Clinical Research Coordinator Jobs in Middle East
- Create a job alert for similar positions

Clinical Research Associate Manager
Senior clinical research associate, clinical research associate, copy rights specialist - 0424-ch-105.
- Build your CV in just a few clicks
Graphics Coordinator
Acceptance coordinator, clinical research associate ii, vendor management coordinator, clinical trial coordinator, project support coordinator ii, logistics coordinator / clinical trial coordinator, field coordinator, department of medicine, risk management coordinator, books risk management, risk mgmt coordinator-arabic, kindle-brm, nurse midwife, clinical research associate (biopharma), people also search for....
- root Jobs in Türkiye
- root Jobs in India
- Clinical Research Coordinator Jobs

Clinical Research Coordinator Average Salary in Egypt 2024
How much money does a person working as clinical research coordinator make in egypt.

A person working as Clinical Research Coordinator in Egypt typically earns around 9,640 EGP . Salaries range from 5,210 EGP (lowest) to 14,600 EGP (highest).
Salary Variance
This is the average salary including housing, transport, and other benefits. Clinical Research Coordinator salaries in Egypt vary drastically based on experience, skills, gender, or location. Below you will find a detailed breakdown based on many different criteria.
Clinical Research Coordinator Pay Scale and Salaries in Egypt

Salary Structure and Pay Scale Comparison
Median salary, maximum and minimum salary, minimum wage, starting salary, and the salary range, salary range, minimum wage, and starting salary.
Salaries for the position Clinical Research Coordinator in Egypt range from 5,210 EGP (starting salary) to 14,600 EGP (maximum salary). It should be noted that the given figure is not the legally mandated minimum wage; rather, it represents the lowest figure reported in a salary survey that included thousands of participants and professionals from all regions of the country.
Median Salary
With a median salary of 8,610 EGP , half of the professionals who work as Clinical Research Coordinator in Egypt earn less than this amount, and the other half earn more. The median salary denotes the middle value of salaries. Ideally, you would want to belong to the group earning more than the median salary, located on the right side of the salary distribution graph.
Percentiles and Salary Scale
The median is closely associated with two other values known as the 25th and 75th percentiles. By examining the salary distribution chart, it can be determined that 25% of professionals employed as Clinical Research Coordinator in Egypt earn less than 7,210 EGP, while 75% earn more. Similarly, the chart shows that 75% earn less than 9,730 EGP while 25% earn more.
Pay Scale Structure
To provide a better understanding of expected salaries, we categorized the frequently occurring salaries into different ranges. This approach provides a more precise representation of salary distribution for the job title Clinical Research Coordinator in Egypt compared to simply calculating the average. The majority of reported salaries, approximately 65%, fall within the range of 5,720 EGP to 7,690 EGP. About 20% of salaries are below the 5,720 EGP mark, while 10% fall within the range of 7,690 EGP to 8,590 EGP. Only 5% of individuals have salaries exceeding 8,590 EGP.
Salary Comparison by Years of Experience / Clinical Research Coordinator / Egypt
How do experience and age affect pay.
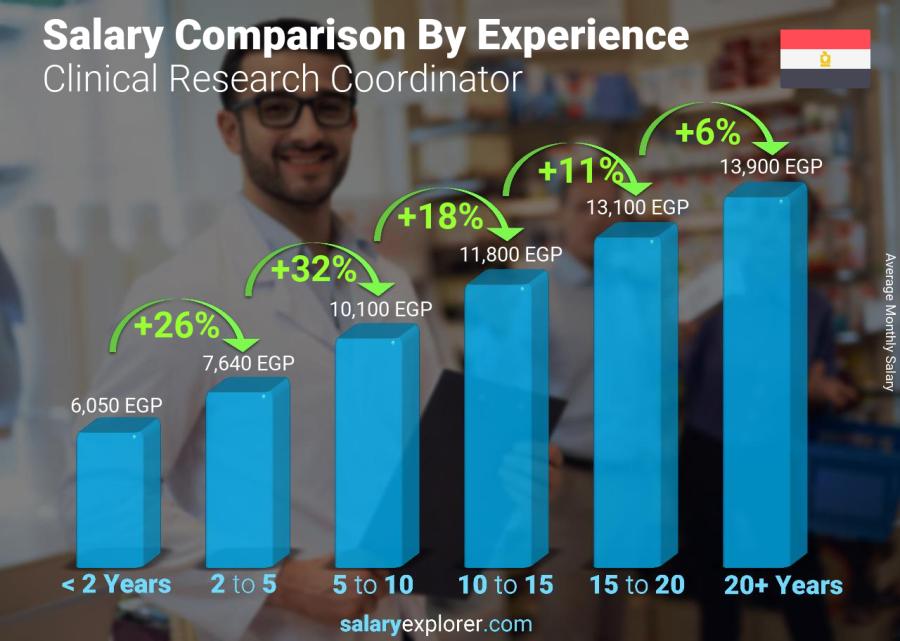
The experience level is the most important factor in determining the salary. Naturally, the more years of experience the higher the wage. We broke down salaries by experience level for people working as Clinical Research Coordinator and this is what we found.
Employees with less than two years of experience earn approximately 6,050 EGP.
While someone with an experience level between two and five years is expected to earn 7,640 EGP, 26% more than someone with less than two year's experience.
Moving forward, an experience level between five and ten years lands a salary of 10,100 EGP, 32% more than someone with two to five years of experience.
Additionally, professionals whose expertise span anywhere between ten and fifteen years get a salary equivalent to 11,800 EGP, 18% more than someone with five to ten years of experience.
If the experience level is between fifteen and twenty years, then the expected wage is 13,100 EGP, 11% more than someone with ten to fifteen years of experience.
Lastly, employees with more than twenty years of professional experience get a salary of 13,900 EGP, 6% more than people with fifteen to twenty years of experience.
Typical Salary Progress for Most Careers

Salary Comparison By Education / Clinical Research Coordinator / Egypt
How do education levels affect salaries, displayed below is the average salary variance between different education levels of professionals working as clinical research coordinator..
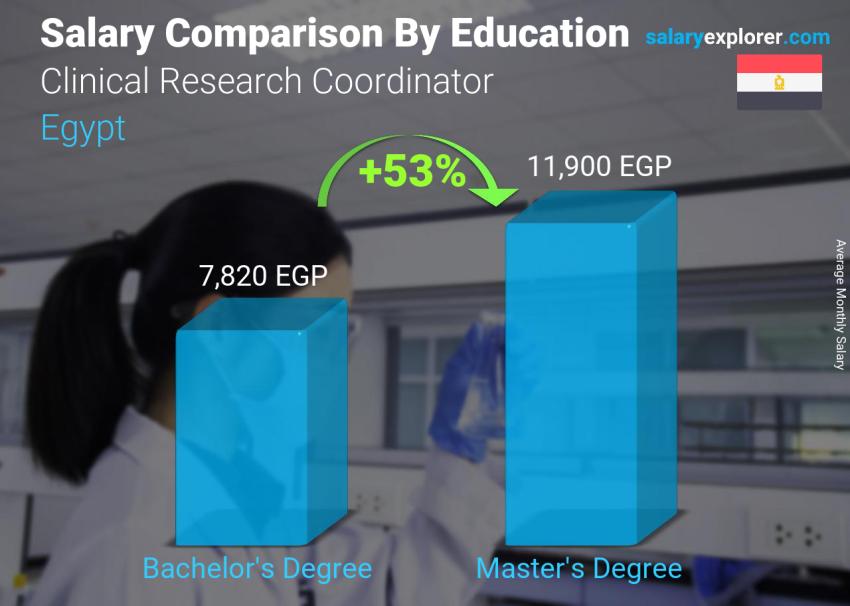
We all know that higher education equals a bigger salary, but how much more money can a degree add to your income? We broke down salaries by education level for the position Clinical Research Coordinator in order to make a comparison.
Level 1: Bachelor's Degree
Employees at this education level have an average salary of 7,820 EGP.
Level 2: Master's Degree
At this level, the average salary becomes 11,900 EGP, 53% more than the previous level.
Is a Master's degree or an MBA worth it? Should you pursue higher education?
A Master's degree program or any post-graduate program in Egypt costs anywhere from 46,200 EGP to 138,000 EGP and lasts approximately two years. That is quite an investment. You can't really expect any salary increases during the study period, assuming you already have a job. In most cases, a salary review is conducted once education is completed and the degree has been attained. Many people pursue higher education as a tactic to switch to a higher-paying job. The numbers seem to support the theory. The average increase in compensation while changing jobs is approximately 10% more than the customary salary increment. If you can afford the costs of higher education, the return on investment is definitely worth it. You should be able to recover the costs in roughly a year or so.
Typical Salary Difference by Education for Most Careers

Salary and Compensation Comparison By Gender / Clinical Research Coordinator / Egypt

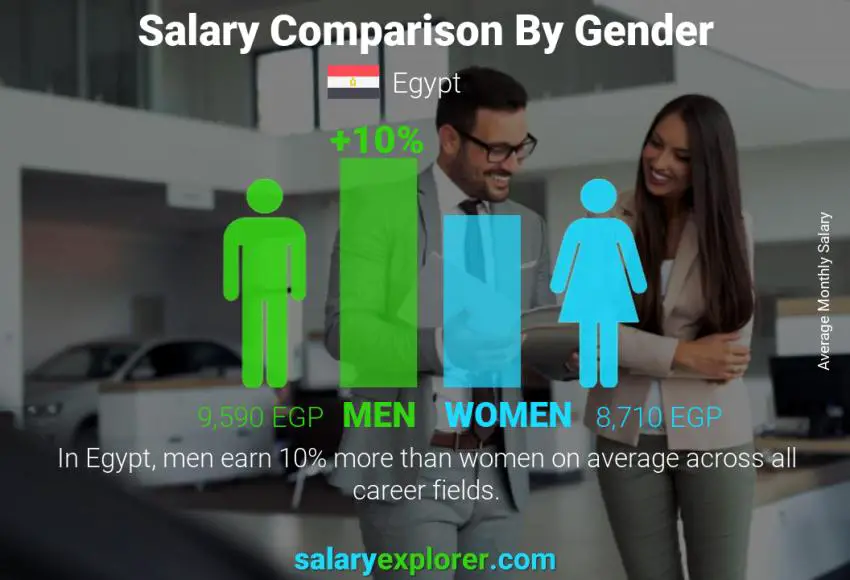
Though gender should not have an effect on pay, in reality, it does. So who gets paid more: men or women? For the people who work as Clinical Research Coordinator in Egypt, the average difference between the salary of male and female employees is 10%.
Salary Comparison By Gender in Egypt for all Careers
43 Careers That Pay Women More Than Men!
Average Annual Salary Increment Percentage / Clinical Research Coordinator / Egypt
How much are annual salary increments in egypt for individuals working as clinical research coordinator how often do employees get salary raises.
Individuals working as Clinical Research Coordinator in Egypt are likely to observe a salary increase of approximately 10% every 17 months. The national average annual increment for all professions combined is 9% granted to employees every 17 months.

Egypt / All Professions

The term Annual Salary Increase usually refers to the increase in 12 calendar month period, but because it is rare that people get their salaries reviewed exactly on the one-year mark, it is more meaningful to know the frequency and the rate at the time of the increase.
How to calculate the salary increment percentage?
The annual salary Increase in a calendar year (12 months) can be easily calculated as follows: Annual Salary Increase = Increase Rate x 12 / Increase Frequency
Worldwide Salary Raises: All Countries and All Jobs

Salary Packages and Schemes
Not all compensation increases are reflected directly in the salary. Some companies offer upgraded packages to their staff instead of cash money. The figures displayed here account only for direct increments to the base salary.
Bonus and Incentive Rates / Clinical Research Coordinator / Egypt
How much and how often are bonuses being awarded share this chart tweet get chart link http://www.salaryexplorer.com/charts/egypt/health-and-medical/healthcare-technical/clinical-research-coordinator/annual-salary-bonus-rate-egypt-clinical-research-coordinator.jpg 50% of surveyed staff reported that they haven't received any bonuses or incentives in the previous year while 50% said that they received at least one form of monetary bonus. those who got bonuses reported rates ranging from 4% to 5% of their annual salary. received bonus 50% no bonus 50% types of bonuses considered.
The most standard form of bonus, where the employee is awarded based on their exceptional performance.
Occasionally, some companies like to celebrate excess earnings and profits with their staff collectively in the form of bonuses that are granted to everyone. The amount of the bonus will probably be different from person to person depending on their role within the organization.
Granted upon achieving an important goal or milestone.
These types of bonuses are given without a reason and usually resemble an appreciation token.
Bonuses Are Not Commissions!
People tend to confuse bonuses with commissions. A commission is a prefixed rate at which someone gets paid for items sold or deals completed while a bonus is in most cases arbitrary and unplanned.
What makes a position worthy of good bonuses and a high salary?
Revenue generators usually get more and higher bonuses, higher salaries, and more frequent salary increments. The reason is quite simple: it is easier to quantify your value to the company in monetary terms when you participate in revenue generation.
Bonus Comparison by Seniority Level
Top management personnel and senior employees naturally exhibit higher bonus rates and frequencies than juniors. This is very predictable due to the inherent responsibilities of being higher in the hierarchy. People in top positions can easily get double or triple bonus rates than employees down the pyramid.
Average Hourly Wage / Clinical Research Coordinator / Egypt
The average hourly wage (pay per hour) for individuals working as clinical research coordinator in egypt is 56 egp.this is the rate they get paid for every worked hour., about the hourly pay rate.
The hourly wage is the salary paid in one worked hour. Usually, jobs are classified into two categories: salaried jobs and hourly jobs. Salaried jobs pay a fixed amount regardless of the hours worked. Hourly jobs pay per worked hour. To convert salary into hourly wage the above formula is used (assuming 5 working days in a week and 8 working hours per day which is the standard for most jobs). The hourly wage calculation may differ slightly depending on the worked hours per week and the annual vacation allowance. The figures mentioned above are good approximations and are considered to be the standard. One major difference between salaried employees and hourly paid employees is overtime eligibility. Salaried employees are usually exempt from overtime as opposed to hourly paid staff.
What is the minimum hourly rate of pay?
The minimum pay rate per hour for people working as Clinical Research Coordinator in Egypt is 30 EGP. This is the minimum as per the gathered data in the salary survey not the minimum hourly rate mandated by law.
Salary comparison with similar jobs

Salary Comparison By City
Government vs private sector salary comparison.
Where can you get paid more, working in a private company or the government? The difference between the public or government sector salaries and the private sector salaries in Egypt is 6% on average across all career fields.
Salary Statistics and Calculation Guide
What is considered to be a good and competitive salary for the job title clinical research coordinator in egypt.
A good and competitive compensation would range anywhere between 8,610 EGP and 9,730 EGP. This is a very rough estimate. Experience and education play a very huge part in the final earnings.
Gross Salary (before tax) and Net Salary (after tax)
All salary and compensation figures displayed here are gross salary figures, that is the salary before tax deductions. Because taxes may differ across sectors and locations, it is difficult to accurately calculate the net salary after tax for every career.
Base / Basic Salary
The base salary for a careers like Clinical Research Coordinator in Egypt ranges from 5,210 EGP to 7,210 EGP. The base salary depends on many factors including experience and education. It is not easy to provide a figure with very little information, so take this range with a grain of salt.
What is the difference between the median and the average salary?
Both are indicators. If your salary is higher than both the average and the median then you are doing very well. If your salary is lower than both, then many people earn more than you and there is plenty of room for improvement. If your wage is between the average and the median, then things can be a bit complicated. We wrote a guide to explain all about the different scenarios. How to compare your salary

©Salary Explorer 2024
- Mayo Clinic Careers
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Internal Medicine
- Lung Transplant
- Psychiatry & Psychology
- Nurse Practitioner & Physician Assistant
- Ambulance Service
- Clinical Labs
- Radiology Imaging
- Clinical Research Coordinator
- Respiratory Care
- Senior Care
- Surgical Services
- Travel Surgical Tech
- Practice Operations
- Administrative Fellowship Program
- Administrative Internship Program
- Career Exploration
- Nurse Residency and Training Program
- Nursing Intern/Extern Programs
- Residencies & Fellowships (Allied Health)
- Residencies & Fellowships (Medical)
- SkillBridge Internship Program
- Training Programs & Internships
- Diversity, Equity & Inclusion
- Employees with Disabilities
You're using Internet Explorer - therefore, some pages or features may not display properly. We recommend switching to a modern browser such as Chrome, Microsoft Edge, or Firefox for a smoother experience.
Search life-changing careers..
Search by Role or Keyword
Enter Location
- United States Applicants
- United Kingdom Applicants
- Current Employees
Clinical Research Coordinator – General Comprehensive
- Jacksonville, FL
Not ready to apply? Join our talent community
Receives direction from principal investigator, supervisor, or other staff involved in research protocol(s). Gives direction to and works cooperatively with other research staff. Collaborates with various departments within the institution. Works cooperatively with other investigators and personnel at all levels. Interacts with research participants, other research centers, and sponsoring companies to resolve problems and ensure efficient completion of research studies. Position Overview: Independently coordinates complex (i.e. interventional, therapeutic greater than minimal risk) clinical research protocols with minimal direction from the principal investigator and/or supervisor in compliance with regulatory laws and institutional guidelines. Collaborates with research team to assess feasibility and management of research protocols. Ensures implementation of research protocols after IRB approval and provides information as appropriate for progress reports. Screens, enrolls, and recruits research participants. Coordinates schedules and monitors research activities and subject participation. Identifies, reviews, and reports adverse events, protocol deviations, and other unanticipated problems appropriately. Manages, monitors, and reports research data to maintain quality and compliance. Provides education/training for others within the department. Performs administrative and regulatory duties related to the study as appropriate. Some travel may be required. ADDENDUM (if applicable) Protocol Development and Maintenance Activities Responsibilities may include, but are not limited to: ongoing management of the protocol document and process through editing, amendments, proofing, coordination of study logistics (i.e. blood collection kits, data collection booklets, use of CRU, etc.), and verification of content to meet institutional and federal standards; communication with study sites and/or federal agencies regarding study status changes; Federal and Institutional Review Board (IRB) document preparation and submission; and provides consultative expertise regarding regulatory and policy requirements. Accurately applies investigators' scientific data into a cohesive format for the protocol document and associated procedures that are consistent with internal and external policies and regulatory requirements. Participates in other protocol development activities and executes other assignments as warranted and assigned.
During the selection process you may participate in an OnDemand (pre-recorded) interview that you can complete at your convenience. During the OnDemand interview, a question will appear on your screen, and you will have time to consider each question before responding. You will have the opportunity to re-record your answer to each question – Mayo Clinic will only see the final recording. The complete interview will be reviewed by a Mayo Clinic staff member and you will be notified of next steps.
Minimum Education and/or Experience Required:
HS Diploma with at least 5 years of clinical research coordination/related experience OR Associate's degree/college Diploma/Certificate Program with at least 3 years of experience, Associate's in Clinical Research from an accredited academic institution without experience OR Bachelor's with at least 1 year of experience or completion of a Mayo Clinic-sponsored clinical research internship in lieu of 1 year of experience. Experience should be in the clinical setting or related experience. Additional Experience and/or Qualifications: Graduate or diploma from a study coordinator training program is preferred. One year of clinical research experience is preferred. Medical terminology course is preferred. Licensure/Certification Required: N/A
About our location
Jacksonville, Florida
We would love to connect with you.
Click the button for a list of our upcoming events.

Join Our Talent Community
Sign up, stay connected and get opportunities that match your skills sent right to your inbox
Email Address
Phone Number
Upload Resume/CV (Must be under 1MB) Remove
Job Category* Select One Advanced Practice Providers Business Education Engineering Executive Facilities Support Global Security Housekeeping Information Technology Internship Laboratory Nursing Office Support Patient Care - Other Pharmacy Phlebotomy Physician Post Doctoral Radiology Imaging Research Scientist Surgical Services Therapy
Location Select Location Albert Lea, Minnesota Arcadia, Wisconsin Austin, Minnesota Barron, Wisconsin Bloomer, Wisconsin Caledonia, Minnesota Cannon Falls, Minnesota Chippewa Falls, Wisconsin Decorah, Iowa Duluth, Minnesota Eau Claire, Wisconsin Fairmont, Minnesota Faribault, Minnesota Holmen, Wisconsin Jacksonville, Florida Kasson, Minnesota La Crosse, Wisconsin Lake City, Minnesota London, England Mankato, Minnesota Menomonie, Wisconsin Minneapolis-St. Paul-Bloomington, Minnesota New Prague, Minnesota Onalaska, Wisconsin Osseo, Wisconsin Owatonna, Minnesota Phoenix, Arizona Prairie du Chien, Wisconsin Red Wing, Minnesota Rice Lake, Wisconsin Rochester, Minnesota Saint Augustine, Florida Saint Cloud, Minnesota Saint James, Minnesota Saint Peter, Minnesota Scottsdale, Arizona Sparta, Wisconsin Waseca, Minnesota Zumbrota, Minnesota
Area of Interest Select One Nursing Research Laboratory Medicine & Pathology Radiology Licensed Practical Nurse (LPN) Pharmacy Facilities Surgery Physical Medicine & Rehabilitation Psychiatry & Psychology Neurology Cardiovascular Medicine Respiratory Therapy General Services Surgical Technician Emergency Medicine Finance Ambulance Services Anesthesiology & Perioperative Medicine Environmental Services Social Work Gastroenterology & Hepatology Mayo Collaborative Services Medical Oncology Global Security Orthopedics Radiation Oncology Cardiovascular Surgery Family Medicine Pediatrics Hospital Internal Medicine International Patient Scheduling Transplant Biochemistry & Molecular Biology Hematology Obstetrics & Gynecology Ophthalmology Administration Desk Operations Hospice & Palliative Care Information Technology Urology Critical Care General Internal Medicine Healthcare Technology Management Mayo Clinic Laboratories Nephrology & Hypertension Office Support Oncology Senior Care Artificial Intelligence & Informatics Community Internal Medicine Dermatology Digital Education Linen & Central Services Quality Endocrinology Engineering Otolaryngology (ENT) Clinical Genomics Pulmonary/Sleep Medicine Rheumatology Sports Medicine Cancer Center Epidemiology Immunology Infectious Diseases Molecular Medicine Primary Care Surgical Assistant Business Development Clinical Nutrition Development/Philanthropy Molecular Pharmacology & Experimental Therapeutics Neurologic Surgery Physiology & Biomedical Engineering Travel Clinical Trials & Biostatistics Dental Specialities Executive Office Health Care Delivery Research Health Information Management Services Informatics Information Security Neurosciences Regenerative Biotherapeutics Spiritual Care Volunteer Services Center for Individualized Medicine Communications Comparative Medicine Computational Biology Geriatric Medicine & Gerontology Human Resources Legal Mayo Clinic Platform Media Support Services Occupational/Preventative Medicine Pain Medicine Spine Center Women's Health
- Research, Jacksonville, Florida, United States Remove
Confirm Email
By submitting your information, you consent to receive email communication from Mayo Clinic.
Join our talent community.
Join our global talent community to receive alerts when new life-changing opportunities become available.

If you want to know what it's really like at Mayo Clinic, just ask. You'll find that our pride–in where we work, and in what we do–is a common trait. You will also find a lot of inspiring stories about lives changed for the better.
National Surgical Assistant Week
The Nurse Residency Program (NRP) is for all nurses with less than 12 months of experience, to be completed within the first year. NRP provides a framework for a successful transtion to a professional nurse by promoting educational and personal advancement.

As your career evolves, our compensation and benefits packages are designed to change with you — meeting needs now, and anticipating what comes next. We know that when Mayo Clinic takes care of you, you can take better care of our patients.
Equal opportunity
All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, gender identity, sexual orientation, national origin, protected veteran status, or disability status. Learn more about "EEO is the Law." Mayo Clinic participates in E-Verify and may provide the Social Security Administration and, if necessary, the Department of Homeland Security with information from each new employee's Form I-9 to confirm work authorization.
Reasonable accommodations
Mayo Clinic provides reasonable accommodations to individuals with disabilities to increase opportunities and eliminate barriers to employment. If you need a reasonable accommodation in the application process; to access job postings, to apply for a job, for a job interview, for pre-employment testing, or with the onboarding process, please contact HR Connect at 507-266-0440 or 888-266-0440.
Job offers are contingent upon successful completion of a post offer placement assessment including a urine drug screen, immunization review and tuberculin (TB) skin testing, if applicable.
Recruitment Fraud
Learn more about recruitment fraud and job scams
Advertising
Mayo Clinic is a not-for-profit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
Advertising and sponsorship policy | Advertising and sponsorship opportunities
Reprint permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
Terms and Conditions | Privacy Policy | Notice of Privacy Practices | Notice of Nondiscrimination
© 1998-2024 Mayo Foundation for Medical Education and Research (MFMER). All rights reserved.

Clinical Research Coordinator Associate
🔍 school of medicine, stanford, california, united states.
The Stanford Center for Clinical Research (SCCR) is a growing academic research organization within the Stanford Department of Medicine. Our mission is to conduct and promote high-impact, innovative clinical research to improve human health.
In collaboration with the Division of Infectious Diseases and others at Stanford, SCCR is seeking multiple Clinical Research Coordinator Associates (CRCAs) to perform duties related to the coordination of a large, complex COVID study. This exciting Team Science project involves multiple disciplines and units, including Infectious Diseases, Hospital Medicine, and Population Health. The CRCAs will work in a dynamic atmosphere and as part of a large team in a supportive environment. There will be the opportunity to work closely with fellow coordinators and research assistants, physicians, nurses, and technicians. Direct patient contact is a primary responsibility of this role. The CRCAs are required to have the ability to multi-task in a high-energy environment.
A flexible work schedule, outstanding communication, organizational skills, and attention to detail are required in a successful candidate. The CRCA will act as a point person for participants and staff, present at meetings and huddles, and troubleshoot research related issues. The CRCA may engage with a pool of coordinators to support other research studies across the Department of Medicine. The CRCA will be responsible for ensuring compliance with federal, state, local, and sponsor regulations and work under the direction of their supervisor. The CRCA will work under close direction of the principal investigator and/or study coordinator/supervisor. Other duties may also be assigned.
At SCCR, we strive to find team members who are passionate about their work, flexible, fun, and want to deliver results. We place a high priority on equipping our staff to perform their job efficiently, helping them acquire new skills and grow within the organization. We encourage our team to have a healthy balance between work commitments and life outside of work and provide support to achieve this balance. If you are looking to make a large impact through global-reaching clinical research, we encourage you to apply!
This is an on-site role.
Duties include:
- Serve as primary contact with research participants, sponsors, and regulatory agencies. Coordinate studies from start-up through close-out.
- Determine eligibility of and gather consent from study participants according to protocol. Assist in developing recruitment strategies.
- Coordinate collection of study specimens and processing.
- Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms.
- Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents. Prepare regulatory submissions, and ensure Institutional Review Board renewals are completed.
- Assemble study kits for study visits, monitor scheduling of procedures and charges, coordinate documents, and attend monitoring meetings with sponsors, acting as primary contact.
- Monitor expenditures and adherence to study budgets and resolve billing issues in collaboration with finance and/or management staff.
- Interact with the principal investigator regularly, ensuring patient safety and adherence to proper study conduct.
- Ensure essential documentation and recording of patient and research data in appropriate files per institutional and regulatory requirements.
- Participate in monitor visits and regulatory audits.
DESIRED QUALIFICATIONS:
- Clinical research coordinator experience.
- Clinical research operations certificate or interest in working toward one.
- Valid CA driver's license.
EDUCATION & EXPERIENCE (REQUIRED):
- Two-year college degree and two years related work experience or a Bachelor's degree in a related field or an equivalent combination of related education and relevant experience.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
- Strong interpersonal skills.
- Proficiency with Microsoft Office.
- Knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
- Working toward certification(s) to perform basic patient measurements and tests, such as phlebotomy and EKG.
PHYSICAL REQUIREMENTS:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
WORKING CONDITIONS:
- Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
- May require extended or unusual work hours based on research requirements and business needs.
WORKING STANDARDS:
- Interpersonal Skills: Demonstrates the ability to work well with Stanford colleagues and clients and with external organizations.
- Promote Culture of Safety: Demonstrates commitment to personal responsibility and value for safety; communicates safety concerns; uses and promotes safe behaviors based on training and lessons learned.
- Subject to and expected to comply with all applicable University policies and procedures, including but not limited to the personnel policies and other policies found in the University’s Administrative Guide, http://adminguide.stanford.edu/ .
The expected pay range for this position is $31.73 to $36.54 per hour.
Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Why Stanford is for You Imagine a world without search engines or social platforms. Consider lives saved through first-ever organ transplants and research to cure illnesses. Stanford University has revolutionized the way we live and enrich the world. Supporting this mission is our diverse and dedicated 17,000 staff. We seek talent driven to impact the future of our legacy. Our culture and unique perks empower you with:
- Freedom to grow . We offer career development programs, tuition reimbursement, or audit a course. Join a TedTalk, film screening, or listen to a renowned author or global leader speak.
- A caring culture . We provide superb retirement plans, generous time-off, and family care resources.
- A healthier you . Climb our rock wall, or choose from hundreds of health or fitness classes at our world-class exercise facilities. We also provide excellent health care benefits.
- Discovery and fun . Stroll through historic sculptures, trails, and museums.
- Enviable resources . Enjoy free commuter programs, ridesharing incentives, discounts and more.
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
Consistent with its obligations under the law, the University will provide reasonable accommodations to applicants and employees with disabilities. Applicants requiring a reasonable accommodation for any part of the application or hiring process should contact Stanford University Human Resources at [email protected]. For all other inquiries, please submit a contact form .
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
- Schedule: Full-time
- Job Code: 1013
- Employee Status: Regular
- Requisition ID: 102995
- Work Arrangement : On Site
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Research
Post Date: Jan 29, 2024
Post Date: Feb 15, 2023
Post Date: Aug 05, 2022
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs
Clinical Research Coordinator I - 129377
Job description, #129377 clinical research coordinator i.
UCSD Layoff from Career Appointment : Apply by 04/22/2024 for consideration with preference for rehire. All layoff applicants should contact their Employment Advisor.
Special Selection Applicants : Apply by 05/01/2024. Eligible Special Selection clients should contact their Disability Counselor for assistance.
DESCRIPTION
The Moores Cancer Center (MCC) is one of just 53 NCI-designated Comprehensive Cancer Centers in the United States and the only one in San Diego County. As a consortium cancer center, it is a collaborative partnership between the UCSD: encompassing 28 departments, 6 schools School of Medicine, Skaggs School of Pharmacy and Pharmaceutical Sciences, School of Public Health, Jacobs School of Engineering, School of Biological Sciences & the School of Physical Sciences), UCSD Health oncology hospitals and clinics; the basic and public health research and outreach of San Diego State University (SDSU), and the basic and translational research of the La Jolla Institute of Immunology (LJI). These various programs and units are all dedicated to fulfilling the Moores Cancer Center's mission of reducing cancer's burden. As such, it ranks among the top centers in the nation conducting the continuum of cancer research, providing advanced patient care, and serving the community through outreach and education programs. As a top-ranking, future-oriented organization, we offer challenging career opportunities in a fast-paced and innovative environment. Moores Cancer Center follows a progressive philosophy of career-path development for its employees including opportunities for cross-training, professional development, and progressive responsibility.
The Clinical Trials Office (CTO) at UCSD's Moores Cancer Center administers many clinical trials on behalf of its member investigators, including trials sponsored by National Cancer Institute cooperative groups, pharmaceutical companies, and physician investigators.
Reporting directly to the Disease Team Project Manager, the Clinical Research Coordinator I is responsible for coordinating and managing clinical trials including providing all aspects of protocol management, including screening for patient eligibility, data collection and analysis, ensuring protocol compliance, adverse drug reaction reports, monitoring patient treatment and toxicities, laboratory and specimen submission, and maintenance of accurate and complete clinical research files. Assist the regulatory department with Human subjects submissions, renewals, and safety reports. Directly communicate with assigned physicians and disease groups, including attending weekly meetings and tumor boards. Provide direct assistance to the Project Manager in reviewing and verifying university research account statements, professional fee statements, and invoicing. Other duties assigned as needed.
MINIMUM QUALIFICATIONS
Theoretical knowledge of biology, microbiology, social sciences, clinical sciences as typically attained by a Bachelor's degree, or an equivalent combination of education and experience.
Experience performing clinical research duties in a clinical research environment.
Experience using statistical software applications. Knowledge of database, word processing and spreadsheet applications such as Velos, Access, Excel and MS Word.
Experience with laboratory procedures and values and experience in interpreting them to determine patient eligibility and potential toxicities.
Experience working with FDA policies regulating clinical trials.
Experience in medical assessment and patient interviewing to determine toxicities related to protocol management.
Experience interpreting medical charts and abstracting data from medical records.
Knowledge of National Institute of Health (NIH), Good Clinical Practice (GCP), Injury and Illness Prevention Program (IIPP), Human Resource Protection Program (HRPP), IATA Shipping of Blood Specimens, and Bloodborne Pathogens.
Experience with clinical trials participant or study subject recruitment.
Experience coordinating study startup activities.
Experience providing in-service training to various research personnel on protocols, processes, and procedures.
Knowledge of x-rays, scans, and other diagnostic procedures.
Experience maintaining files and keeping records.
Excellent planning and organizational skills and ability to work in a changing, multiple-demand setting in order to prioritize a large volume of work and meet deadlines efficiently and accurately.
Excellent interpersonal, as well as written and verbal communication skills (using grammatically correct written English and accurate typing) to interact with a diverse population. Excellent phone etiquette skills.
Ability to work independently. Ability to maintain confidentiality.
Experience completing clinical trials case report forms via hard copy and online.
Demonstrated ability to interact effectively with diverse groups, including professional and non- professional staff and clients.
PREFERRED QUALIFICATIONS
Bachelor's degree in related area and / or equivalent experience / training.
Certification as a Clinical Research Associate or Coordinator.
Fluency in English and at least one of the following languages: Spanish, Korean, Vietnamese, Mandarin or Cantonese.
Experience working with research bulk accounts.
Experience with investigational drug authorization criteria.
SPECIAL CONDITIONS
Employment is subject to a criminal background check and pre-employment physical.
Must be able to travel to different locations and work weekends and evenings as needed.
Pay Transparency Act
Annual Full Pay Range: $64,812 - $104,275 (will be prorated if the appointment percentage is less than 100%)
Hourly Equivalent: $31.04 - $49.94
Factors in determining the appropriate compensation for a role include experience, skills, knowledge, abilities, education, licensure and certifications, and other business and organizational needs. The Hiring Pay Scale referenced in the job posting is the budgeted salary or hourly range that the University reasonably expects to pay for this position. The Annual Full Pay Range may be broader than what the University anticipates to pay for this position, based on internal equity, budget, and collective bargaining agreements (when applicable).
If employed by the University of California, you will be required to comply with our Policy on Vaccination Programs, which may be amended or revised from time to time. Federal, state, or local public health directives may impose additional requirements. If applicable, life-support certifications (BLS, NRP, ACLS, etc.) must include hands-on practice and in-person skills assessment; online-only certification is not acceptable.
UC San Diego Health Sciences is comprised of our School of Medicine, Skaggs School of Pharmacy and Pharmaceutical Sciences, The Herbert Wertheim School of Public Health and Human Longevity Science, and our Student Health and Well-Being Department. We have long been at the forefront of translational - or "bench-to-bedside" - research, transforming patient care through discovery and innovation leading to new drugs and technologies. Translational research is carried out every day in the hundreds of clinical trials of promising new therapies offered through UC San Diego Health, and in the drive of our researchers and clinician-scientists who are committed to having a significant impact on patient care. We invite you to join our team!
Applications/Resumes are accepted for current job openings only. For full consideration on any job, applications must be received prior to the initial closing date. If a job has an extended deadline, applications/resumes will be considered during the extension period; however, a job may be filled before the extended date is reached.
To foster the best possible working and learning environment, UC San Diego strives to cultivate a rich and diverse environment, inclusive and supportive of all students, faculty, staff and visitors. For more information, please visit UC San Diego Principles of Community .
UC San Diego is an Equal Opportunity/Affirmative Action Employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, age or protected veteran status.
For the University of California’s Affirmative Action Policy please visit: https://policy.ucop.edu/doc/4010393/PPSM-20 For the University of California’s Anti-Discrimination Policy, please visit: https://policy.ucop.edu/doc/1001004/Anti-Discrimination
UC San Diego is a smoke and tobacco free environment. Please visit smokefree.ucsd.edu for more information.
UC San Diego Health maintains a marijuana and drug free environment. Employees may be subject to drug screening.
Application Instructions
Please click on the link below to apply for this position. A new window will open and direct you to apply at our corporate careers page. We look forward to hearing from you!
Share This Page
Posted : 4/17/2024
Job Reference # : 129377
JOIN OUR TALENT COMMUNITY
Interested in working at UC San Diego and UC San Diego Health but can't find a position that's right for you? Submit your resume to our Talent Community to be considered for future opportunities that may align with your expertise. Please note, by joining our Talent Community, you are not applying for a position with UC San Diego Campus and Health. Rather, this is an additional way for our Talent Acquisition team to find candidates with specific credentials, if an opportunity arises. You are still encouraged to regularly check back on our career site or sign up for Job Alerts to apply for openings that are a match for your background.
- Career Sites by Recruiting.com
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Clinical Research Coordinator
- Ophthalmology
- Columbia University Medical Center
- Opening on: Apr 23 2024
- Job Type: Officer of Administration
- Regular/Temporary: Regular
- Hours Per Week: 35
- Salary Range: $62,400 - $65,000
Position Summary
Under the direction of the Director of the Clinical Trials Unit (CTU) and Principal Investigators, the Clinical Research Coordinator will conduct clinical research studies (industry-sponsored and investigator-initiated) within the Columbia University Irving Medical Center (CUIMC) Department of Ophthalmology in adherence with assigned study protocols and manuals of operation and in accordance with clinical research principles.
Responsibilities
- Serve as the contact person for those interested in study participation and assist with recruitment activities including pre-screening electronic medical records for eligibility, contacting potential subjects, explaining all study procedures, and consenting eligible subjects or assenting parents or guardians for children enrolled in research studies.
- Coordinate day-to-day aspects of study related procedures, including, but not limited to scheduling visits and procedures, data entry, preparing for research visits, research visit documentation, maintenance of regulatory binders and study files, creation and/or maintenance of source documentation, preparation for monitoring visits, site initiation/closeout visits and audits as needed.
- Be able to coordinate and perform research testing and imaging for clinical research studies including but not limited to visual acuity, refraction, dark adaptation, visual field, microperimetry, fluorescein angiography, fundus photography, optical coherence tomography (OCT), ICG angiography, slit lamp photography, MP1, corneal mapping, specular biomicroscopy including confocal imaging, HRT Analyzer (glaucoma), and ERGs.
- Be able to administer surveys, such as the National Eye Institute Vision Function Questionnaire (NEI-VFQ-25), EuroQOL-5 Dimension, Reading speed, Health Utilities Index.
- Work with the research team and ocular photography department to ensure that all required eye exams and ocular testing are scheduled and completed according to protocol.
- Obtain and maintain study certifications for ETDRS, OCT, and photography for clinical trials.
- Obtain access to sponsors’ electronic data capture (EDC) systems, complete EDC trainings, and enter data into the EDC within 5 days of seeing the study patient.
- Maintain and organize study-related documentation and records using the EDC platforms, including capturing adverse events and serious adverse events and preparing for monitoring visits.
- Respond to all sponsor-related queries in a timely manner.
- Ensure that all aspects of Good Clinical Practice are followed at all times by developing and ensuring adherence with Standard Operating Procedure (SOP) for clinical studies being conducted in the Ophthalmology Clinical Trials Unit.
- Work with the Regulatory Manager to gain CUIMC Institutional Review Board (IRB) approval in a timely manner by creating informed consent forms using sponsors’ templates, responding to IRB correspondents, submitting amendments, renewals, modifications, and other regulatory documents required by the sponsor and FDA, including progress reports.
- Ensure that all appropriate Institutional, State, and Federal regulations are followed throughout the course of the study according to study-related protocols and manuals.
- Work directly with sponsors’ designated Clinical Research Organizations (CRO) to complete all required study start-up documents including FDA 1572 forms, investigator signatures, CVs, medical licenses, Conflict of Interest, HIPAA, and Human Subjects Trainings in a timely manner.
- Complete feasibility forms requested by sponsors in a timely manner to assess ophthalmic equipment and examination rooms to conduct the studies.
Minimum Qualifications
- Bachelor’s degree or equivalent in education and experience, plus minimum of 1 to 2 years of related experience.
- Conform to all applicable HIPAA, billing compliance and safety requirements.
- Must be able to work effectively with minimal supervision.
- Prior research experience to include recruiting study participants, conducting standardized protocol visits and data entry.
- Excellent verbal and written communication skills and attention to detail required.
- Computer skills (Word, Excel) required.
- Excellent interpersonal skills.
- Willingness to travel to different sites.
Preferred Qualifications
- Working knowledge of Spanish
- Phlebotomy license
- Prior experience in ophthalmology
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Research Assistant
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .
Thanks for starting your application to {{companyName}}.
To complete your application you must do one of the following:
Forward an email from your mobile device with your resume attached to {{fromEmail}}
Reply to this email from your laptop or desktop computer with your resume attached.
Thank you for your interest, The Recruiting Team
Reply to this email from your laptop or desktop computer with your cover letter attached.
Dear ${user.firstName},
Thanks for choosing to apply for a job with ${client.display.name}! Please verify ownership of your email address by clicking this link .
Alternatively, you can verify your account by pasting this URL into your browser: ${page.url}?id=${user.id}&ptoken=${user.token}
Please note that your job application will not be submitted to ${client.display.name} until you have successfully verified ownership of your email address.
The ${client.display.name} Recruiting Team
Nice to meet you. 👋
Lets quickly set up your profile
to start having tailored recommendations.
In order to create an account with us and submit applications for positions with our company you must read the following Terms and Agreements and select to agree before registering.
In the event that you do not accept our Terms and Agreements you will not be able to submit applications for positions with our company.
You agree to the storage of all personal information, applications, attachments and draft applications within our system. Your personal and application data and any attached text or documentation are retained by Sequoia Apply in accordance with our record retention policy and applicable laws.
You agree that all personal information, applications, attachments and draft applications created by you may be used by us for our recruitment purposes, including for automated job matching. It is specifically agreed that we will make use of all personal information, applications, attachments and draft applications for recruitment purposes only and will not make this information available to any third party unconnected with the our recruitment processes.
Your registration and access to our Careers Web Site indicates your acceptance of these Terms and Agreements.
This career site protects your privacy by adhering to the European Union General Data Protection Regulation (GDPR). We will not use your data for any purpose to which you do not consent.
We store anonymized interaction data in an aggregated form about visitors and their experiences on our site using cookies and tracking mechanisms. We use this data to fix site defects and improve the general user experience.
We request use of your data for the following purposes:
Job Application Data
This site may collect sensitive personal information as a necessary part of a job application. The data is collected to support one or more job applications, or to match you to future job opportunities. This data is stored and retained for a default period of 12 months to support job matching or improve the user experience for additional job applications. The data for each application is transferred to the Applicant Tracking System in order to move the application through the hiring process. \nYou have the right to view, update, delete, export, or restrict further processing of your job application data. To exercise these rights, you can e-mail us at [email protected] . \nConversion Tracking \nWe store anonymized data on redirects to the career site that is used to measure the effectiveness of other vendors in sourcing job candidates.
Consent and Data Privacy
This application protects your privacy by adhering to the European Union General Data Protection Regulation (GDPR). Jibe will not use your data for any purpose to which you do not consent.\n
We request use of your data for the following purposes:\n \n User Authentication \n
\n This site retains personally identifiable information, specifically e-mail addresses, as a necessary part of user login. This data is retained for the duration of the user profile lifecycle and enables user authentication.\n
\n \n Usage Analytics \n
We store anonymized usage data to measure and improve the effectiveness of this CRM application in filling job requisitions and managing talent communities.\n
\n \n E-mails to Candidates \n
We collect your personal information such as name and email address. This information is used when you send marketing or contact emails to candidates.\n\n
Click here for the ‘EEOC Know Your Rights’ Poster
Click here to view the deod 310 equal opportunity poster(ny.gov).
Enter your email address to continue. You'll be asked to either log in or create a new account.
There was an error verifying your account. Please click here to return home and try again.
You are about to enter an assessment system which is proprietary software developed and produced by Kenexa Technology, Inc. The content in this questionnaire has been developed by Kenexa Technology, Inc., Kenexa’s Suppliers and/or Yum Restaurant Services Group, Inc.’s (“Company”) third party content providers and is protected by International Copyright Law. Under no condition may the content be copied, transmitted, reproduced or reconstructed, in whole or in part, in any form whatsoever, without express written consent by Kenexa Technology, Inc. or the applicable third party content provider. Under no circumstances will Kenexa Technology, Inc. be responsible for content created or provided by Company’s third party content providers.
IN NO EVENT SHALL KENEXA, AN IBM COMPANY, KENEXA’S SUPPLIERS OR THE COMPANY’S THIRD PARTY CONTENT PROVIDERS, BE LIABLE FOR ANY DAMANGES WHATSOEVER INCLUDING, WITHOUT LIMITATION, DAMAGES FOR LOSS OF VOCATIONAL OPPORTUNITY ARISING OUT OF THE USE OF, THE PERFORMANCE OF, OR THE INABILITY TO USE THIS KENEXA ASSESSMENT SYSTEM OR THE CONTENT, REGARDLESS OF WHETHER OR NOT THEY HAVE BEEN ADVISED ABOUT THE POSSIBILITY OF SUCH DAMAGES.
By clicking below, you are also confirming your identity for purposes of the questionnaire. You may not receive assistance, refer to any written material, or use a calculator (or similar device) while completing the questionnaire.
Unless otherwise directed by the Questionnaire Administrator, you are only authorized to take each requested questionnaire once. Failure to comply may result in disqualification. All Kenexa SelectorTM questionnaires are monitored.
- ICU Registered Nurse
- Registered Respiratory Therapist
A web browser is a piece of software on your computer. It lets you visit webpages and use web applications.
It's important to have the latest version of a browser. Newer browsers save you time, keep you safer, and let you do more online.
Try a different browser - all are free and easy to install. Visit whatbrowser.org for more information.
If you are using a later version of Internet Explorer, please make sure you are not in compatibility mode of an older version of the browser.

- Sign In / Create Account
- Employee Login
- Employee Referral
- Join Our Community
- Facts & Figures
- Mount Sinai Experience
- Advanced Practice Clinicians
- Behavioral Health
- Digital Technology Partners
- Mount Sinai Health Partners
- Non-clinical Professionals
- PA Fellowship
- Diversity & Inclusion
- Search Careers
- Physician Jobs
- Development & Fundraising
- Specialties
- Careers Home
150 East 42nd Street 4th Floor New York, NY 10017
P: 646-605-4310
Mount Sinai Health System
© 2020 Icahn School of Medicine at Mount Sinai
User Agreement and Privacy Policy
Mount Sinai Health System endeavors to make Mount Sinai's Career Center accessible to any and all users. If you would like to contact us regarding the accessibility of our website or need assistance completing the application process, please contact our Talent Acquisition team at P: 646-605-4310 or click on the Live Chat icon below! | LCA Notices
Cookies are used on this site to assist in continually improving the candidate experience and all the interaction data we store of our visitors is anonymous. Learn more about your rights on our Privacy Policy page.
- Current Employees
- Duke & Durham
- Human Resources
- Connect With Us
- External Applicants
- Current Duke Employees
- Duke Health Careers
CLINICAL RESEARCH NURSE COORDINATOR
Durham, NC, US, 27710
School of Medicine
Established in 1930, Duke University School of Medicine is the youngest of the nation's top medical schools. Ranked sixth among medical schools in the nation, the School takes pride in being an inclusive community of outstanding learners, investigators, clinicians, and staff where interdisciplinary collaboration is embraced and great ideas accelerate translation of fundamental scientific discoveries to improve human health locally and around the globe. Composed of more than 2,600 faculty physicians and researchers, nearly 2,000 students, and more than 6,200 staff, the Duke University School of Medicine along with the Duke University School of Nursing, and Duke University Health System comprise Duke Health, a world-class academic medical center. The Health System encompasses Duke University Hospital, Duke Regional Hospital, Duke Raleigh Hospital, Duke Health Integrated Practice, Duke Primary Care, Duke Home Care and Hospice, Duke Health and Wellness, and multiple affiliations.
Occupational Summary
Participates in or leads day to day operations of clinical research studies conducted by principal investigator(s) at Duke Medicine; performs a variety of duties involved in the collection, compilation, documentation, and analysis of clinical research data. May oversee the work of junior staff and train or mentor others in clinical research tasks. Provides and documents professional nursing care for research participants.
Work Performed
Nursing activities
- Plan, provide, supervise, and document professional nursing care utilizing the nursing process for patients in accordance with physician orders and established policies and procedures.
- Use professional nursing judgment when conducting nursing research activities to patients. May delegate tasks and supervise the activities of other licensed and unlicensed research staff.
- Monitor and initiate corrective action to maintain the environment of care, including equipment and material resources.
- Participate in the identification of clinical or operational performance improvement opportunities and in performance improvement activities.
- Research Operations
- Recognizes when typical agreements (MTAs, CDAs, DUAs, DTAs, etc.) are necessary and alerts appropriate parties.
- May prepare Federal Drug Administration (FDA) regulatory submissions in collaboration with Office of Regulatory Affairs and Quality (ORAQ), including development, submission, and maintenance of relevant documentation. Addresses FDA review and potential hold issues in collaboration with the Principal Investigator (PI).
- Knowledgeable in regulatory and institutional policies and processes; applies appropriately in study documentation, protocol submissions, and standard operating procedures (SOPs).
- Is responsible for all aspects of managing and documenting Investigational Product (IP); including arrival, storage, tracking, and provision to research participants. Serves as the primary liaison with sponsors, Investigational Drug Service (IDS), and other parties as necessary. Follows protocol schema for randomization and blinding and unbinding.
- May maintain study level documentation for international studies and develop resources and tools for management of international studies, and coordinate with other entities or offices.
- Prepares for and provides support for study monitoring and audit visits, including support for the reviewer. Addresses and corrects findings. Maintains participant level and study level documentation for all studies, including those that are complex in nature (e.g., procedural and interventional studies) and require access to the Duke electronic health record (EHR).
- Employs and may develop strategies to maintain recruitment and retention rates and evaluate processes to identify problems. Escalates issues.
- Screens participants for complex studies (e.g., procedural and interventional studies). Develops or helps develop SOPs.
- Collects, prepares, processes, ships, and maintains the inventory of human research specimens, primarily those requiring complex procedures. Conducts activities for study visits in compliance with the protocol.
- Contributes to the effective facilitation of team meetings to achieve predetermined objectives. May lead multidisciplinary meetings with various stakeholders.
Safety and Ethics
- Identifies all AEs, and determines whether or not they are reportable. Collaborates with the PI to determine AE attributes, including relatedness to study.
- Conducts and documents consent for participants for all types of studies, including those that are complex in nature and require any orders in Maestro Care.
- Develops consent plans and documents for participants in a variety of studies.
- Develops and submits documentation and information for Institutional Review Board (IRB) review. Communicates with the IRB staff and reviewers and handle issues appropriately.
- Prepares and submits documents needed for regulatory and safety reporting to sponsors and other agencies. Responsible for adherence to clinical research policies to ensure ethical conduct and protect vulnerable populations.
- Communicates the difference between clinical activities and research activities, and the risks and benefits of study participation to research participants.
- Enters and collects data, and develops data entry or collection SOPs or tools. May provide oversight or training to study team members collecting or entering data.
- Ensures accuracy and completeness of data for all studies, including those that are complex in nature. Recognizes data quality trends and escalates as appropriate. May develop tools for, and train others in, data quality assurance procedures.
- Recognizes and reports security of physical and electronic data vulnerabilities. May develop or review data lifecycle and management plans for multiple study protocols.
- Maps a protocols data flow plan including data capture, storage, transfer, management, quality, and preparation for analysis (may include data from EDCs, EHR, mobile apps, etc.).
- Innovatively uses technology to enhance a research process.
- Prepares tables, data visualizations, and lay summaries to communicate study results to participants.
- Develops reports on study progress for the PI and other study team members and collaborators. Creates clear visualizations to help communicate key information to stakeholders.
Scientific Concepts
- Assists with or contributes to the development of funding proposals. Independently conducts literature searches and reviews.
- Demonstrates and applies a basic understanding of open science practices and the FAIR data principals. Using scientific proposals from the PI, develops elements of research protocols.
- Demonstrates a basic understanding of the elements of research study designs.
- Contributes to the development of scientific publications or presentations and serves as an author on poster presentations or publications.
Site and Study Management
- Prepares for, coordinates, and actively participates in site visits. Communicates effectively with sponsors and contract research organizations (CROs).
- Uses clinical research management system and its reports to manage research participants activities, calendars, tracking and marking financial milestones, and all aspects of study visits. Uses required EMR functionalities to manage participants and study visits.
- Uses clinical research management system and its reports to manage all protocol activities, including minimum footprint, SIP counsel, and all aspects of maintaining current protocol information.
- Collects appropriate information to determine whether the study teams participation in a specific trial is feasible. May make feasibility recommendations.
- For studies with supplies or equipment, ensures that there are ample supplies and that equipment is in good working order. May forecast effort needs.
- Ensures that studies are conducted in compliance with institutional requirements and other policies.
- Follows, and may develop or implement, operational plans (e.g. protocol specific systems and documents including process flows).
- Prepares studies for closeout and document storage. Leadership and professionalism
- May train or oversee others in the above tasks. Proactively seeks opportunities to add relevant skills and certifications to own portfolio.
- Keeps current with research updates by attending key external offerings (i.e. Research Wednesday, RPN, events outside of Duke, etc.) and applies the learned material to the job. May disseminate information to others.
- May serve on committees and workgroups internal to Duke or externally in therapeutic area of research.
- Navigates processes and people involved in Duke clinical research, demonstrates the organizational awareness, and has the interpersonal skills necessary to get work done efficiently.
- Demonstrates resilience and is adaptive to change.
- Uses advanced subject matter expertise in the therapeutic area or clinical research to solve problems. Communicates effectively with others, regardless of reporting relationship, to accomplish shared work objectives.
Required Qualifications at this Level
Education/Training:
- Work requires graduation from an accredited BSN or Associates Degree in Nursing or Nursing Diploma program.
- All registered nurses without a Bachelors degree in Nursing (or higher) will be required to enroll in an appropriate BSN program within two years of their start date and to complete the program within five years of their start date.
- Must have current or compact RN licensure in the state of North Carolina. BLS required.
- Maintain compliance with required hospital and unit specific training competencies as well as an active RN status with the North Carolina Board of Nursing (NCBON).
Experience:
Twelve months of appropriate clinical nursing experience is required.
- Can easily use computing software and web based applications (e.g., Microsoft Office products and internet browsers).
The intent of this job description is to provide a representative and level of the types of duties and responsibilities that will be required of positions given this title and shall not be construed as a declaration of the total of the specific duties and responsibilities of any particular position. Employees may be directed to perform job-related tasks other than those specifically presented in this description.
Duke University is an Affirmative Action/Equal Opportunity Employer committed to providing employment opportunity without regard to an individual's age, color, disability, gender, gender expression, gender identity, genetic information, national origin, race, religion, sex, sexual orientation, or veteran status.
Duke aspires to create a community built on collaboration, innovation, creativity, and belonging. Our collective success depends on the robust exchange of ideas-an exchange that is best when the rich diversity of our perspectives, backgrounds, and experiences flourishes. To achieve this exchange, it is essential that all members of the community feel secure and welcome, that the contributions of all individuals are respected, and that all voices are heard. All members of our community have a responsibility to uphold these values.
Essential Physical Job Functions
Certain jobs at Duke University and Duke University Health System may include essential job functions that require specific physical and/or mental abilities. Additional information and provision for requests for reasonable accommodation will be provided by each hiring department.
Minimum Qualifications
Work requires graduation from an accredited BSN or Associates Degree in Nursing or Nursing Diploma program. All registered nurses without a Bachelors degree in Nursing (or higher) will be required to enroll in an appropriate BSN program within two years of their start date and to complete the program within five years of their start date. Must have current or compact RN licensure in the state of North Carolina. BLS required. Maintain compliance with required hospital and unit specific training competencies as well as an active RN status with the North Carolina Board of Nursing (NCBON).
Duke is an Affirmative Action/Equal Opportunity Employer committed to providing employment opportunity without regard to an individual's age, color, disability, gender, gender expression, gender identity, genetic information, national origin, race, religion, sex, sexual orientation, or veteran status.
Duke aspires to create a community built on collaboration, innovation, creativity, and belonging. Our collective success depends on the robust exchange of ideas—an exchange that is best when the rich diversity of our perspectives, backgrounds, and experiences flourishes. To achieve this exchange, it is essential that all members of the community feel secure and welcome, that the contributions of all individuals are respected, and that all voices are heard. All members of our community have a responsibility to uphold these values.
Essential Physical Job Functions: Certain jobs at Duke University and Duke University Health System may include essentialjob functions that require specific physical and/or mental abilities. Additional information and provision for requests for reasonable accommodation will be provided by each hiring department.
Nearest Major Market: Durham Nearest Secondary Market: Raleigh
Duke is an Affirmative Action / Equal Opportunity Employer committed to providing employment opportunity without regard to an individual’s age, color, disability, gender, gender expression, gender identity, genetic information, national origin, race, religion, sex, sexual orientation, or veteran status. Read more about Duke’s commitment to affirmative action and nondiscrimination at hr.duke.edu/eeo.
Clinical Research Coordinator Jobs in Egypt .css-uzlk74{font-size:14px;font-weight:400;font-style:normal;letter-spacing:-0.35px;line-height:22px;margin-left:8px;} 5 Jobs found
.css-o171kl{-webkit-text-decoration:none;text-decoration:none;color:inherit;} medical science liaison immunology, head of accounting - egypt, director business development, payers, providers & government, middle east, physiotherapy specialist, what are the top 10 popular jobs in egypt now, egypt's top 5 companies hiring in egypt now, what are the top 5 cities with open jobs in egypt, browse new jobs in egypt, accounting/finance, administration, analyst/research, android jobs, business development, c-level executive/gm/director, creative/design/art, customer service/support, education/teaching, engineering - construction/civil/architecture, engineering - mechanical/electrical, engineering - oil & gas/energy, engineering - other, engineering - telecom/technology, hospitality/hotels/food services, human resources, it/software development, installation/maintenance/repair, internships in egypt, logistics/supply chain, manufacturing/production, marketing/pr/advertising, media/journalism/publishing, medical/healthcare, operations/management, pharmaceutical, project/program management, purchasing/procurement, r&d/science, sales/retail, sports and leisure, startup jobs, strategy/consulting, tourism/travel, training/instructor, writing/editorial, browse jobs by location, jobs in cairo, jobs in dubai, jobs in riyadh, jobs in giza, jobs in alexandria, jobs in doha, jobs in makkah, jobs in kuwait city, jobs in sharqia, jobs in jeddah, jobs in ras al-khaimah, jobs in abu dhabi, jobs in monufya, jobs in qalubia, jobs in dakahlia, jobs in gharbia, jobs in tabuk, jobs in red sea, jobs in suez, jobs in beheira, jobs in south sinai, jobs in beni suef, jobs in matruh, jobs in minya, jobs in port said, jobs in new cairo, jobs in maadi, jobs in nasr city, jobs in 6th of october, jobs in heliopolis, jobs in sheikh zayed, jobs in sheraton, jobs in mohandessin, jobs in dokki, jobs in obour city, jobs in 10th of ramadan city, jobs in haram, jobs in downtown, jobs in mokattam, jobs in madinaty, jobs in new capital, jobs in bourj alarab, jobs in smart village, jobs in katameya, jobs in abu rawash, jobs in new nozha, jobs in ameria, jobs in badr city, jobs in zamalek.
Clinical Research Coordinator Technician / Assistant
How to apply.
A cover letter is required for consideration for this position and should be attached as the first page of your resume. The cover letter should address your specific interest in the position and outline skills and experience that directly relate to this position.
This position may independently provide study coordination for simple and moderately complex clinical research studies. As a member of a coordination team, this position may help support a portfolio of projects with varying levels of complexity. Mastery of all job duties from the CRC-Assistant position on the Michigan Medicine CRC Career Ladder is required.
Mission Statement
Michigan Medicine improves the health of patients, populations and communities through excellence in education, patient care, community service, research and technology development, and through leadership activities in Michigan, nationally and internationally. Our mission is guided by our Strategic Principles and has three critical components; patient care, education and research that together enhance our contribution to society.
Responsibilities*
Experience as part of a team with all 8 competency domains is expected:
- Scientific Concepts and Research Design
- Ethical Participant Safety Considerations
- Investigational Products Development and Regulation
- Clinical Study Operations (GCPs)
- Study and Site Management
- Data Management and Informatics
- Leadership and Professionalism
- Communication and Teamwork
Supervision Received:
This position receives direct supervision and reports directly to the unit Administrator, CRC-Lead, or CRC-Project Manager.
Supervision Exercised:
Required Qualifications*
Clinical Research Technician
- Associate's Degree in Health Science or an equivalent combination of related education and experience is necessary.
- Minimum 1 year of directly related experience in clinical research and clinical trials is necessary. (Please review SoCRA's Definition of a Clinical Research Professional for qualifying experience prior to applying.) or An advanced degree in a health-related areas such as: Health Sciences, Behavioral Sciences, Public Health, Health Care Administration, Clinical Research Administration, Social Work, Psychology, Epidemiology, Foreign MD. or Minimum 3 years of human subject experience (clinical, lab or health regulations) such as related patient care, related community health and wellness, related clinical information, and research.
Clinical Research Assistant
- High School Diploma or GED
Desired Qualifications*
Bachelor's degree in Health Science or an equivalent combination of related education and experience is desirable. An understanding of medical terminology, experience in a large complex health care setting, ability to effectively communicate with staff and faculty of all levels, and knowledge of university policies and procedures is desirable.
Work Schedule
This position will primarily support work M-F during normal business hours.
Work Locations
Underfill statement.
This position may be underfilled at the CRC-Assistant title based on selected candidates’ qualifications.
Additional Information
Michigan Medicine is firmly committed to advancing inclusion, diversity, equity, accessibility, and belonging, which are core to the culture and values of the Medical School Office of Research. Our community supports recruiting and cultivating a diverse workforce as a reflection of our commitment to serve the diverse people of Michigan and the world. We strive to create a work culture where each team member feels respected, valued, and safe.
Background Screening
Michigan Medicine conducts background screening and pre-employment drug testing on job candidates upon acceptance of a contingent job offer and may use a third party administrator to conduct background screenings. Background screenings are performed in compliance with the Fair Credit Report Act. Pre-employment drug testing applies to all selected candidates, including new or additional faculty and staff appointments, as well as transfers from other U-M campuses.
Application Deadline
Job openings are posted for a minimum of seven calendar days. The review and selection process may begin as early as the eighth day after posting. This opening may be removed from posting boards and filled anytime after the minimum posting period has ended.
U-M EEO/AA Statement
The University of Michigan is an equal opportunity/affirmative action employer.

IMAGES
VIDEO
COMMENTS
People who searched for clinical research jobs in Egypt also searched for senior cra, in house cra, irb administrator, clinical project manager, clinical investigator, disability case manager, study coordinator, statistical programmer, study manager, clinical coordinator. If you're getting few results, try a more general search term.
Apply now to over 2 Clinical Research Coordinator jobs in Egypt and make your job hunting simpler. Find the latest Clinical Research Coordinator job vacancies and employment opportunities in Egypt. Clinical Research Coordinator Jobs in Egypt (August 2023) - Bayt.com
30+ days ago. Apply now to over 10 Clinical Research jobs in Egypt and make your job hunting simpler. Find the latest Clinical Research job vacancies and employment opportunities in Egypt.
GE Renewable Energy. Cairo, Cairo, Egypt. Actively Hiring. 2 weeks ago. Today's top 4,000+ Research jobs in Cairo, Cairo, Egypt. Leverage your professional network, and get hired. New Research ...
Apply now to over 8 Clinical Research Coordinator jobs in Egypt and make your job hunting simpler. Find the latest Clinical Research Coordinator job vacancies and employment opportunities in Egypt. Clinical Research Coordinator Jobs in Egypt (January 2023) - Bayt.com
Clinical Research Jobs in Egypt 20 Jobs found. Filters. 0 filters selected. Workplace New. Country. City. Area. Career Level. Job Category. Job Type. Date Posted. Head of Accounting - Egypt. Siemens Technology and Services Private Limited - Cairo, Egypt . 4 days ago. Full Time.
EGP 8KEGP 8K. Most Likely Range. The estimated salary for a Clinical Research Coordinator is EGP 7,976 per year in the Egypt area. This number represents the median, which is the midpoint of the ranges from our proprietary Total Pay Estimate model and based on salaries collected from our users. The "Most Likely Range" represents values that ...
Find how to become a clinical research coordinator. Discover qualification needed, the knowledge, skills, ability and personality required for the job. Check salaries, benefits and job outlook and how to apply and get your first job.
Apply now to over 10 Clinical Research Coordinator jobs in Middle East and Gulf and make your job hunting simpler. Find the latest Clinical Research Coordinator job vacancies and employment opportunities in Middle East and Gulf.
Apply to Clinical Research Coordinator job at ClinGroup Holding | Research, Analysis, Business Analysis, Science, Nursing, Pharmacy. STAND WITH PALESTINE. Browse Jobs. ... Egypt 16 days ago. Search Engine Optimization Spe... STIO Life Science - Heliopolis, Cairo 20 days ago. Medical Sales Representative (... STIO Life Science - Heliopolis ...
Apply for Clinical Research Coordinator at Force 10 Uae Llc in United Arab Emirates, Egypt today! Find the best Full Time jobs on GrabJobs and browse the latest Nurse jobs in United Arab Emirates. ... Most In-Demand Jobs in Egypt in 2023 Competition for jobs has increased. How can you stand out? Find out what skills and jobs are in high demand ...
Clinical Files Specialist. IQVIA - Cairo, Egypt. 16 days ago. Full Time. Not specified · R&D/Science · Medical/Healthcare · Pharmaceutical · Medical · CTD · Healthcare · Pharmacy · Regulatory Affairs · Science · B2B.
Clinical Research Coordinator - Comprehensive Cancer Center 331289 Rochester, Minnesota. Lead - Clinical Research Coordinator - Cardiovascular Diseases 330211 Rochester, Minnesota. Associate Clinical Research Coordinator - Cardiovascular Diseases 329950 Rochester, Minnesota. Associate Clinical Research Coordinator - NW Wisconsin 328244 Eau ...
The Poston Lab seeks a full-time Clinical Research Coordinator Associate. The desired candidate is self-motivated, detail-oriented, relatively independent, patient, punctual, and conscientious, with excellent interpersonal skills and excellent communication skills in English.
A person working as Clinical Research Coordinator in Egypt typically earns around 9,640 EGP.Salaries range from 5,210 EGP (lowest) to 14,600 EGP (highest).. Salary Variance. This is the average salary including housing, transport, and other benefits. Clinical Research Coordinator salaries in Egypt vary drastically based on experience, skills, gender, or location.
If you need a reasonable accommodation in the application process; to access job postings, to apply for a job, for a job interview, for pre-employment testing, or with the onboarding process, please contact HR Connect at 507-266-0440 or 888-266-0440. Job offers
Our mission is to conduct and promote high-impact, innovative clinical research to improve human health. In collaboration with the Division of Infectious Diseases and others at Stanford, SCCR is seeking multiple Clinical Research Coordinator Associates (CRCAs) to perform duties related to the coordination of a large, complex COVID study.
Reporting directly to the Disease Team Project Manager, the Clinical Research Coordinator I is responsible for coordinating and managing clinical trials including providing all aspects of protocol management, including screening for patient eligibility, data collection and analysis, ensuring protocol compliance, adverse drug reaction reports ...
Job details. As the world's oldest and largest private cancer center, everything that we do is focused on changing the way that the world treats cancer. ... Clinical Research Coordinator, Spanish Fluency Required. Department: Research - Clinical. Location: New York, NY. Find out more
Job Type: Officer of Administration Regular/Temporary: Regular Hours Per Week: 35 Salary Range: $62,400 - $65,000 The salary of the finalist selected for this role will be set based on a variety of factors, including but not limited to departmental budgets, qualifications, experience, education, licenses, specialty, and training. The above hiring range represents the University's good faith ...
Job Description . Strength Through Diversity. Ground breaking science. Advancing medicine. Healing made personal. The Clinical Research Coordinator is an entry research position, responsible for conducting and assisting in clinical research studies, obtaining informed consent, collecting, maintaining and organizing study information.
Durham CLINICAL RESEARCH NURSE COORDINATOR - NC, 27710. School of Medicine . Established in 1930, Duke University School of Medicine is the youngest of the nation's top medical schools.
Apply for Project Support Coordinator job with Thermo Fisher Scientific in Remote, Mexico. Clinical Research jobs at Thermo Fisher Scientific
Apply for Clinical Trial Coordinator (CTC) job with Thermo Fisher Scientific in Remote, Korea, Republic of. Clinical Research jobs at Thermo Fisher Scientific
Searching for jobs in Egypt? Wuzzuf helps you in your online job search to find Jobs in Egypt and Middle East. Choose the right job using our online recruitment services. STAND WITH PALESTINE. ... No results found for "Clinical Research Coordinator Jobs in Egypt ...
Apply for Project Support Coordinator II job with Thermo Fisher Scientific in Remote, Mexico. Clinical Research jobs at Thermo Fisher Scientific
1,606 Clinical Research jobs available in Egypt, MA on Indeed.com. Apply to Clinical Research Coordinator, Clinical Research Associate, Operations Manager and more!
(Please review SoCRA's Definition of a Clinical Research Professional for qualifying experience prior to applying.) or An advanced degree in a health-related areas such as: Health Sciences, Behavioral Sciences, Public Health, Health Care Administration, Clinical Research Administration, Social Work, Psychology, Epidemiology, Foreign MD. or