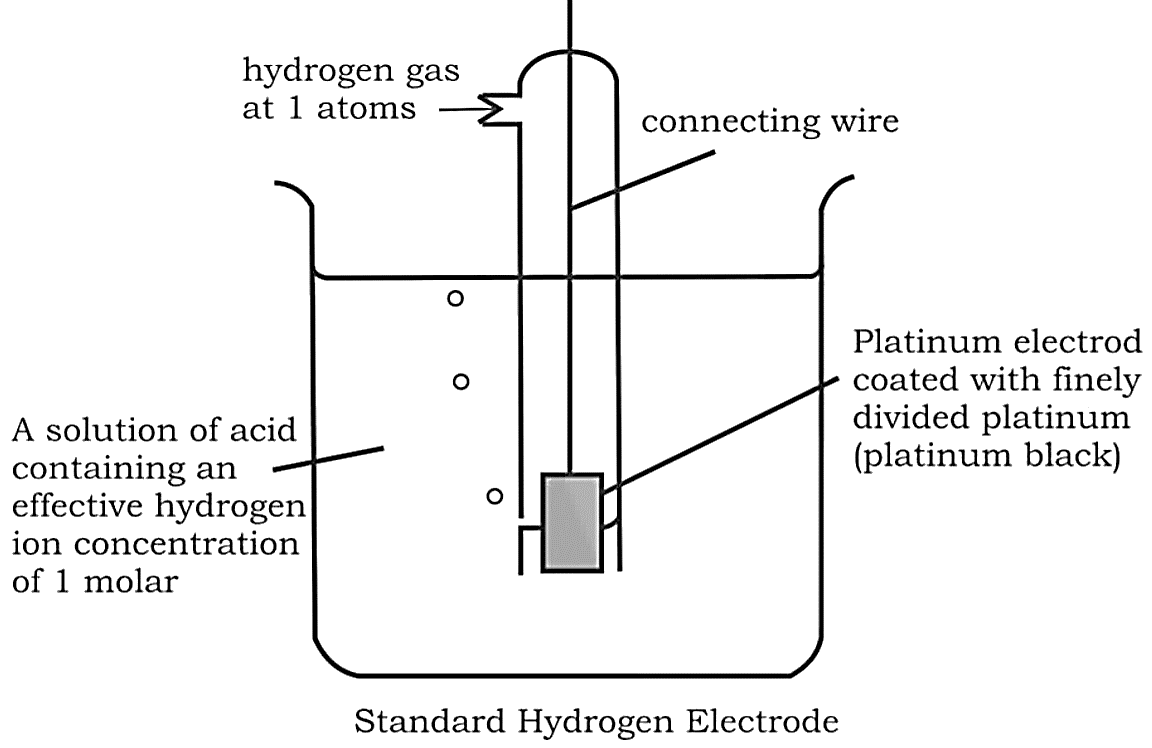
Anode: ${H_2} \to 2{H^ + } + 2{e^ - }$
Cathode: $2{H^ + } + 2{e^ - } \to {H_2}$
The hydrogen electrode is also used as a standard for measuring the potentials of other electrodes. As a reference, its own potential is set at $0\;V$ . The concentration of the HCl used as a reference is 1 M, and the electrode is known as the "Standard Hydrogen Electrode (SHE)".
Metal – Insoluble Salt Electrode
As electrodes, we use salts of several metals that are only sparingly soluble with the metal itself. When we employ $AgCl$ with $Ag$ , for example, there is a potential gap between these two phases, as seen in the following reaction:
$AgCl(s) + {e^ - } \to Ag(s) + C{l^ - }$
This electrode is made by dipping a silver rod in a solution containing $AgCl(s)$ and $C{l^ - }$ ions.
Calomel Electrode
Mercury is combined with two other phases: calomel paste $(H{g_2}C{l_2})$ and a $C{l^ - }$ ions containing electrolyte.
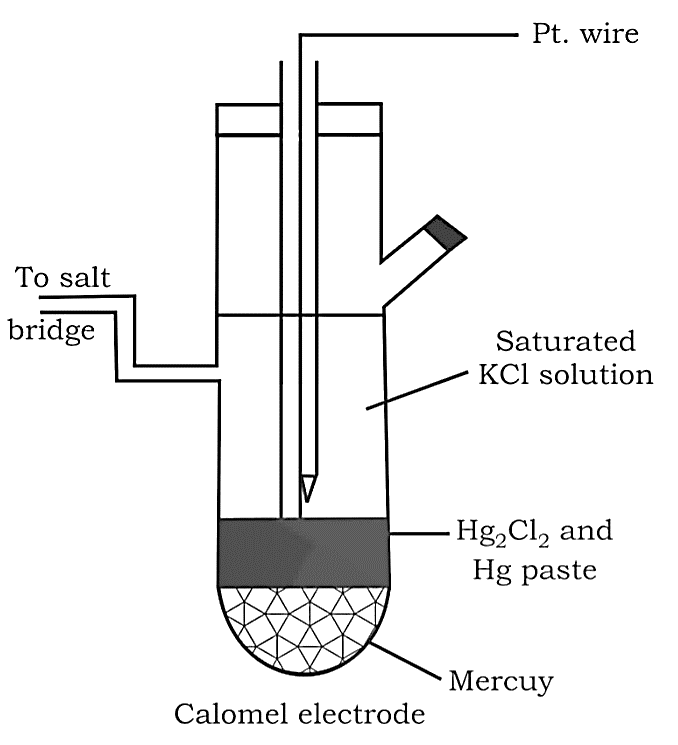
Cathode: $H{g_2}C{l_2}(s) + 2{e^ - } \to 2Hg(l) + 2C{l^ - }(aq)$
Anode: $2Hg(l) + 2C{l^ - }(aq) \to H{g_2}C{l_2}(s) + 2{e^ - }$
This electrode is also utilised as a reference point for determining other potentials. It's also known as Standard Calomel Electrode in its standard form (SCE).
Redox Electrode
Two distinct oxidation states of the same metal are used in the same half cell in these electrodes. For example, $F{e^{2 + }}$ and $F{e^{3 + }}$ are dissolved in the same container and the electron transfer is performed using a platinum inert electrode.
The following reactions may occur:
Anode: $F{e^{2 + }} \to F{e^{3 + }} + {e^ - }$
Cathode: $F{e^{3 + }} + {e^ - } \to F{e^{2 + }}$
Nernst Equation
It establishes a link between electrode voltage and ion concentration. When a result, as the concentration of ions rises, so does the reduction potential. For a type of generic electrochemical reaction.
$aA + bB\xrightarrow{{n{e^ - }}}cC + dD$
Nernst equation can be given as:
${E_{{\text{cell}}}} = E_{{\text{call}}}^0 - \dfrac{{RT}}{{nF}}\ln \dfrac{{{{[C]}^c}{{[D]}^d}}}{{{{[A]}^a}{{[B]}^b}}}$
${E_{c \in l}} = E_{cdl}^ \circ - \dfrac{{2303}}{{nF}}RT\log \dfrac{{{{[C]}^c}{{[D]}^d}}}{{{{[A]}^a}{{[B]}^b}}}$
Substituting the values of R and F we get:
${E_{{\text{cell}}}} = E_{ccll}^0 - \dfrac{{0.0591}}{n}\log \dfrac{{{{[C]}^c}{{[D]}^d}}}{{{{[A]}^a}{{[B]}^b}}}$
Applications of Nernst Equation
Equilibrium Constant from Nernst Equation
For a Daniel Cell, at equilibrium
${{E_{{\text{cell}}}} = 0 = E_{{\text{cell}}}^0 - \dfrac{{2.303{\text{RT}}}}{{2{\text{F}}}}\log \dfrac{{\left[ {{\text{Z}}{{\text{n}}^{2 + }}} \right]}}{{\left[ {{\text{C}}{{\text{u}}^{2 + }}} \right]}}}$
${{\text{E}}_{{\text{cdl}}}^{\text{o}} = \dfrac{{2.303{\text{RT}}}}{{2{\text{F}}}}\log \dfrac{{\left[ {{\text{Z}}{{\text{n}}^{2 + }}} \right]}}{{\left[ {{\text{C}}{{\text{u}}^{2 + }}} \right]}}}$
But at equilibrium:
$\dfrac{{\left[ {Z{n^{2 + }}} \right]}}{{\left[ {C{u^{2 + }}} \right]}} = {K_c}$
${{\text{E}}_{cell}^{\text{a}} = \dfrac{{2.303{\text{RT}}}}{{2{\text{F}}}}\log {{\text{K}}_{\text{c}}}}$
${{\text{E}}_{cell}^{\text{o}} = \dfrac{{2.303 \times 8.314 \times 298}}{{2 \times 96500}}\log {{\text{K}}_{\text{c}}}}$
${ = \dfrac{{0.0591}}{2}\log {{\text{K}}_{\text{c}}}}$
In general:
${{\text{E}}_{{\text{cell}}}^ \circ = \dfrac{{0.0591}}{{\text{n}}}\log {{\text{K}}_{\text{c}}}}$
${\log {{\text{K}}_{\text{c}}} = \dfrac{{{\text{n}}E_{{\text{cell}}}^ \circ }}{{0.0591}}}$
Concentration Cells
Concentration cells are formed when two electrodes of the same metal are dipped individually into two solutions of the same electrolyte with varying concentrations and the solutions are connected by a salt bridge. As an example:
${H_2}|{H^ + }({C_1})||{H^ + }({C_2})|{H_2}$
$Cu|C{u^{ + 2}}({C_1})||C{u^{2 + }}({C_2})|Cu$
These Are of Two Types:
Electrode concentration cells.
${H_2}({P_1})|{H^ + }(C)||{H^ + }(C)|{H_2}({P_2})$
${E_{{\text{cell}}}} = 0 - \dfrac{{0.059}}{n}\log \dfrac{{{P_2}}}{{{P_1}}}$
Where, ${P_2} < {P_1}$ for spontaneous reaction.
Electrolyte Concentration Cell
The EMF of concentration cell at 298 K is given by:
$Zn|Z{n^{2 + }}({C_1})||Z{n^{2 + }}({C_2})|Zn$
${{\text{E}}_{{\text{cell}}}} = \dfrac{{0.0591}}{{{{\text{n}}_1}}}\log \dfrac{{{{\text{c}}_2}}}{{{{\text{c}}_{\text{l}}}}}$
Where, ${C_2} > {C_1}$ for spontaneous reaction
Cases of Electrolysis
Electrolysis of molten sodium chloride.
$2NaCl(l) \rightleftharpoons 2N{a^ + }(l) + 2C{l^ - }(l)$
The reactions occurring at the two electrodes may be shown as follows:
At cathode: $2N{a^ + } + 2{e^ - } \to 2Na$ , ${E^ \circ } = - 2.71\;V$
At anode: $2C{l^ - } \to C{l_2} + 2{e^ - }$ , ${E^ \circ } = - 1.36\;V$
Overall reaction:
$2N{a^ + }(l) + 2C{l^ - }\xrightarrow{{electrolysis}}2Na(l) + C{l_2}(g)$ OR
$2NaCl(l)\xrightarrow{{electrolysis}}2Na(l) + C{l_2}(g)$
Electrolysis of an aqueous solution of Sodium Chloride
$NaCl(aq) \to N{a^ + }(aq) + C{l^ - }(aq)$
${H_2}O(l) \rightleftharpoons {H^ + }(aq) + O{H^ - }(aq)$
At cathode:
$2N{a^ + } + 2{e^ - } \to 2Na$ , ${E^ \circ } = - 2.71\;V$
$2{H_2}O + 2{e^ - } \to {H_2} + 2O{H^ - }$ , ${E^ \circ } = - 0.83\;V$
Thus ${H_2}$ gas is evolved at cathode value $N{a^ + }$ ions remain in solution.
$2{H_2}O \to {O_2} + 4{H^ + } + 4{e^ - }$ , ${E^ \circ } = - 1.23\;V$
$2C{l^ - } \to C{l_2} + 2{e^ - }$ , ${E^ \circ } = - 1.36\;V$
Thus, $C{l_2}$ gas is evolved at the anode by over voltage concept while $O{H^ - }$ ions remain in the solution.
The term "battery" refers to a configuration in which Galvanic cells are connected in series to achieve a higher voltage.
Primary Batteries
Primary cells can be employed indefinitely as long as active components are present. When they're gone, the cell stops working and can't be used again. For instance, a Dry Cell or a Leclanche Cell, as well as a Mercury Cell.
Anode: Zinc container
Cathode: Carbon (graphite) rod surrounded by powdered $Mn{O_2}$ and carbon
Electrolyte: $N{H_4}Cl$ and $ZnC{l_2}$
Anode: $Zn \to Z{n^{2 + }} + 2{e^ - }$
Cathode: $Mn{O_1} + NH_{_4}^ + + {e^ - } \to MnO(OH) + N{H_3}$
The standard potential of this cell is 1.5 V, which decreases as the battery is repeatedly discharged, and it cannot be refilled once used.
Mercury Cells
These are used in small equipments like watches, hearing aids.
Anode: $Zn - Hg$ Amalgam
Cathode: Paste of $HgO$ and carbon
Electrolyte: Paste of $KOH$ and $ZnO$
Anode: $Zn(Hg) + 2O{H^ - } \to ZnO(s) + {H_2}O + 2{e^ - }$
Cathode: $HgO(s) + {H_2}O + 2{e^ - } \to Hg(l) + 2O{H^ - }$
Overall Reaction: $Zn(Hg) + HgO(s) \to ZnO(s) + Hg(l)$
The cell potential is approximately 1.35 V and remains constant during its life.
Secondary Batteries
Secondary batteries are rechargeable for many applications and can be recharged multiple times. Lead storage batteries and $Ni - Cd$ batteries, for example.
Lead Storage Battery
Anode: Lead $(Pb)$
Cathode: Grid of lead packed with lead oxide $(Pb{O_2})$
Electrolyte: 38% solution of ${H_2}S{O_4}$
Discharging Reaction
Anode: $Pb(s) + SO_4^{2 - }(aq) \to PbS{O_4}(s) + 2{e^ - }$
Cathode: $Pb{O_2}(s) + 4{H^ + }(aq) + SO_4^{2 - }(aq) + 2{e^ - } \to PbS{O_4}(s) + 2{H_2}O(l)$
Overall Reaction: $Pb(s) + Pb{O_2}(s) + 2{H_2}S{O_4}(aq) \to 2PbS{O_4}(s) + 2{H_2}O(l)$
To recharge the cell, it is connected to a higher-potential cell, which acts as an electrolytic cell and reverses the processes. At the relevant electrodes, $Pb(s)$ and $Pb{O_2}(s)$ are regenerated. These cells produce a voltage that is nearly constant.
Recharging Reaction: $2PbS{O_4}(s) + 2{H_2}O(l) \to Pb(s) + Pb{O_2}(s) + 2{H_2}S{O_4}(aq)$
A fuel cell varies from a traditional battery in that the reactants are supplied externally from a reservoir rather than being stored inside the cell. In space vehicles, fuel cells are employed, and the two gases are supplied from external storage. The electrodes in this cell are carbon rods, and the electrolyte is $KOH$ .
Cathode: ${O_2}(g) + 2{H_2}O(l) + 4{e^ - } \to 4O{H^ - }(aq)$
Anode: $2{H_2}(g) + 4O{H^ - }(aq) \to 4{H_2}O(l) + 4{e^ - }$
Overall Reaction: $2{H_2}(g) + {O_2}(g) \to 2{H_2}O(l)$
On the surface of iron or any other metal, it entails a redox process and the development of an electrochemical cell.
The oxidation of iron (anode) occurs at one point, while the reduction of oxygen to generate water occurs at another (cathode). $Fe$ is first oxidised to $F{e^{2 + }}$ , which is then converted to $F{e^{3 + }}$ in the presence of oxygen, which subsequently combines with water to generate rust, which is represented by $F{e_2}{O_3}.x{H_2}O$ .
Anode: $2Fe(s) \to 2F{e^{2 + }} + 4{e^ - }$ , ${E^ \circ } = + 0.44\;V$
Cathode: ${O_2}(g) + 4{H^ + } + 4{e^ - } \to 2{H_2}O(l)$ , ${E^ \circ } = 1.23\;V$
Overall Reaction: $2Fe(s) + {O_2}(g) + 4{H^ + } \to 2F{e^{2 + }} + 2{H_2}O$ , $E_{Cell}^ \circ = 1.67\;M$
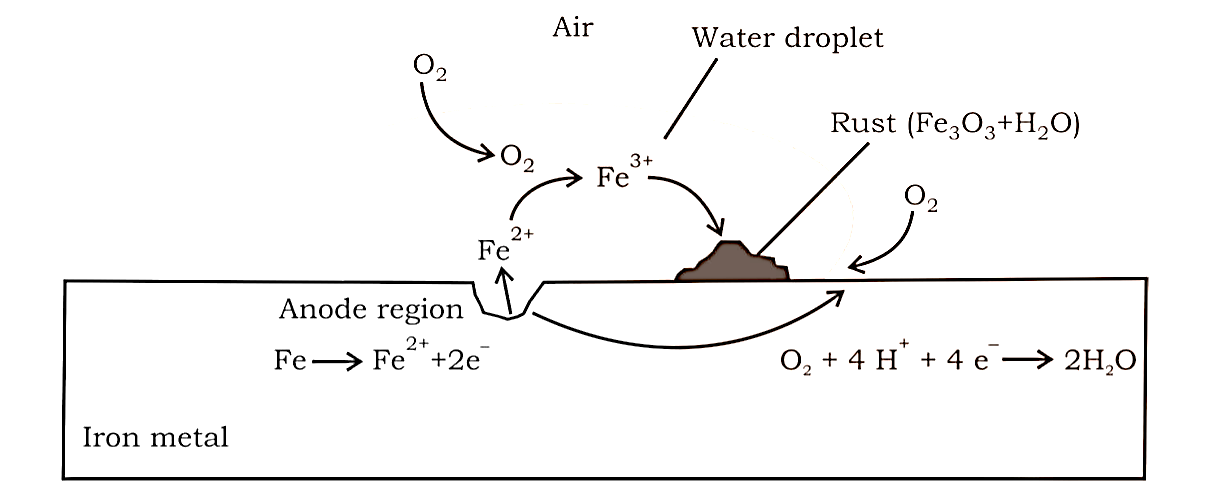
Painting or coating iron with other metals, such as zinc, helps prevent it from rusting. Galvanisation is the name for the latter procedure. Because $Zn$ has a higher potential to oxidise than iron, it is oxidised first, while iron is protected. Cathodic Protection is another name for this approach of shielding one metal by the other.
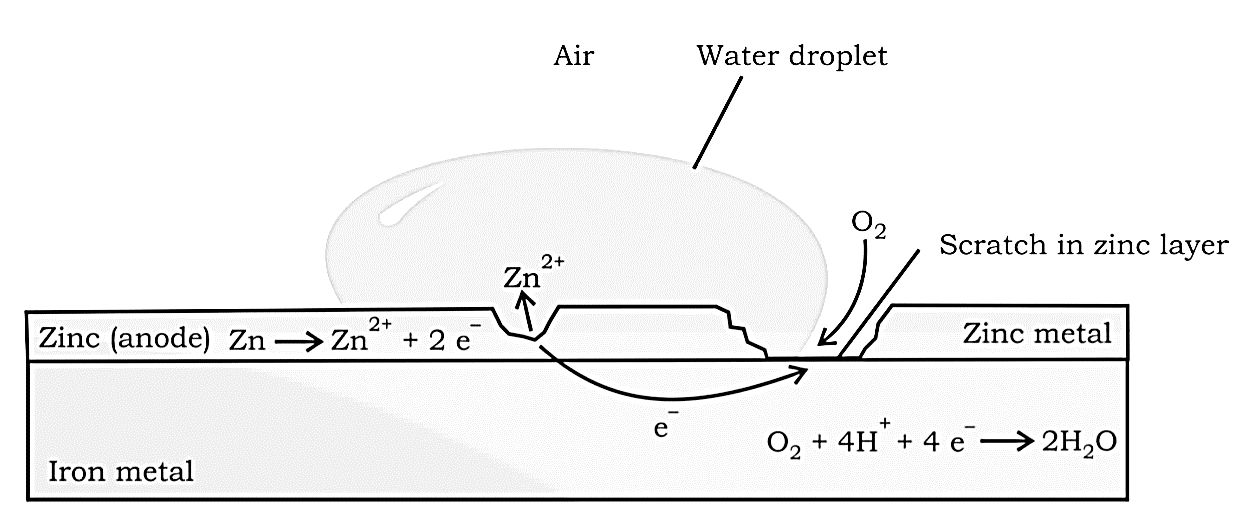
Conductance (G)
It is defined as the ease with which electric current passes through a conductor and is the reciprocal of resistance.
$G = \dfrac{1}{R}$
SI unit is Siemen (S).
$1\;S = 1\;oh{m^{ - 1}}(mho)$
Conductivity
It is the reciprocal of resistivity $(\rho )$ .
$\kappa = \dfrac{1}{\rho } = \dfrac{1}{R} \times \dfrac{\ell }{A} = G \times \dfrac{\ell }{A}$
Now is $l = 1\;cm$ and $A = 1\;c{m^2}$ , then $\kappa = G$
As a result, the conductivity of an electrolytic solution can be defined as the conductance of a $1\;cm$ long solution with a $1\;c{m^2}$ cross-sectional area.
Factors Affecting Electrolyte Conductance
Electrolyte.
In a dissolved or molten form, an electrolyte is a substance that dissociates in solution to produce ions and hence conducts electricity.
Examples: $HCl,\;NaOH,\;KCl$ are strong electrolytes and $C{H_3}COOH,\;N{H_4}OH$ are weak electrolytes.
Electrolytic or ionic conductance refers to the conductance of electricity by ions present in solutions. The flow of electricity through an electrolyte solution is governed by the following factors.
Electrolyte Nature or Interionic Attractions: The lower the solute-solute interactions, the larger the freedom of ion mobility and the higher the conductance.
Ion Solvation: As the amount of solute-solvent interactions increases, the extent of solvation increases, and the electrical conductance decreases.
The Nature of the Solvent and its Viscosity: The larger the solvent-solvent interactions, the higher the viscosity, and the greater the solvent's resistance to ion flow, and thus the lower the electrical conductance.
Temperature: As the temperature of an electrolytic solution rises, solute-solute, solute-solvent, and solvent-solvent interactions diminish, causing electrolytic conductance to rise.
Measurement of Conductance
As we know, $\kappa = \dfrac{1}{{\text{R}}} \times \dfrac{\ell }{{\text{A}}}$
If we measure $l$ , $A$ , and $R$ , we can figure out what the value of $\kappa $ is. Using the ‘Wheatstones' bridge method, the resistance of the solution $R$ between two parallel electrodes is calculated.
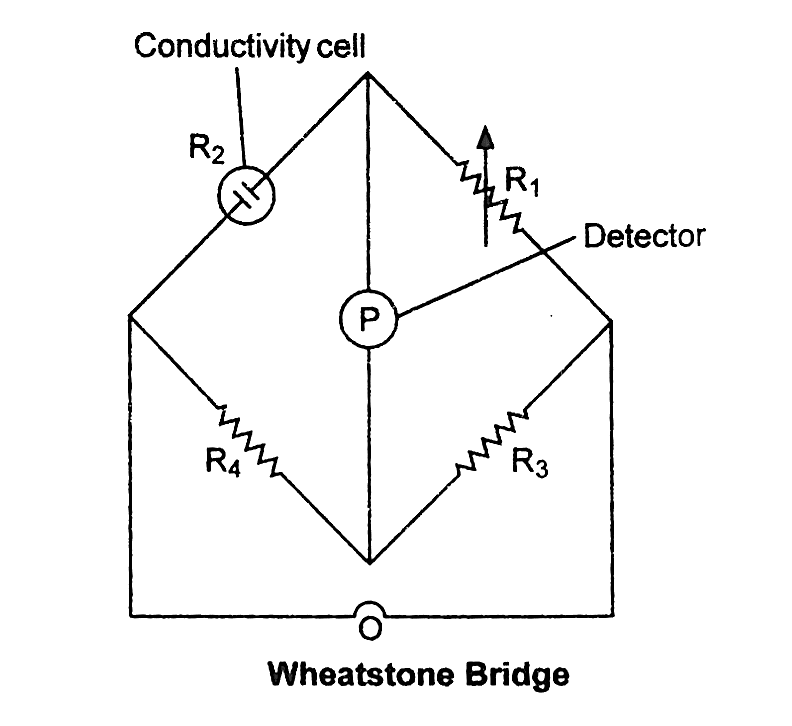
It is made up of two fixed resistances, R3 and R4, a variable resistance R1, and a conductivity cell with an unknown resistance, R2. When no current goes through the detector, the bridge is balanced. Then, in these circumstances:
$\dfrac{{{R_1}}}{{{R_2}}} = \dfrac{{{R_3}}}{{{R_4}}}$ or ${R_2} = \dfrac{{{R_1}{R_4}}}{{{R_3}}}$
Molar Conductivity
It's the total conducting power of all the ions created by dissolving one mole of an electrolyte between two big electrodes separated by one centimetre.
Mathematically,
\[\Lambda_{m} = \kappa \times V, \Lambda_{m} = \frac{\kappa \times V}{C}\]
where, V is the volume of solution in $c{m^3}$ containing 1 mole of electrolyte and C is the molar concentration.
Units: \[\Lambda_{m} = \frac{\kappa \times V}{C} = \frac{\text{S }cm^{-1}}{\text{mol } cm^{-1}}\]
${ = {\text{oh}}{{\text{m}}^{ - 1}}{\text{c}}{{\text{m}}^2}{\text{mo}}{{\text{l}}^{ - 1}}{\text{orSc}}{{\text{m}}^2}{\text{mo}}{{\text{l}}^{ - 1}}}$
Equivalent Conductivity
It is the electrical conductivity of one equivalent electrolyte placed between two big electrodes separated by one centimetre.
Mathematically:
${{{{\Lambda }}_{{\text{eq}}}} = \kappa \times {\text{v}} = }$
${{{{\Lambda }}_{{\text{eq}}}} = \dfrac{{\kappa \times 1000}}{{\text{N}}}}$
Where, v is the volume of solution in $c{m^3}$ containing 1 equivalent of electrolyte and N is normality.
${ = \dfrac{{{\text{Sc}}{{\text{m}}^{ - 1}}}}{{{{\;equivalent\;c}}{{\text{m}}^{ - 3}}}} = \dfrac{{{\text{Oh}}{{\text{m}}^{ - 1}}{\text{c}}{{\text{m}}^2}{{\;equivalent}}{{{\;}}^{ - 1}}}}{{{\text{Sc}}{{\text{m}}^2}{{\;equivalent}}{{{\;}}^{ - 1}}}}}$
Variation of Conductivity and Molar Conductivity with Dilution
Because the number of ions per unit volume that carry the current in the solution reduces as concentration lowers, conductivity drops. With a decrease in concentration, molar conductivity rises. This is due to an increase in the total volume V of a solution containing one mole of electrolyte. The drop in $\kappa $ as a result of dilution of a solution has been found to be more than compensated by increases in its volume.
Graphical representation of the variation of ${\Lambda _m}$ vs $\sqrt c $ .
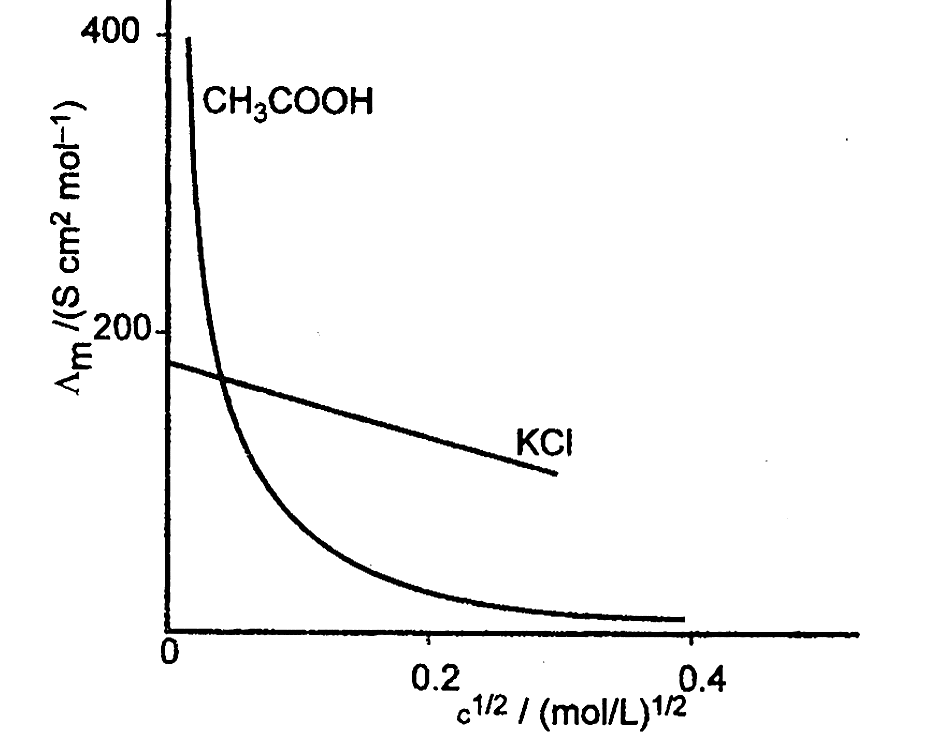
Limiting Molar Conductivity $({\Lambda _m})$
Limiting molar conductivity, also known as molar conductivity at infinite dilution, is the value of molar conductivity as the concentration approaches zero. In the case of a strong electrolyte, extrapolation of the ${\Lambda _m}$ vs $\sqrt c $ curve can be used to derive the molar conductivity at infinite dilution. Extrapolation of the curve, on the other hand, cannot be used to calculate the value of molar conductivity of a weak electrolyte at infinite dilution since the curve becomes practically parallel to the y-axis as concentration approaches zero.
The mathematical relationship between ${\Lambda _m}$ and $\Lambda _m^ \circ $ for a strong electrolyte was developed by Debye, Huckel and Onsagar.
In simplified form the equation can be given as:
${{t{\Lambda }}_{\text{m}}} = {{\Lambda }}_{\text{m}}^\infty - {\text{b}}{{\text{c}}^{1/2}}$
Kohlrausch’s Law
It asserts that an electrolyte limiting molar conductivity can be described as the total of the individual contributions of the electrolyte's anion and cation.
In general, if an electrolyte produces ${v_ + }$ cations and ${v_ - }$ anions upon dissociation, its limiting molar conductivity is given by:
${{\Lambda }}_{\text{m}}^\infty = {{\text{v}}_ + }\lambda _ + ^ \circ + {{\text{v}}_ - }\lambda _ - ^ \circ $
Applications of Kohlrausch’s Law
Calculation of molar conductivities of weak electrolyte at infinite dilution
For example, the molar conductivity of acetic acid at infinite dilution can be calculated using the molar conductivities of strong electrolytes like $HCl$ , $C{H_3}COONa$ , and $NaCl$ at infinite dilution, as shown below.
${{\Lambda }}_{{\text{m}}\left( {{\text{C}}{{\text{H}}_3} - {\text{COOH}}} \right)}^{\text{o}} = {{\Lambda }}_{{\text{m}}\left( {{\text{C}}{{\text{H}}_3} - {\text{cooNa}}} \right)}^{\text{o}} + {{\Lambda }}_{{\text{m}}({\text{HCl}})}^{\text{o}} - {{\Lambda }}_{{\text{m}}({\text{NaCl}})}^ \circ $
Determination of Degree of Dissociation of Weak Electrolytes
Degree of dissociation $\alpha = \dfrac{{\Lambda _m^c}}{{\Lambda _m^ \circ }}$
Determination of Dissociation Constant of Weak Electrolytes:
${{\text{K}} = \dfrac{{{\text{c}}{\alpha ^2}}}{{1 - \alpha }}}$
${\alpha = \dfrac{{{{\Lambda }}_{\text{m}}^{\text{c}}}}{{{{\Lambda }}_{\text{m}}^\infty }}}$
${{\text{K}} = \dfrac{{{\text{c}}{{\left( {{{\Lambda }}_{\text{m}}^{\text{c}}/{{\Lambda }}_{\text{m}}^\infty } \right)}^2}}}{{1 - {{\Lambda }}_{\text{m}}^c/{{\Lambda }}_{\text{m}}^\infty }} = \dfrac{{{\text{C}}{{\left( {{{\Lambda }}_{\text{m}}^{\text{c}}} \right)}^2}}}{{{{\Lambda }}_{\text{m}}^\infty \left( {{{\Lambda }}_{\text{m}}^ * - {{\Lambda }}_{\text{m}}^{\text{c}}} \right)}}}$
Use of $\Delta G$ in Relating EMF values of Half Cell Reactions
When we have two half-cell reactions that produce another half-cell reaction when we combine them, their emfs cannot be mixed directly. However, thermodynamic functions such as $\Delta G$ can be added and EMF values can be connected through them in any scenario. Take a look at the three half-cell responses below:
$F{e^{2 + }} + 2{e^ - } \to Fe$ , ${E_1}$
$F{e^{3 + }} + 3{e^ - } \to Fe$ , ${E_2}$
$F{e^{3 + }} + {e^ - } \to F{e^{2 + }}$ , ${E_3}$
We can clearly see that subtracting the first reaction from the second yields the third reaction. However, the same relationship does not hold true for EMF values.
That is: ${E_3} \ne {E_2} - {E_1}$ . But the $\Delta G$ values can be related according to the reactions:
${{{\Delta }}{{\text{G}}_3} = {\text{\Delta }}{{\text{G}}_2} - {{\Delta }}{{\text{G}}_1}}$
${ - {{\text{n}}_3}{\text{F}}{{\text{E}}_3} = - {{\text{n}}_2}{\text{F}}{{\text{E}}_2} + {{\text{n}}_1}{\text{F}}{{\text{E}}_1}}$
${ - {{\text{E}}_3} = - 3{{\text{E}}_2} + 2{{\text{E}}_1}}$
${ \Rightarrow {{\mathbf{E}}_3} = 3{{\mathbf{E}}_2} - {\mathbf{2}}{{\mathbf{E}}_1}}$
${\text{R}} = \rho \left( {\dfrac{\ell }{{\text{A}}}} \right) = \rho \times {\text{Cell constant}}$
Where, $R$ = Resistance,
$A$ = Area of cross-section of the electrodes
$\rho $ = Resistivity
$\kappa = \dfrac{1}{{\text{R}}} \times {\text{\;cell constant\;}}$
Where, $\kappa $ = Conductivity or specific conductance
${{{\Lambda }}_{\text{m}}} = \dfrac{{\kappa \times 1000}}{{\text{M}}}$
Where, ${\Lambda _m}$ = Molar conductivity
$M$ = Molarity of the solution.
${{\Lambda }}_m^\infty \left( {{A_x}{B_y}} \right) = x{{\Lambda }}_m^\infty \left( {{A^y}} \right) + y{\text{\Lambda }}_m^\infty \left( {{B^{x - }}} \right)$
$\alpha = \dfrac{{\Lambda _m^c}}{{\Lambda _m^ \circ }}$
Where, $\alpha $ = Degree of dissociation
$\Lambda _m^c$ = Molar conductivity at a given concentration
For a weak binary electrolyte AB
${\text{K}} = \dfrac{{{\text{c}}{\alpha ^2}}}{{1 - \alpha }} = \dfrac{{{\text{c}}{{\left( {{{\Lambda }}_{\text{m}}^{\text{c}}} \right)}^2}}}{{{{\Lambda }}_{\text{m}}^\infty \left( {{{\Lambda }}_{\text{m}}^\infty - {{\Lambda }}_{\text{m}}^{\text{c}}} \right)}}$
Where, K is the Dissociation constant
${{\text{E}}_{{\text{edl}}}^ \circ = {\text{E}}_{{\text{cathode}}}^ \circ + {\text{E}}_{{\text{anode}}}^ \circ }$
${ = {{\text{E}}^ \circ }{\text{Right}} + {{\text{E}}^{\text{o}}}{\text{left}}}$
Nernst equation for a generation electrochemical reation
${E_{{\text{ofll}}}} = E_{{\text{cell}}}^ \circ - \dfrac{{0.059}}{n}\log \dfrac{{{{[A]}^2}{{[B]}^b}}}{{{{[C]}^c}{{[D]}^d}}}$
$\log {{\text{K}}_{\text{c}}} = \dfrac{{\text{n}}}{{0.0591}}{\text{E}}_{{\text{cell}}}^ \circ $
Where, ${K_c}$ = Equilibrium constant.
${{{\Delta }}_r}{{\text{G}}^ \circ } = - {\text{nFE}}_{{\text{cell}}}^ \circ $
${{{\Delta }}_{\text{r}}}{{\text{G}}^ \circ } = - 2.303{\text{RT}}\log {{\text{K}}_{\text{c}}}$
${\Delta _r}{G^ \circ }$ = Standard Gibbs energy of a reaction
$Q = I \times t$
Where, $Q$ = Quantity of charge in coulombs
$I$ = Current in amperes
$t$ = Time in seconds
$m = Z \times I \times t$
Where, $m$ = mass of the substance liberated at the electrodes
$Z$ = Electrochemical equivalent
Standard Reduction Potential At 298 K. In Electrochemical Order
$\mathrm{H}_{4} \mathrm{XeO}_{6}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{XeO}_{3}+3 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad+3.0$
$\mathrm{~F}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-} \quad\quad\quad\quad+2.87$
$\mathrm{O}_{3}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}+\mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +2.07$
$\mathrm{~S}_{2} \mathrm{O}_{8}^{2-}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{SO}_{4}^{2-} \quad\quad\quad\quad +2.05$
$\mathrm{Ag}^{2+}+\mathrm{c}^{-} \rightarrow \mathrm{Ag}^{+} \quad\quad\quad\quad +1.98$
$\mathrm{Co}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Co}^{2+} \quad\quad\quad\quad +1.81$
$\mathrm{H}_{2} \mathrm{O}_{2}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.78$
$\mathrm{Au}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Au} \quad\quad\quad\quad +1.69$
$\mathrm{~Pb}^{4+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}^{2+} \quad\quad\quad\quad +1.67$
$2 \mathrm{HClO}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cl}_{2}+2 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.63$
$\mathrm{Ce}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Ce}^{3+} \quad\quad\quad\quad +1.61$
$2 \mathrm{H} \mathrm{IBrO}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Br}_{2}+2 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.60$
$\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.51$
$\mathrm{Mn}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+} \quad\quad\quad\quad +1.51$
$\mathrm{Au}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Au} \quad\quad\quad\quad +1.40$
$\mathrm{Cl}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cl}^{-} \quad\quad\quad\quad +1.36$
$\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.33$
$\mathrm{O}_{3}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}+2 \mathrm{OH}^{-} \quad\quad\quad\quad +1.24$
$\mathrm{O}_{2}+4 \mathrm{H}^{+} 4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.23$
$\mathrm{Hg}_{2} \mathrm{SO}_{4}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Hg}+\mathrm{SO}_{4}^{2-} \quad \quad \quad \quad +0.62$
$\mathrm{MnO}_{4}^{2-}+2 \mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{MnO}_{2}+4 \mathrm{OH}^{-} \quad \quad \quad \quad +0.60$
$\mathrm{MnO}_{4}^{-}+\mathrm{e}^{-} \rightarrow \mathrm{MnO}_{4}^{2-} \quad \quad \quad \quad +0.56$
$\mathrm{I}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{I}^{-} \quad \quad \quad \quad +0.54$
$\mathrm{Cu}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Cu} \quad \quad \quad \quad +0.52$
$\mathrm{I}_{3}^{-}+2 \mathrm{e}^{-} \rightarrow 3 \mathrm{I}^{-} \quad \quad \quad \quad +0.53$
$\mathrm{NiOOH}+\mathrm{H}_{2} \mathrm{O}+\mathrm{e}^{-} \rightarrow \mathrm{Ni}(\mathrm{OH})_{2}+\mathrm{OH}^{-} \quad \quad \quad \quad +0.49$
$\mathrm{Ng}_{2} \mathrm{CrO}_{4}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Ag}+\mathrm{CrO}_{4}^{2-} \quad \quad \quad \quad +0.45$
$\mathrm{O}_{2}+2 \mathrm{H}_{2} \mathrm{O}+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}^{-} \quad \quad \quad \quad +0.40$
$\mathrm{ClO}_{4}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{ClO}_{3}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.36$
${\left[\mathrm{Fe}(\mathrm{CN})_{6}^{3-}+\mathrm{e}^{-} \rightarrow\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{+}\right.} \quad \quad \quad \quad +0.36$
$\mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu} \quad \quad \quad \quad +0.34$
$\mathrm{Hg}_{2} \mathrm{Cl}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Hg}+2 \mathrm{Cl}^{-} \quad \quad \quad \quad +0.27$
$\mathrm{AgCl}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{Cl}^{-} \quad \quad \quad \quad +0.22$
$\mathrm{Bi}+3 \mathrm{e}^{-} \rightarrow \mathrm{Bi} \quad \quad \quad \quad +0.20$
$\mathrm{Cu}^{2+}+\mathrm{e}^{-} \rightarrow \mathrm{Cu}^{+} \quad \quad \quad \quad +0.16$
$\mathrm{Sn}^{4+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sn}^{2+} \quad \quad \quad \quad +0.15$
$\mathrm{AgBr}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{Br}^{-} \quad \quad \quad \quad +0.07$
$\mathrm{ClO}_{4}^{-}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{ClO}_{3}^{-}+\mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.23$
$\mathrm{MNO}_{2}+4 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.23$
$\mathrm{Br}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-} \quad \quad \quad \quad +1.09$
$\mathrm{Pu}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Pu}^{3+} \quad \quad \quad \quad +0.97$
$\mathrm{NO}_{3}^{-}+4 \mathrm{H}^{+}+3 \mathrm{e}^{-} \rightarrow \mathrm{NO}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +0.96$
$2 \mathrm{Hg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Hg}_{2}^{2+} \quad \quad \quad \quad +0.92$
$\mathrm{ClO}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cl}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.89$
$\mathrm{Hg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Hg} \quad \quad \quad \quad +0.86$
$\mathrm{NO}_{3}^{-}+2 \mathrm{H}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{NO}_{2}+\mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +0.80$
$\mathrm{Ag}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Ag} \quad \quad \quad \quad +0.80$
$\mathrm{Hg}_{2}^{2+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Hg} \quad \quad \quad \quad +0.79$
$\mathrm{Fe}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Fe}^{2+} \quad \quad \quad \quad +0.77$
$\mathrm{BrO}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{Br}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.76$
$\mathrm{Ti}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Ti}^{3+} \quad \quad \quad \quad 0.00 2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2} \quad \quad \quad \quad 0, \text { by definition }$
$\mathrm{Fe}^{3-}+3 \mathrm{e}^{-} \rightarrow \mathrm{Fe} \quad \quad \quad \quad -0.04$
$\mathrm{O}_{2} \mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{HO}_{2}^{-}+\mathrm{OH}^{-} \quad \quad \quad \quad -0.08$
$\mathrm{~Pb}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb} \quad \quad \quad \quad -0.13$
$\mathrm{In}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{In} \quad \quad \quad \quad -0.14$
$\mathrm{Sn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sn} \quad \quad \quad \quad -0.14$
$\mathrm{AgI}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{F}^{-} \quad \quad \quad \quad -0.15$
$\mathrm{Ni}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ni} \quad \quad \quad \quad -0.23$
$\mathrm{Co}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Co} \quad \quad \quad \quad -0.28$
$\mathrm{In}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{In} \quad \quad \quad \quad -0.34$
$\mathrm{Tl}^{+}\mathrm{e}^{-} \rightarrow \mathrm{Tl} \quad \quad \quad \quad -0.34$
$\mathrm{PbSO}_{4}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}+\mathrm{SO}_{4}^{2-} \quad \quad \quad \quad -0.36$
$\mathrm{Ti}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Ti}^{2+} \quad \quad \quad \quad -0.37$
$\mathrm{Cd}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cd} \quad \quad \quad \quad -0.40$
$\mathrm{In}^{2+}+\mathrm{e}^{-} \rightarrow \mathrm{In}^{+} \quad \quad \quad \quad -0.40$
$\mathrm{Cr}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Cr}^{2+} \quad \quad \quad \quad -0.41$
$\mathrm{Fe}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Fe} \quad \quad \quad \quad -0.44$
$\mathrm{In}^{3+}+2 \mathrm{e}^{-} \rightarrow \mathrm{In}^{+} \quad \quad \quad \quad -0.44$
$\mathrm{~S}+2 \mathrm{e}^{-} \rightarrow \mathrm{S}^{2-} \quad \quad \quad \quad -0.48$
$\mathrm{In}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{In}^{2+} \quad \quad \quad \quad -0.49$
$\mathrm{U}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{U}^{3+} \quad \quad \quad \quad -0.61$
$\mathrm{Cr}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Cr} \quad \quad \quad \quad -0.74$
$\mathrm{Zn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Zn} \quad \quad \quad \quad -0.76$
$\mathrm{Cd}(\mathrm{OH})_{2}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cd}+2 \mathrm{OH}^{-} \quad \quad \quad \quad -0.81$
$2 \mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2}+2 \mathrm{OH}^{-} \quad \quad \quad \quad -0.83$
$\mathrm{Cr}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cr} \quad \quad \quad \quad -0.91$
$\mathrm{Mn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn} \quad \quad \quad \quad -1.18$
$\mathrm{V}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{V} \quad \quad \quad \quad -1.19$
$\mathrm{Ti}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ti} \quad \quad \quad \quad -1.63$
$\mathrm{Al}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Al} \quad \quad \quad \quad -1.66$
$\mathrm{U}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{U} \quad \quad \quad \quad -1.79$
$\mathrm{Sc}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Sc} \quad \quad \quad \quad -2.09$
$\mathrm{Mg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mg} \quad \quad \quad \quad -2.36$
$\mathrm{Ce}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Ce} \quad \quad \quad \quad -2.48$
$\mathrm{La}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{La} \quad \quad \quad \quad -2.52$
$\mathrm{Na}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Na} \quad \quad \quad \quad -2.71$
$\mathrm{Ca}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ca} \quad \quad \quad \quad -2.87$
$\mathrm{Sr}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sr} \quad \quad \quad \quad -2.89$
$\mathrm{Ba}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ba} \quad \quad \quad \quad -2.91$
$\mathrm{Ra}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ra} \quad \quad \quad \quad -2.92$
$\mathrm{Cs}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Cs} \quad \quad \quad \quad -2.92$
$\mathrm{Rb}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Rb} \quad \quad \quad \quad -2.93$
$\mathrm{~K}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{K} \quad \quad \quad \quad -2.93$
$\mathrm{Li}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Li} \quad \quad \quad \quad -3.05$
Reduction Potential in Alphabetical Order:
$\mathrm{Ag}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}$
$\mathrm{Ag}^{2+}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}^{+}$
$\mathrm{AgBr}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{Br}^{-}$
$\mathrm{AgCl}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{Cl}^{-}$
$\mathrm{Ag}_{2} \mathrm{CrO}_{4}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Ag}+\mathrm{CrO}_{4}^{2-}$
$\mathrm{AgF}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{F}^{-}$
$\mathrm{Agl}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{I}^{-}$
$\mathrm{Al}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Al}$
$\mathrm{Au}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Au}$
$\mathrm{Au}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Au}$
$\mathrm{Ba}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ba}$
$\mathrm{Be}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Be}$
$\mathrm{Bi}^{-3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Bi}$
$\mathrm{Br}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-}$
$\mathrm{BrO}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{Br}^{-}+2 \mathrm{OH}^{-}$
$\mathrm{Ca}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ca} \quad \quad \quad \quad -2.87$
$\mathrm{Ce}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Ce} \quad \quad \quad \quad -2.48$
$\mathrm{Ce}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Ce}^{3+} \quad \quad \quad \quad +1.61$
$\mathrm{Cl}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cl}^{-} \quad \quad \quad \quad +1.36$
$\mathrm{Co}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Co}^{2+} \quad \quad \quad \quad +1.81$
$\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.33$
$\mathrm{Cs}^{+} \mathrm{e}^{-} \rightarrow \mathrm{Cs} \quad \quad \quad \quad -2.92$
$\mathrm{~F}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-} \quad \quad \quad \quad +2.87$
$\mathrm{Fe}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Fe} \quad \quad \quad \quad -0.04$
${\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3}+\mathrm{e}^{-} \rightarrow\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{+}} \quad \quad \quad \quad +0.36$
$2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2} \quad \quad \quad \quad 0, \text { by definition }$
$2 \mathrm{HBrO}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Br}_{2}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.60$
$2 \mathrm{HClO}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cl}_{2}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.63$
$\mathrm{H}_{2} \mathrm{O}_{2}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.78$
$\mathrm{H}_{4} \mathrm{XeO}_{6}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{XeO}_{3}+3 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +3.0$
$\mathrm{MnO}_{4}^{-}+2 \mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{MnO}_{2}+4 \mathrm{OH}^{-} \quad \quad \quad \quad +0.60$
$\mathrm{Na}^{-}+\mathrm{e}^{-} \rightarrow \mathrm{Na} \quad \quad \quad \quad -2.71$
$\mathrm{NO}_{3}^{-}+2 \mathrm{H}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{NO}_{2}+\mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad -0.80$
$\mathrm{NO}_{3}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{NO}_{2}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.10$
$\mathrm{O}_{2}+4 \mathrm{H}^{+}+4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.23$
$\mathrm{O}_{2}+\mathrm{e}^{-} \rightarrow \mathrm{O}_{2}^{-} \quad \quad \quad \quad -0.56$
$\mathrm{O}_{2}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{HO}_{2}^{-}+\mathrm{OH}^{-} \quad \quad \quad \quad -0.08$
$\mathrm{O}_{3}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}+\mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +2.07$
$\mathrm{O}_{3}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +1.24$
$\mathrm{Hg}_{2} \mathrm{SO}_{4}+2 \mathrm{e}-\rightarrow 2 \mathrm{Hg}+\mathrm{SO}_{4}^{2-} \quad \quad \quad \quad +0.62$
$\mathrm{In}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{In} \quad \quad \quad \quad -0.34$
$\mathrm{In}^{3+}+\mathrm{c}^{-} \rightarrow \mathrm{In}^{2+} \quad \quad \quad \quad -0.49$
$\mathrm{~K}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{K} \quad \quad \quad \quad -2.93$
$\mathrm{La}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{La} \quad \quad \quad \quad -2.52$
$\mathrm{Li}+\mathrm{e}^{-} \rightarrow \mathrm{Li} \quad \quad \quad \quad -3.05$
$\mathrm{Mg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mg} \quad \quad \quad \quad -2.36$
$\mathrm{Mn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{M} \quad \quad \quad \quad -1.18$
$\mathrm{Mn}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+} \quad \quad \quad \quad +1.51$
$\mathrm{MnO}_{2}+4 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.23$
$\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.51$
$\mathrm{~Pb}^{4+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}^{2+} \quad \quad \quad \quad +1.67$
$\mathrm{Pt}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pt} \quad \quad \quad \quad +1.20$
$\mathrm{Ra}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ra} \quad \quad \quad \quad -2.92$
$\mathrm{Rb}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Rb} \quad \quad \quad \quad -2.93$
$\mathrm{~S}_{2} \mathrm{O}_{8}^{2-}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{SO}_{4}^{2-} \quad \quad \quad \quad +2.05$
$\mathrm{SC}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Sc} \quad \quad \quad \quad -2.09$
$\mathrm{Sr}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sr} \quad \quad \quad \quad -2.89$
$\mathrm{Ti}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ti} \quad \quad \quad \quad -1.63$
$\mathrm{Ti}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Ti}^{3+} \quad \quad \quad \quad 0.00$
$\mathrm{Tl}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Tl} \quad \quad \quad \quad -0.34$
$\mathrm{U}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{U} \quad \quad \quad \quad -1.79$
$\mathrm{~V}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{V} \quad \quad \quad \quad -1.19$
$\mathrm{~V}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{V}^{2+} \quad \quad \quad \quad -0.26$
$\mathrm{Zn}^{2+}+2 \mathrm{e}-\rightarrow \mathrm{Zn} \quad \quad \quad \quad -0.76$
Some Important Questions on Electrochemistry
1. What is the meaning of the negative sign in the expression $\dfrac{E_0 Zn^{2+}}{Zn} = – 0.76 V$ ?
Ans. The negative sign in the given expression implies that Zn is more reactive than hydrogen or that it is a stronger reducing agent than hydrogen. Zinc will be oxidised to $Zn^{2+}$ ions, while the $H^+$ ions will get reduced to hydrogen in a cell that contains a zinc electrode and a standard hydrogen electrode present in two half-cells.
2. What are the conditions under which $E_0$ cell = 0 and $\Delta rG_0 = 0$ ?
Ans. When at equilibrium, $E_0$cell = 0 and $\Delta rG_0 = 0$ .
3. Can we measure the absolute electrode potential of an electrode?
Ans. No, it is not possible to measure the absolute potential of an electrode since the half-cell that contains a single electrode cannot work on its own, it can only work in combination with another half-cell.
Topics Related to Electrochemistry Class 12 Notes PDF Download
Explore our curated collection of resources on Electrochemistry Class 12. Dive into concise notes, explanations, and practice questions to bolster your understanding of this essential subject. Whether you're preparing for exams or aiming to deepen your knowledge, these resources are created to support your learning journey effectively. Here is the links for a few Topics Related to Electrochemistry Class 12 Notes, that you can learn along with Class 12 Electrochemistry Notes.
Standard Electrode Potential
EMF of Cell
Reduction Potential
For Further Assistance Watch our Master Teacher Shilpi mam Explaining Electrochemistry Full Chapter in 60 Minutes | Class 12 Chemistry.
You can also watch Electrochemistry Class 12 One Shot by Aravind Arora Sir.
Other Related Links
Chapter 1 - The Solid State (Not in the updated syllabus)
Chapter 2 - Solutions
Chapter 3 - Electrochemistry
Chapter 4 - Chemical Kinetics
Chapter 5 - Surface Chemistry (Not in the updated syllabus)
Chapter 6 - General Principles and Processes of Isolation of Elements (Not in the updated syllabus)
Chapter 7 - The p-Block Elements (Not in the updated syllabus)
Chapter 8 - The d and f Block Elements
Chapter 9 - Coordination Compounds
Chapter 10 - Haloalkanes and Haloarenes
Chapter 11 - Alcohols, Phenols and Ethers
Chapter 12 - Aldehydes, Ketones and Carboxylic Acids
Chapter 13 - Amines
Chapter 14 - Biomolecules
Chapter 15 - Polymers (Not in the updated syllabus)
Chapter 16 - Chemistry in Everyday life (Not in the updated syllabus)
Important Electrochemistry Related Links
Explore a compilation of valuable links related to Electrochemistry topic, offering comprehensive study materials, solved examples, and practice questions for Class 12 students studying chemistry.
Important Class 12 Study Materials Links
Find a curated selection of study resources for Class 12 subjects, helping students prepare effectively and excel in their academic pursuits.
This article on revision notes for CBSE Class 12 Chemistry Chapter 3, which is on Electrochemistry, has been offered by Vedantu's expert professors to assist students in their never-ending hunt for exam-appropriate study resources as test dates approach. We urge that students look through the resources in this page as well as the ones in the connected links in order to achieve good grades in their class 12 chemistry examinations.

FAQs on Electrochemistry Class 12 Notes CBSE Chemistry Chapter 3 (Free PDF Download)
1. What is the Difference Between a Galvanic Cell and Electrolytic Cell?
The prime disparity between a galvanic cell and electrolytic cells are-
An electrolytic cell has a non-spontaneous reaction which transforms into an electrical form of energy. In a galvanic cell, input energy is put to function in a redox form of response in spontaneous form.
In an electrolytic cell, the anode remains positive electrode and cathode is in negative form while it is opposite in the case of a galvanic cell. In a galvanic cell, electrons create from the class that experience oxidation while the oxidation process happens at the cathode of an electrolytic cell.
2. What is a Cell Notation?
An electrochemical cell has various uses in the general world. Therefore, a user needs to follow specific rules while representing them. The crucial parts like the cathode should always be right while an anode should stay on the left.
Here the cell is shown by following a specific rule where metals are written first, followed by metal ions. These metal ions can be found in electrolyte forms. They are further required to be separated via a vertical structure of the line.
An example will be $Zn | Zn^{2+}$ . Here one can find a molar concentration that is represented via brackets. The result is $Zn | Zn^{2+}(1M)$ .
3. What are the applications of electrochemical cells?
An electrolytic cell is used to refine electrically many of the non-ferrous metals. They are also used for electrowinning. Apart from melting metals and creating new structures, an electrochemical cell performs different reactions.
They are also used to produce high-grade metals like aluminium, zinc, copper, etc., for general use. Furthermore, one can extract metallic sodium out of molten sodium chloride. This is possible by placing an electrolytic cell in a solution and bypassing electric current over it.
Moreover, these cells are used to produce large batteries which are used commercially like galvanic cells. The best part is that these cells can also be eco-friendly as they save the environment in the form of fuel cells.
4. How are Electrochemistry Class 12 Notes PDF Download helpful for Class XII board examinations?
Revision notes for any subject are an extremely important study tool particularly for a subject like chemistry. Class XII Chemistry is an extensive subject that requires loads of cognizance and memorization. These revision notes can easily serve as a quick reference manual close to examinations. Students can study important points from them quickly. Revision notes for Chapter 3 "Electrochemistry" contain all important topics, which students need to refer to for Class XII Board exams.
5. Are Class 12 Chemistry Chapter 3 Notes available to download? If so, are they free?
Vedantu provides extremely useful notes for all the chapters of Class XII Chemistry. These notes can easily be downloaded at no cost from the Vedantu website or the Vedantu Mobile app. To download Class 12 Chemistry Chapter 3 Notes:
Visit the page Class 12 Chemistry Chapter 3 Notes.
You will be taken to the page containing the required revision notes.
As you scroll down you will find the option to "Download pdf." Click on it.
You will be redirected to a page containing the link to download the pdf of these revision notes.
6. Is Chapter 3 “Electrochemistry” a difficult chapter?
Class XII Chapter 3 "Electrochemistry" is a pretty important chapter for board exams. Electrochemistry along with solutions, surface chemistry, and chemical kinetics carry a total weightage of 23 marks. The chapter carries important topics like Electrochemical cell, Galvanic cell, Electrolysis, etc. The chapter, if understood well, is not hugely difficult. Vedantu provides useful study material like NCERT Solutions , revision notes, question papers, and conceptual videos for this chapter.
7. What are some useful tips to ace the Class XII Chemistry Board exam?
Chemistry of Class XII may seem like an intimidating subject. But with some useful strategies, you can nail the CBSE board exams:
Pay attention during class.
Be thorough with the NCERT textbook.
Make crisp and short notes.
Refer to NCERT Solutions , revision notes, sample papers, and previous years' question papers and practice well. Solve loads of question papers for practice.
Revise extensively.
8. What are the important topics from Class 12 Electrochemistry Notes?
Electrochemistry is a pretty important chapter from the perspective of Class XII Board examinations. It contains significant topics like Electrochemical cells, Galvanic cells, Measurement of electrode potential, Nernst equation, The equilibrium constant from the Nernst equation, Electrochemical cells and Gibbs free energy of the reaction, The conductance of the electrolytic cells, Measurement of the conductivity of the ionic solution, Variation in conductivity and molar conductivity, and Kohlrausch's law and batteries.
Students can refer to important questions on the page Important Questions for CBSE Class 12 Chemistry Chapter 3 .
9. What is electrochemistry class 12 notes?
Electrochemistry Class 12 notes cover the fundamental concepts and principles related to the study of chemical reactions involving electricity and the transfer of electrons between substances.
10. What is an electrode in Class 12 Electrochemistry Notes?
Electrode Class 12 Electrochemistry Notes explain the role and function of electrodes in electrochemical cells, detailing their importance in facilitating electron transfer during redox reactions.
11. What is the most important topics of electrochemistry class 12?
The most important topics of electrochemistry Class 12 include oxidation-reduction reactions, galvanic cells, electrolytic cells, electrode potentials, and the Nernst equation.
12. What are the points to remember in class 12 Electrochemistry Notes PDF?
Points to remember in Class 12 Electrochemistry Notes PDF include understanding the difference between oxidation and reduction, grasping the concept of electrode potentials, recognizing the components of a galvanic cell and an electrolytic cell, and mastering the application of the Nernst equation in various scenarios.
Previous Year Question Papers CBSE Class 12
- NCERT Solutions
- NCERT Class 12
- NCERT 12 Chemistry
- Chapter 3: Electrochemistry
NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry
Ncert solutions for class 12 chemistry chapter 3 – free pdf download.
NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry play a pivotal role in the CBSE Class 12 Chemistry board examination. NCERT Solutions for Class 12 Chemistry are comprehensive materials that have answers to the exercise present in the NCERT Textbook. These solutions are developed by subject experts at BYJU’S, following the latest CBSE Syllabus for 2023-24 and its guidelines.
By studying these NCERT Solutions for Class 12 Chemistry, students will be able to solve different kinds of questions that might appear in the board examination and entrance examinations. These solutions are presented in a clear and step-wise format for ease of understanding. To download the NCERT Solutions for Class 12 Chemistry Chapter 3 PDF, click the link given below.
carouselExampleControls112

Previous Next
Class 12 Chemistry NCERT Solutions Chapter 3 Electrochemistry – Important Questions
Arrange the following metals in the order in which they displace each other from the solution of their salts. Al, Cu, Fe, Mg and Zn.
According to their reactivity, the given metals replace the others from their salt solutions in the said order: Mg, Al, Zn, Fe, and Cu.
Mg: Al: Zn: Fe: Cu
Given the standard electrode potentials. K + /K = –2.93V
Ag + /Ag = 0.80V
Hg 2+ /Hg = 0.79V
Mg 2+ /Mg = –2.37 V
Cr 3+ /Cr = – 0.74V Arrange these metals in their increasing order of reducing power.
The reducing power increases with the lowering of the reduction potential. In order of given standard electrode potential (increasing order): K + /K < Mg 2+ /Mg < Cr 3+ /Cr < Hg 2+ /Hg < Ag + /Ag
Thus, in the order of reducing power, we can arrange the given metals as Ag< Hg < Cr < Mg < K
Depict the galvanic cell in which the reaction Zn(s) + 2Ag + (aq) →Zn 2+ (aq) + 2Ag(s) takes place. Further show: (i) Which of the electrode is negatively charged? (ii) The carriers of the current in the cell. (iii) Individual reaction at each electrode.
The galvanic cell in which the given reaction takes place is depicted as
(i) The negatively charged electrode is the Zn electrode (anode).
(ii) The current carriers in the cell are ions. Current flows to zinc from silver in the external circuit.
(iii) Reaction at the anode is given by
Reaction at the anode is given by
Calculate the standard cell potentials of the galvanic cell in which the following reactions take place. (i) 2Cr(s) + 3Cd 2+ (aq) → 2Cr 3+ (aq) + 3Cd (ii) Fe 2+ (aq) + Ag + (aq) → Fe 3+ (aq) + Ag(s) Calculate the ∆rGJ and equilibrium constant of the reactions.
(i) \(\begin{array}{l}E^{\Theta}_{Cr^{3+}/Cr}\end{array} \) = 0.74 V
The galvanic cell of the given reaction is depicted as
Now, the standard cell potential is
= – 0.40 – ( -0.74 )
In the given equation, n = 6
F = 96487 C mol −1
Then, \(\begin{array}{l}\Delta_rG^{\Theta}\end{array} \) = −6 × 96487 C mol −1 × 0.34 V
= −196833.48 CV mol−1
= −196833.48 J mol−1
= −196.83 kJ mol−1
K = antilog (34.496) = 3.13 × 10 34
Here, n = 1
Then, \(\begin{array}{l}\Delta_t G^0 = -nFE^0_{cell}\end{array} \)
= −1 × 96487 C mol −1 × 0.03 V
= −2894.61 J mol −1
= −2.89 kJ mol −1
Again, \(\begin{array}{l}\Delta_t G^0 = -2.303 RT \; ln K\end{array} \) \(\begin{array}{l}ln K = \frac{\Delta_t G}{ 2.303 RT }\end{array} \) \(\begin{array}{l}= \frac{-2894.61 }{ 2.303 \times 8.314 \times 298 }\end{array} \)
K = antilog (0.5073)
= 3.2 (approximately)
Write the Nernst equation and emf of the following cells at 298 K. (i) Mg(s)|Mg 2+ (0.001M)||Cu 2+ (0.0001 M)|Cu(s) (ii) Fe(s)|Fe 2+ (0.001M)||H + (1M)|H2(g)(1bar)| Pt(s) (iii) Sn(s)|Sn 2+ (0.050 M)||H + (0.020 M)|H2(g) (1 bar)|Pt(s) (iv) Pt(s)|Br–(0.010 M)|Br 2 (l )||H + (0.030 M)| H 2 (g) (1 bar)|Pt(s)
(i) For the given reaction, the Nernst equation can be given as
= 2.7 − 0.02955
= 2.67 V (approximately)
(ii) For the given reaction, the Nernst equation can be given as
= 0 – ( – 0.14) – \(\begin{array}{l}\frac{0.0591}{n}log\frac{0.050}{(0.020)^{2}}\end{array} \)
= 0.52865 V
= 0.53 V (approximately)
(iii) For the given reaction, the Nernst equation can be given as
= 0 – ( – 0.14) – \(\begin{array}{l}\frac{0.591}{2}log\frac{0.050}{(0.020)^2}\end{array} \)
= 0.14 − 0.0295 × log125
= 0.14 − 0.062
= 0.08 V (approximately)
(iv) For the given reaction, the Nernst equation can be given as
= 0 – 1.09 – \(\begin{array}{l}\frac{0.591}{2}log\frac{1}{(0.010)^2(0.030)^2}\end{array} \)
= -1.09 – 0.02955 x \(\begin{array}{l}log\frac{1}{0.00000009}\end{array} \)
= -1.09 – 0.02955 x \(\begin{array}{l}log\frac{1}{9\times 10^{-8}}\end{array} \)
= -1.09 – 0.02955 x \(\begin{array}{l}log{ (1.11 \times 10^{7} )}\end{array} \)
= -1.09 – 0.02955 x (0.0453 + 7)
= -1.09 – 0.208
In the button cells widely used in watches and other devices, the following reaction takes place:

Determine ∆ r GJ and EJ for the reaction.
We know that,
= −2 × 96487 × 1.04
= −213043.296 J
= −213.04 kJ
Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
The conductivity of a solution is defined as the conductance of a solution of 1 cm in length and area of cross-section 1 sq. cm. Specific conductance is the inverse of resistivity, and it is represented by the symbol κ. If ρ is resistivity, then we can write
At any given concentration, the conductivity of a solution is defined as the unit volume of solution kept between two platinum electrodes with the unit area of the cross-section at a distance of unit length.
When concentration decreases, there will be a decrease in Conductivity. It is applicable for both weak and strong electrolytes. This is because the number of ions per unit volume that carry the current in a solution decreases with a decrease in concentration.
Molar conductivity –
The molar conductivity of a solution at a given concentration is the conductance of volume V of a solution containing 1 mole of the electrolyte, kept between two electrodes with the area of cross-section A and distance of unit length.
Now, l = 1 and A = V (volume containing 1 mole of the electrolyte)
Molar conductivity increases with a decrease in concentration. This is because the total volume V of the solution containing one mole of the electrolyte increases on dilution. The variation of \(\begin{array}{l}\Lambda_m\end{array} \) with \(\begin{array}{l}\sqrt{c}\end{array} \) for strong and weak electrolytes is shown in the following plot :

The conductivity of the 0.20 M solution of KCl at 298 K is 0.0248 S cm –1 . Calculate its molar conductivity.
Given, κ = 0.0248 S cm −1 c
Molar conductivity, \(\begin{array}{l}\Lambda_m = \frac{k \times 1000}{c}\end{array} \) \(\begin{array}{l}= \frac{0.0248 \times 1000}{0.2}\end{array} \)
= 124 Scm 2 mol -1
The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if the conductivity of 0.001M KCl solution at 298 K is 0.146 × 10 –3 S cm –1
Conductivity, k = 0.146 × 10 −3 S cm−1
Resistance, R = 1500 Ω
Cell constant = k × R
= 0.146 × 10 −3 × 1500
= 0.219 cm −1
The conductivity of sodium chloride at 298 K has been determined at different concentrations, and the results are given below.
Concentration/M 0.001 0.010 0.020 0.050 0.100
10 2 × k/S m −1 1.237 11.85 23.15 55.53 106.74
Calculate Λm for all concentrations and draw a plot between Λm and c½. Find the value of 0 Λ m.
κ = 1.237 × 10 −2 S m−1, c = 0.001 M
Then, κ = 1.237 × 10 −4 S cm −1 , c 1⁄2 = 0.0316 M 1/2
= 123.7 S cm 2 mol −1
κ = 11.85 × 10 −2 S m −1 , c = 0.010M
Then, κ = 11.85 × 10 −4 S cm −1 , c 1⁄2 = 0.1 M 1/2
= 118.5 S cm 2 mol −1
κ = 23.15 × 10 −2 S m −1 , c = 0.020 M
Then, κ = 23.15 × 10 −4 S cm −1 , c 1/2 = 0.1414 M 1/2
= 115.8 S cm 2 mol −1
κ = 55.53 × 10 −2 S m −1 , c = 0.050 M
Then, κ = 55.53 × 10 −4 S cm −1 , c 1/2 = 0.2236 M 1/2
= 111.1 1 S cm 2 mol −1
κ = 106.74 × 10 −2 S m −1 , c = 0.100 M
Then, κ = 106.74 × 10 −4 S cm −1 , c 1/2 = 0.3162 M 1/2
= 106.74 S cm 2 mol −1
Now, we have the following data:

Since the line interrupts \(\begin{array}{l}\Lambda_m\end{array} \) at 124.0 S cm 2 mol −1 , \(\begin{array}{l}\Lambda^0_m\end{array} \) = 124.0 S cm 2 mol −1
The conductivity of 0.00241 M acetic acid is 7.896 × 10 –5 S cm –1 . Calculate its molar conductivity. If 0 Λ m for acetic acid is 390.5 S cm 2 mol –1 , what is its dissociation constant?
Given, κ = 7.896 × 10 −5 S m −1 c
= 0.00241 mol L −1
Then, molar conductivity, \(\begin{array}{l}\Lambda_m = \frac{k}{c}\end{array} \)
= \(\begin{array}{l}\frac{7.896 \times 10^{-5} S cm^{-1}}{0.00241 \; mol \; L^{-1}}\times \frac{1000 cm^3}{L}\end{array} \)
= 32.76S cm 2 mol −1
= \(\begin{array}{l}= \frac{32.76 \; S\; cm^2 \; mol^{-1} }{390.5 \; S\; cm^2 \; mol^{-1} }\end{array} \)
Dissociation constant, \(\begin{array}{l}K_a = \frac{c\alpha^2}{(1-\alpha)}\end{array} \)
= \(\begin{array}{l}\frac{ ( 0.00241 \; mol \; L^{-1} )( 0.084 )^2}{ ( 1 – 0.084 ) }\end{array} \)
= 1.86 × 10 −5 mol L −1
How much charge is required for the following reductions? (i) 1 mol of Al 3+ to Al (ii) 1 mol of Cu 2+ to Cu (iii) 1 mol of MnO 4 – to Mn 2+
Ans :
(i) \(\begin{array}{l}Al^{3+} + 3e^- \rightarrow Al\end{array} \)
Required charge = 3 F
= 3 × 96487 C
(ii) \(\begin{array}{l}Cu^{2+} + 2e^- \rightarrow Cu\end{array} \)
Required charge = 2 F
= 2 × 96487 C
(iii) \(\begin{array}{l}MnO^-_4 \rightarrow Mn^{2+}\end{array} \)
i.e \(\begin{array}{l}Mn^{7+} + 5e^-\rightarrow Mn^{2+}\end{array} \)
Required charge = 5 F
= 5 × 96487 C
How much electricity in terms of Faraday is required to produce (i) 20.0 g of Ca from molten CaCl 2 ? (ii) 40.0 g of Al from molten Al 2 O 3 ?
(i) From the given data,
Electricity required to produce 40 g of calcium = 2 F
Therefore, electricity required to produce 20 g of calcium = (2 x 20 )/ 40 F
(ii) From the given data,
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al = ( 3 x 40 )/27 F
How much electricity is required in coulomb for the oxidation of (i) 1 mol of H 2 O to O 2 ? (ii) 1 mol of FeO to Fe 2 O 3 ?
(i) From the given data,
We can say that
Electricity required for the oxidation of 1 mol of H 2 O to O 2 = 2 F
Electricity required for the oxidation of 1 mol of FeO to Fe 2 O 3 = 1 F
A solution of Ni(NO 3 ) 2 is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Current = 5A
Time = 20 × 60 = 1200 s
Charge = current × time
According to the reaction,
Nickel deposited by 2 × 96487 C = 58.71 g
Therefore, nickel deposited by 6000 C = \(\begin{array}{l}\frac{58.71 \times 6000}{2 \times 96487}g\end{array} \)
Hence, 1.825 g of nickel will be deposited at the cathode.
Three electrolytic cells, A, B, and C, containing solutions of ZnSO 4 , AgNO 3 and CuSO 4 , respectively, are connected in series. A steady current of 1.5 amperes was passed through them until 1.45 g of silver was deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
i.e., 108 g of Ag is deposited by 96487 C.
Therefore, 1.45 g of Ag is deposited by = \(\begin{array}{l}\frac{96487\times 1.45}{107}C\end{array} \)
= 1295.43 C
Current = 1.5 A
Time = 1295.43/ 1.5 s
= 14.40 min
i.e., 2 × 96487 C of charge deposit = 63.5 g of Cu
Therefore, 1295.43 C of charge will deposit \(\begin{array}{l}\frac{63.5 \times 1295.43}{2 \times 96487}\end{array} \)
= 0.426 g of Cu
i.e., 2 × 96487 C of charge deposit = 65.4 g of Zn
Therefore, 1295.43 C of charge will deposit \(\begin{array}{l}\frac{65.4 \times 1295.43}{2 \times 96487}\end{array} \)
= 0.439 g of Zn
Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible. (i) Fe 3+ (aq) and I – (aq) (ii) Ag + (aq) and Cu(s) (iii) Fe 3+ (aq) and Br – (aq) (iv) Ag(s) and Fe 3+ (aq) (v) Br 2 (aq) and Fe 2+ (aq)

E 0 is positive; hence, the reaction is feasible.

E 0 is negative; hence, the reaction is not feasible.

Predict the products of electrolysis in each of the following. (i) An aqueous solution of AgNO 3 with silver electrodes (ii) An aqueous solution of AgNO 3 with platinum electrodes (iii) A dilute solution of H 2 SO 4 with platinum electrodes (iv) An aqueous solution of CuCl 2 with platinum electrodes
(i) At the cathode,
The following reduction reactions compete to take place at the cathode.
The reaction with a higher value of E 0 takes place at the cathode. Therefore, the deposition of silver will take place at the cathode.
At the anode,
The Ag anode is attacked by \(\begin{array}{l}NO^+_3\end{array} \) ions. Therefore, the silver electrode at the anode dissolves in the solution to form Ag + .
(ii) At the cathode,
Since Pt electrodes are inert, the anode is not attacked by \(\begin{array}{l}NO^+_3\end{array} \) ions. Therefore, OH − or \(\begin{array}{l}NO^+_3\end{array} \) ions can be oxidised at the anode. But OH − ions have a lower discharge potential and get preference and decompose to liberate O 2 .
(iii) At the cathode, the following reduction reaction occurs to produce H 2 gas.
At the anode, the following processes are possible.
For dilute sulphuric acid, reaction (i) is preferred to produce O 2 gas. But for concentrated sulphuric acid, reaction (ii) occurs.
(iv) At the cathode,
The reaction with a higher value takes place at the cathode. Therefore, the deposition of copper will take place at the cathode.
The following oxidation reactions are possible at the anode.
At the anode, the reaction with a lower value of E 0 is preferred. But due to the overpotential of oxygen, Cl − gets oxidised at the anode to produce Cl 2 gas.
Electrochemistry is the branch of chemistry that deals with the relationship between chemical energy and electrical energy produced in a redox reaction and how they can be converted into each other. NCERT Solutions for Class 12 are prepared by the best subject experts. In essence, these solutions can be useful for those students preparing for Class 12 exams , JEE Advance and other medical entrance exams. Students can successfully answer the numerical problems based on electrochemistry by downloading the free PDF.
Class 12 NCERT Solutions for Chemistry Chapter 3 Electrochemistry
Chapter 3 Electrochemistry of Class 12 Chemistry, is prepared as per the CBSE Syllabus for 2023-24. Class 12 Chemistry NCERT Solutions for Chapter 3 – Electrochemistry have been designed to help the students prepare well and score good marks in the CBSE Class 12 Chemistry exam. Further, the solutions consist of well thought and structured questions, along with detailed explanations, to help students learn and remember concepts easily.
Subtopics for Class 12 Chemistry Chapter 3 – Electrochemistry
- Electrochemical Cells
- Measurement of Electrode Potential
- Equilibrium Constant from Nernst Equation
- Electrochemical Cell and Gibbs Energy of Reaction
- Measurement of the Conductivity of Ionic Solutions
- Variation of Conductivity and Molar Conductivity with Concentration
- Products of Electrolysis
- Primary Batteries
- Secondary Batteries
After studying Electrochemistry Class 12 important textbook questions solutions , students will be able to describe an electrochemical cell and differentiate between galvanic and electrolytic cells. They will also study the application of the Nernst equation for calculating the emf of a galvanic cell and define the standard potential of the cell.
This chapter has derivations of the relation between the standard potential of the cell, Gibbs energy of cell reaction and its equilibrium constant. This solution will give the definition of resistivity (p), conductivity (K) and molar conductivity ( Am) of ionic solutions; differentiate between ionic (electrolytic) and electronic conductivity; describe the method for measurement of conductivity of electrolytic solutions and calculation of their molar conductivity; justify the variation of conductivity and molar conductivity of solutions with change in their concentration and define Aom (molar conductivity at zero concentration or infinite dilution); enunciate Kohlrausch law and learn its applications; understand quantitative aspects of electrolysis; describe the construction of some primary and secondary batteries and fuel cells and explain corrosion as an electrochemical process.
About BYJU’S NCERT Solutions
BYJU’S provides a comprehensive set of NCERT Solutions for students that have been designed to offer several benefits to the users. BYJU’S solutions offer the opportunity to learn NCERT Class 12 Chemistry syllabus including Chapter 3 from anywhere and within the comfort zone of the students. Students can learn, practise and revise the different chemistry chapters’ topics right from their homes or from any place. NCERT Solutions for Class 12 Chemistry Chapter 3 are easily accessible, and students can view the solutions right on the website, or they can download and use BYJU’S – The Learning App for a more enhanced learning experience.
With these NCERT Solutions for Class 12 , students can easily customise how they learn. Apart from these NCERT Solutions, BYJU’S also has the best subject experts who can guide students to learn the concepts in a more simple and precise manner. Further, if students come across any doubts or queries while going through the Class 12 Chemistry NCERT Solutions , they can always approach BYJU’S responsive support team to clear all their doubts. Besides, BYJU’S keeps track of all the progress that students make and offers feedback, as well as counselling via periodic assessments. Moreover, students can bring in all their queries regarding Chemistry, and other subjects, including Physics, Biology and Maths.
Frequently Asked Questions on NCERT Solutions for Class 12 Chemistry Chapter 3
Explain the concept of electrochemistry discussed in chapter 3 of ncert solutions for class 12 chemistry., how should i prepare chapter 3 of ncert solutions for class 12 chemistry for the board exams, why should i download the ncert solutions for class 12 chemistry chapter 3 pdf from byju’s, leave a comment cancel reply.
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Counselling

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- DK Goel Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- ML Aggarwal Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- HOTS Question
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Crash Course
- JEE Previous Year Paper
- Important Info
- JEE Mock Test
- JEE Sample Papers
- SRM-JEEE Mock Test
- VITEEE Mock Test
- BITSAT Mock Test
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- COMEDK Previous Year Paper
- GUJCET Previous Year Paper
- KCET Previous Year Paper
- KEAM Previous Year Paper
- Manipal Previous Year Paper
- MHT CET Previous Year Paper
- WBJEE Previous Year Paper
- AMU Previous Year Paper
- TS EAMCET Previous Year Paper
- SRM-JEEE Previous Year Paper
- VITEEE Previous Year Paper
- BITSAT Previous Year Paper
- UPSEE Previous Year Paper
- CGPET Previous Year Paper
- CUSAT Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
Electrochemistry Class 12 Important Questions with Solutions
Free pdf download.
SHARING IS CARING If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
Chemistry topic Electrochemistry is an important lesson for students to master because various questions are asked from it. However, for assistance in CBSE Class 12 Chemistry board exam preparation here on this page, we have shared the direct link of Electrochemistry Class 12 Important Questions with Solutions.
Along with the PDF link, we have also shared how the important questions on Electrochemistry play an important role.
Why Solve Electrochemistry Class 12 Important Questions?
Due to various reasons, Class 12 students should be thorough with Electrochemistry Class 12 important questions. A few reasons to solve CBSE Class 12 Chemistry Electrochemistry important questions are -
- To Enahce Grip on Electrochemistry: To develop a deeper-level understanding of Electrochemistry students should solve important questions on Class 12 Chemistry Electrochemistry. The important questions are generally considered a good level of question that enable students to use all of their acquired Chemistry knowledge to solve the questions.
- To Practise Numerous Board-Level Chemistry Questions: There is nothing better than solving actual board-level questions and therefore, CBSE Class 12 students should solve the PDF file of Electrochemistry Class 12 Important Questions with Solutions because solving the PDF file will help students to not only practise numerous board-level Chemistry questions but to get instant answers too.
- For Boosting Confidence: Being able to solve actual board-level questions give students confidence, at the same time students refer to the Electrochemistry Class 12 Important Questions with Solutions are able to boost their confidence. Overall, we can say that solving the important Class 12 Chemistry questions using the answers PDF helps students to level up their board exam preparation and gives them the confidence to answer questions in the actual board examination.
- To Prepare for Class 12 Electrochemistry Important Questions: The Electrochemistry important questions are a collection of those questions which have a higher possibility to be asked in the upcoming CBSE Class 12 Chemistry examination. However, students solving the important questions on this topic will be able to better prepare for the Class 12 Chemistry exam.
Features of CBSE Class 12 Chemistry Electrochemistry Class 12 Important Questions PDF
In this section, we are discussing the features of Class 12 Chemistry Important Questions PDF that will help students like you to better understand why the PDF provided here is the best to use.
- All Types of Questions: One mark to 5 marks all types of questions are mentioned in the PDF file of Electrochemistry Class 12 Important Questions with Solutions.
- Chemistry Electrochemistry Questions with Answers: The subject experts have answered the Chemistry Electrochemistry important questions so, the solutions are 100% accurate and can be used by students to solve their unsolved questions.
- Diagrams are Used: Chemistry has lots of diagrams, images and graphics that play a crucial role in understanding the concepts and questions. Therefore, the subject matter experts have included the images and diagram in Electrochemistry Class 12 Important Questions with Solutions PDF.
- Prepared by Chemistry Experts: To maintain the accuracy and authenticity of Class 12 Electrochemistry Important Questions and its solutions PDF, the subject experts have prepared the PDF file’s content.
How to Download Class 12 Electrochemistry Important Questions with Answers?
Here’s a step-by-step process to download the Class 12 Electrochemistry Important Questions with Answers PDF.
- Go to Selfstudys.com using an internet browser
- After reaching the homepage of Selfstudys.com click on the navigation icon/button
- A side pop-up bar will appear, navigate CBSE and click on that
- It will bring more options to choose from, click on the previous year question paper
- A new page will load, click on “12th PYP Chapter Wise”
- Scroll the page a little and click on Chemistry then find the PDF file of Electrochemistry Class 12 Important Questions with Solutions.
How to Use Important Questions for Class 12 Chemistry Electrochemistry PDF?
One can use important questions for Class 12 Chemistry Electrochemistry PDF in whatever way they want, however, there are certain ways that we think are a good way to utilise the important questions for Class 12 Chemistry Electrochemistry PDF.
- To Revise Important Portions of Electrochemistry: The PDF file of Class 12 Chemistry Electrochemistry can be a great study tool to revise the concepts and subtopics discussed in this lesson. Furthermore, the students can also use the same PDF file to recall the questions based on the Electrochemistry.
- To Do Last-Minute Exam Preparation: Students preparing for the CBSE Class 12 Board exam and are in the last-minute exam preparation process can use the PDF file of Electrochemistry important questions undoubtedly.
- Find Weakness to Improve: The Electrochemistry important question class 12 PDF can be a great study resource to find weak areas in this lesson. After finding those weaker areas students can use the answers provided to improve the errors and to learn something new. Students can also ask their elders, teachers or tutors to help them with the Electrochemistry important questions to master them.
When to Use Class 12 Electrochemistry Important Questions with Solutions PDF?
Once a Class 12 student has studied their Electrochemistry lesson completely can go for the important questions to practise. However, there are a few more times when a CBSE Class 12 student can utilise the PDF file of Electrochemistry Class 12 Important Questions with Solutions
- When Doing Board Exam Preparation: One can refer to the Class 12 Chemistry Electrochemistry important questions when they are doing the board exam preparation. At the time of preparation, referring to the PDF file is very helpful because it helps students better understand the types of questions that are asked in the CBSE Class 12 Chemistry Board examination. Also, solving the Class 12 Electrochemistry important questions during board exam preparation helps students refresh their answering methods for a variety of questions that can be asked from the chapter Electrochemistry.
- While Solving Chapter-End Questions: One of the best times to use the PDF file of CBSE Class 12 Chemistry Electrochemistry is when solving the Chapter-end questions. It is the best time because at this time students have a very fresh knowledge of Electrochemistry and therefore, they would be able to answer most of the important questions. However, if one struggles to answer the important questions given in the PDF file, one would be required to read the same chapter again to strengthen their grip.
Precautions to Take When Using Electrochemistry Class 12 Important Questions with Solutions PDF
There are certain points that students should take care of when using Electrochemistry Class 12 Important Questions PDF. Those points are mentioned below:
- Read the Questions Carefully: It is essential to read the questions carefully to better understand the Electrochemistry Class 12 Important Questions and to answer them. Reading the questions carefully, ease the process of answering questions.
- Answer Questions Based on the Exam Pattern: The PDF file of CBSE Class 12 Chemistry Electrochemistry important questions contains details on whether a particular question is one word or long answer types of questions. So, it is advised students answer Electrochemistry Class 12 Important Questions following the exam pattern or as directed in the PDF file.
- Don’t Completely Rely on Electrochemistry Class 12 Important Questions with Solutions PDF: No doubt, Electrochemistry important question Class 12 is a great study tool and it can help students better prepare for the board examination of Chemistry, however, students should make sure not to be dependent on the PDF file of Electrochemistry important question class 12.
How Electrochemistry Class 12 Important Questions with Solutions PDF are Prepared?
Various study materials such as previous year question papers, sample question papers, CBSE Class 12 Chemistry Syllabus and several other study resources are used to prepare the Electrochemistry Class 12 Important Questions with Solutions PDF.
- Using Previous Year Question Papers of Class 12 Chemistry: One of the ideal study materials that are used to prepare the CBSE Class 12 Chemistry Electrochemistry Important Questions are the sets of Previous Year Question Papers. It helps subject experts to find and match the most frequent and high-scoring Electrochemistry questions to add them to the PDF file.
- Taking Help of Class 12 Chemistry Syllabus: The Class 12 Chemistry Syllabus also plays an important role because it helps students better predict the important questions of Electrochemistry. It is possible because the syllabus contains a complete detail of the Electrochemistry weightage for the board examination.
How to Deal With CBSE Class 12 Electrochemistry Important but Difficult Questions?
While solving the CBSE Class 12 Electrochemistry important questions if a student feels stuck, they can use the following methods to deal with them.
- Use the Answers Provided in PDF: The PDF file of Electrochemistry Class 12 Important Questions with Solutions contains very accurate answers prepared by subject experts. The answers are easier to understand too so when feeling stuck at some Electrochemistry questions refer to the answers available in the PDF.
- Consult Teachers/Tutors: If provided answers don’t help much to the students then they should consult teachers or tutors for help. They will guide them in understanding the questions and answering them with ease.
- Use the Internet: If above mentioned things don’t help much the Internet will surely help students to understand and answers the important questions of Class 12 Electrochemistry. There are several resources on the internet that can help students better prepare for the exam.

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.


IMAGES
VIDEO
COMMENTS
The following questions are multiple choice questions. Choose the most appropriate answer. (i) At infinite dilution, the equivalent conductance of CaSO 4 is. (a) 256 x 10-4. (b) 279. (c) 23.7. (d) 2.0 x 10- 8. (ii) If the degree of dissociation of CaSO 4 solution is 10% then equivalent conductance of CaSO 4 is.
There is Case Study Questions in class 12 Chemistry in session 2020-21. For the first time, the board has introduced the case study questions in the board exam. The first two questions in the board exam question paper will be based on Case Study and Assertion & Reason. The first question will have 5 MCQs … Continue reading Case Study Questions for Class 12 Chemistry Chapter 3 Electrochemistry
Download the PDF now and boost your chemistry knowledge! Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th. You need to improve your preparation for the Class 12 Chemistry Case Study Questions exams if you want to achieve a 95+% on the boards.
The PDF file of the Electrochemistry Case Study for Class 12 Chemistry with Solutions is a very important study resource that can help students better prepare for the exam and boost conceptual learning. The solutions are in the hint manner as well as contain full examples too, refer to the link to access the Case Study on Electrochemistry Class ...
Important Questions, MCQ's, NCERT Solutions - Class 12 Chemistry . Get here all the Important questions for Class 12 Chemistry chapter wise as free PDF download. Here you will get Extra Important Questions with answers, Numericals and Multiple Choice Questions (MCQ's) chapter wise in Printable format. Solving Chapter wise questions is one of the best ways to prepare for the examination.
This will help them to understand the type of Case Study questions that can be asked in Grade 12 Chemistry examinations. Our expert faculty for standard 12 Chemistry have designed these questions based on the trend of questions that have been asked in last year's exams. The solutions have been designed in a manner to help the grade 12 ...
However, our subject matter experts have given the solutions of all the Chemistry Case Study for Class 12 Chemistry questions. Passage Based Class 12 Chemistry Case Study Questions in PDF. CBSE Class 12 Case studies are known as Passage Based Questions. These types of problems usually contain a short/long paragraph with 4 to 5 questions.
Class 12th Chemsitry - Electrochemistry Case Study Questions and Answers 2022 - 2023 - Complete list of 12th Standard CBSE question papers, syllabus, exam tips, study material, previous year exam question papers, centum tips, formula, answer keys, solutions etc..
Electrochemistry Class 12 Chemistry MCQs. 1. A new galvanic cell of E ext more than E o (1.1 v)of Daniel cell is connected to Daniel cell in a manner that new cell gives electrons to Zn, what will happen (a) E cell will increase (b) E cell will decrease (c) No change will take place (d) Daniel cell will work as electrolytic cell where Zn will be deposite on zinc rod and copper will dissolve ...
Class 12 Chemistry Case Study Questions for Term 1 exam includes The Solid State, The P block elements, Haloalkanes and Haloarenes, Biomolecules, etc. Questions for all these chapters are given in the PDF file that are available here for free to download. Term 1 exam is about to be held in November-December this year.
Solution: (a) Both assertion and reason are true and the reason is correct explanation for assertion. 4. Assertion : Λ m for weak electrolytes shows a sharp increase when the electrolytic solution is diluted. Reason : For weak electrolytes degree of dissociation increases with dilution of solution.
Electrochemistry Class 12 Notes Chapter 3. According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 2. A device that generates a potential difference between electrodes by chemical reactions is called an electrochemical cell. It comprises two electron conductors, which are separated by an ionic conductor and are ...
Install Now. CBSE will ask Case Study Questions class 12 Chemistry in session 2020-21. These will be the first two questions in the board exam question paper. The first question will have 5 MCQs out of which students will attempt any 4 questions. The second question will carry 5 Assertion & Reason type questions with the choice to attempt any four.
Electrochemistry Notes for CBSE Class 12 Chemistry Chapter 3 - Free PDF Download. Electrochemistry is a vital section of chemistry that determines the function of electrodes and reactors. Vedantu's Electrochemistry notes class 12 tries to situate the ideas behind the chemical reactions. An electrochemical cell is a tool that produces the ...
May 18, 2021 Physics Gurukul Leave a Comment on Case Study Questions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids. ... Case Study Questions for Class 12 Chemistry Chapter 3 Electrochemistry. March 17, 2021 May 17, ...
After studying Electrochemistry Class 12 important textbook questions solutions, students will be able to describe an electrochemical cell and differentiate between galvanic and electrolytic cells. They will also study the application of the Nernst equation for calculating the emf of a galvanic cell and define the standard potential of the cell.
Electrochemistry is all about electricity and chemistry. Yay, fun stuff! In this unit we will explore how different batteries work, the kind of chemical reactions that go on inside, and how we measure/define their potentials! ... Class 12 Chemistry (India) 9 units · 66 skills. Unit 1. The Solid State. Unit 2. Electrochemistry. Unit 3. Chemical ...
Electrochemistry is the branch of chemistry which deals with the relationship between electrical energy and chemical energy and inter-conversion of one form into another. 2. An electrochemical cell consists of two metallic electrodes dipped in electrolytic solutions. The cells are of two types: 3. A galvanic cell consists of two half cells.
Electrochemistry is the study of how electrical energy is used to create non-spontaneous chemical processes. We will also be discussing the fundamentals of electrochemistry and the types of electrochemistry. ... Get all the important information related to the CBSE Class 12 Examination including the process of application, important calendar ...
Follow the below given simple steps to know how to download CBSE Case Study of Class 12:-. Open Selfstudys website in your browser. Go to the navigation menu that look like this. Now, click on CBSE and then Case Study respectively. A new page will open, where you have to click on "Class 12".
Here's a step-by-step process to download the Class 12 Electrochemistry Important Questions with Answers PDF. Go to Selfstudys.com using an internet browser. After reaching the homepage of Selfstudys.com click on the navigation icon/button. A side pop-up bar will appear, navigate CBSE and click on that.