Drug Regimen Boosts Survival of People with Advanced Colorectal Cancer
May 24, 2023 , by Edward Winstead
The SUNLIGHT trial was the first phase 3 study involving people with treatment-resistant metastatic colorectal cancer to show an improvement in overall survival versus an existing treatment.
A new treatment regimen may help improve the survival of some people with advanced colorectal cancer, according to results from an international clinical trial.
The new regimen includes bevacizumab (Avastin) and the combination of trifluridine and tipiracil (Lonsurf) . Both therapies had previously been approved by the Food and Drug Administration (FDA) for the treatment of some people with colorectal cancer.
The trial, called SUNLIGHT , included nearly 500 people with advanced colorectal cancer that had gotten worse after at least two prior treatment regimens. Participants were randomly assigned to receive trifluridine – tipiracil alone or combined with bevacizumab.
After a median follow-up of 14 months, the group that received the combination therapy survived for a median of 10.8 months , compared with 7.5 months for the group that received trifluridine – tipiracil alone.
The combination therapy also increased how long patients lived without their cancer getting worse, called progression-free survival, by several months (a median of 5.6 months versus 2.4 months).
The study’s lead investigator, Josep Tabernero, M.D., Ph.D., of the Vall d’Hebron University Hospital in Barcelona, Spain, and his colleagues reported the findings in the New England Journal of Medicine on May 4.
The results “confirm that trifluridine – tipiracil plus bevacizumab is an effective treatment option” for patients with previously treated metastatic colorectal cancer, Dr. Tabernero said earlier this year when he presented the study’s preliminary findings at the American Society of Clinical Oncology’s GI Cancers Symposium .
SUNLIGHT was the first phase 3 study involving people with treatment-resistant metastatic colorectal cancer to demonstrate an improvement in overall survival versus an existing treatment, he added.
“This study sets a new standard for the care of patients with metastatic colorectal cancers that have stopped responding to other treatments,” said Carmen Allegra, M.D., who works with NCI’s Cancer Therapy Evaluation Program and was not involved in the study.
The new findings demonstrate that the combination therapy is more beneficial for these patients than trifluridine – tipiracil, Dr. Allegra added.
Taiho Oncology, which manufactures trifluridine – tipiracil, funded the trial. FDA is reviewing an application for the use of trifluridine – tipiracil in combination with bevacizumab for previously treated metastatic colorectal cancer, according to the company.

Combining therapies in the SUNLIGHT trial
Several small studies have suggested that adding bevacizumab to trifluridine – tipiracil might benefit patients with previously treated metastatic colorectal cancer . Dr. Tabernero and his colleagues developed the SUNLIGHT trial to confirm these results.
Trifluridine – tipiracil and bevacizumab are given in different ways (as a tablet taken by mouth and intravenously , respectively) and attack cancer cells through different mechanisms.
Trifluridine causes DNA damage that may lead to the death of cancer cells, while tipiracil helps maintain blood concentrations of trifluridine by blocking an enzyme that degrades it.
Bevacizumab blocks the activity of a protein called VEGF. This helps starve tumors of oxygen and nutrients by preventing them from growing new blood vessels —a process known as angiogenesis.
Combination benefits patients treated previously with bevacizumab
Participants in the trial had received prior treatments such as fluoropyrimidine , irinotecan (Camptosar) , oxaliplatin (Eloxatin) , bevacizumab or another drug that blocks VEGF, and/or a drug that blocks EGFR , another protein involved in tumor growth.
About 70% of the study participants in both groups had tumors with a mutation in a RAS gene such as KRAS . These mutations occur in about half of all people with advanced colorectal cancer and may limit a patient’s treatment options.
In the SUNLIGHT trial, however, the combination therapy seemed to be effective regardless of whether a patient’s tumor had a RAS mutation.
Those study participants who had previously received bevacizumab also benefited from the addition of bevacizumab to trifluridine – tipiracil. That finding, the researchers wrote, adds to evidence supporting a role for continuing to use angiogenesis inhibitors after the cancer progresses on regimens that include these drugs.
The most common side effects experienced by patients in both groups were neutropenia (a condition caused by too few neutrophils, a type of white blood cell), nausea, and anemia (a low red blood cell count).
Neutropenia and hypertension were more common in the combination therapy group. Hypertension is associated with the class of drugs that includes bevacizumab.
“The combination did have additional side effects, but it appears that most patients tolerated the agents reasonably well,” Dr. Allegra said.
The use of the combination therapy “would need to be weighed for each patient given the associated side effects and additional [financial] cost,” he added.
Dr. Tabernero and his colleagues answered an important question by documenting the overall survival benefit conferred by this regimen for some patients with advanced colorectal cancer, according to Oladapo Yeku, M.D., Ph.D., and Dan L. Longo, M.D., of Massachusetts General Hospital, the authors of an accompanying editorial . They wrote that improved progression-free survival, which had been shown in phase 2 trials of the combination, “does not necessarily correspond with an effect on overall survival.”
The phase 3 trial, they noted, “provides a rigorous test of the combination on survival in this patient population.”
Featured Posts
February 22, 2024, by Carmen Phillips
January 23, 2024, by Elia Ben-Ari
January 12, 2024, by Shana Spindler
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Colorectal cancer articles from across Nature Portfolio
Colorectal cancer, also known as bowel cancer, is a cancer formed by uncontrolled cell growth in the colon or rectum (parts of the large intestine), or in the appendix. Genetic analysis shows that colon and rectal tumours are genetically the same cancer.
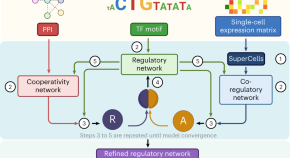
Enabling comparative gene regulatory network analysis on single-cell data with SCORPION
We present SCORPION, a computational tool to model gene regulatory networks based on single-cell transcriptomic data and prior knowledge of gene regulation. SCORPION networks can be modeled for specific cell types in individual samples, and are therefore suitable for conducting comparisons between experimental groups.
Related Subjects
- Colon cancer
- Rectal cancer
Latest Research and Reviews
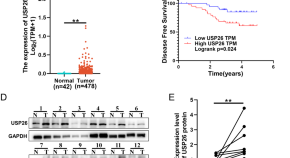
USP26 promotes colorectal cancer tumorigenesis by restraining PRKN-mediated mitophagy
- Zhihong Wang
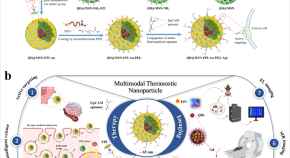
Hybridized quantum dot, silica, and gold nanoparticles for targeted chemo-radiotherapy in colorectal cancer theranostics
Multimodal NPs for cancer theranostics show selective release, uptake, and reduced cancer cell survival when combined with radiation. In vivo tracking confirms efficacy with minimal side effects by targeted NPs containing chemo/radio/contrast agents.
- Amir Abrishami
- Ahmad Reza Bahrami
- Maryam M. Matin
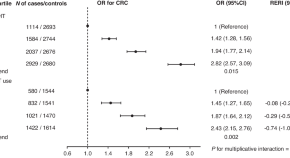
Genetic risk impacts the association of menopausal hormone therapy with colorectal cancer risk
- Jenny Chang-Claude
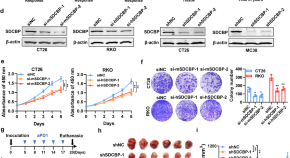

SDCBP modulates tumor microenvironment, tumor progression and anti-PD1 efficacy in colorectal cancer
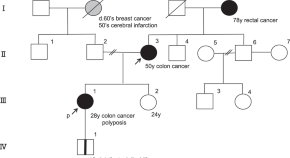
Genomic insights into familial adenomatous polyposis: unraveling a rare case with whole APC gene deletion and intellectual disability
- Hiroki Tanabe
- Masami Ijiri
- Toshikatsu Okumura
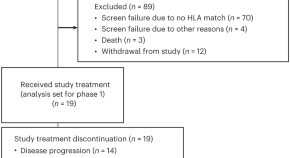
A shared neoantigen vaccine combined with immune checkpoint blockade for advanced metastatic solid tumors: phase 1 trial interim results
In an interim analysis of a phase 1/2 trial, a heterologous prime boost vaccine comprised of a chimpanzee adenovirus and self-amplifying mRNA that encodes neoantigens derived from common oncogenic driver mutations in combination with immune checkpoint blockade was safe and elicited neoantigen-specific T cell responses in patients with advanced solid tumors.
- Amy R. Rappaport
- Chrisann Kyi
- Karin Jooss
News and Comment
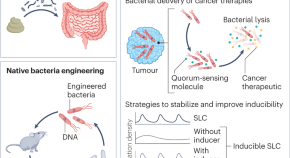
Live bacterial therapeutics for detection and treatment of colorectal cancer
Live microorganisms can be manipulated and engineered for colorectal cancer detection and treatment through methods such as faecal microbiota transplantation, native bacteria engineering and synthetic circuit engineering. Although promising, substantial effort is required to translate these approaches for clinical use.
- Joanna Zhang
- Amir Zarrinpar
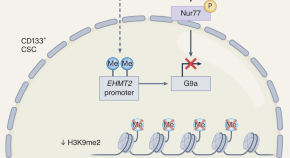
Repurposing the dopamine transporter antagonist vanoxerine to treat colorectal cancer
Self-renewing cancer stem cells drive tumor initiation and progression and represent a major target for therapeutic development. A study now shows that vanoxerine, a dopamine transporter antagonist, precisely inhibits this cell population in colorectal cancer, which leads to attenuation of tumor initiation and increased infiltration by immune cells.
- Winnie Chen
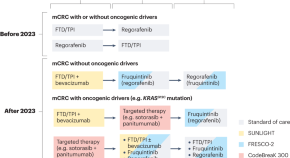
New options for late-line treatment of metastatic colorectal cancer
Metastatic colorectal cancer is a heterogeneous disease associated with poor patient outcomes. Although the past decade has seen few first-line treatment advances, key studies published in 2023 established new options for late-line therapy of the disease with and without oncogenic drivers, thus expanding the continuum of care in metastatic colorectal cancer.
- Sara Lonardi
- Filippo Pietrantonio

Explaining the male bias in cancers
Two studies published in Nature investigate the genetic mechanisms of sex bias in cancers and implicate Y chromosome genes in contributing to the aggressiveness of bladder cancer and colorectal cancer in men.
- Michael Attwaters
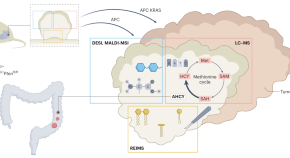
Multimodal metabolomics pinpoint new metabolic vulnerability in colorectal cancer
By combining multimodal metabolomics technologies to investigate colorectal tissues and tumours in situ, Vande Voorde et al. found tumour genotype-specific metabolic profiles and revealed the methionine-cycle enzyme AHCY to be a potential therapeutic target in colorectal cancer.
- May Yin Lee
- Wai Leong Tam
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Find a Doctor
- Appointments and Second Opinions
- Find a Location
- Patient Portals
Immunotherapy with two novel drugs shows activity in colorectal cancer
A combination of two next-generation immunotherapy drugs has shown promising clinical activity in treating patients with refractory metastatic colorectal cancer, a disease which has not previously responded well to immunotherapies, according to a Dana-Farber Cancer Institute researcher.
The results of an expanded phase 1 trial of the two drugs, botensilimab and balstilimab, are to be presented at the ASCO Gastrointestinal Cancers Symposium Jan. 19-21 in San Francisco. The study is led by Benjamin L. Schlechter, MD , a senior physician in the Gastrointestinal Cancer Treatment Center at Dana-Farber.
The trial included 70 patients with metastatic colorectal cancer who had been previously treated with several lines of drugs, including immunotherapies. These patients all had tumors termed microsatellite stable, or MSS, meaning that their genes for repairing certain types of DNA damage were intact. MSS colorectal tumors account for the vast majority of colorectal cancers, and the first generation of immunotherapy drugs have had little effect on them. While immunotherapy has succeeded in microsatellite unstable (MSI) colorectal cancers, only about 3-5% advanced colorectal cancers are MSI and there are no approved immunotherapies for the far more common MSS colorectal cancers.
The two-drug combination being tested in the expanded phase 1a/1b trial of patients with metastatic MSS colorectal cancers were novel, next-generation antibodies. Botensilimab is an antibody directed against the T-cell receptor cytotoxic T-lymphocyte-associated antigen 4, or CTLA-4, which is an immune checkpoint that regulates T-cell activation. Balstilimab is a novel monoclonal antibody designed to block PD-1 – another immune checkpoint protein – from interacting with PD-L1 and PD-L2. By inhibiting this interaction, balstilimab is aimed at freeing the immune system to attack cancers.
The patients in the trial were followed for a median of 7 months after receiving the drug combination. During that period, 23% of the patients had a reduction in the size of their tumors, and the median duration of response was not reached. The disease control rate – the percentage of patients with metastatic cancer who had a complete or partial response and stable disease – was 76%. The 12-month overall survival was 63%. The main population of patients who benefited from the combination were those who did not have active metastatic cancer in their liver.
Treatment-related adverse events occurred in 91% of patients, including grade 3 in 40% and grade 4 in 3%. Twelve percent of patients discontinued both drugs because of adverse events.
The researchers concluded that “in patients with heavily pretreated metastatic MSS colorectal cancer, botensilimab plus balstilimab continues to demonstrate promising clinical activity with durable response, and was well tolerated, with no new immune-mediated safety signals.”
“Harnessing the power of immune therapy in refractory colorectal cancer has been a key goal of multiple clinical trials in advanced colorectal cancer, but in MSS colorectal cancer efforts have been universally disappointing,” said Schlechter. “These data are a meaningful and important advance in the care of this very sick population.”
Based on these findings, a randomized phase 2 trial in patients with MSS colorectal cancer is currently enrolling.
Funding for this research comes from Agenus, Inc.
Media Contacts
If you are a journalist and have a question about this story, please call 617-632-4090 and ask to speak to a member of the media team, or email [email protected] .
The Media Team cannot respond to patient inquiries. For more information, please see Contact Us .

IMAGES
COMMENTS
The new regimen includes bevacizumab (Avastin) and the combination of trifluridine and tipiracil (Lonsurf). Both therapies had previously been approved by the Food and Drug Administration (FDA) for the treatment of some people with colorectal cancer. The trial, called SUNLIGHT, included nearly 500 people with advanced colorectal cancer that had ...
Colorectal cancer, also known as bowel cancer, is a cancer formed by uncontrolled cell growth in the colon or rectum (parts of the large intestine), or in the appendix. Genetic analysis shows that ...
January 21, 2023. A combination of two next-generation immunotherapy drugs has shown promising clinical activity in treating patients with refractory metastatic colorectal cancer, a disease which has not previously responded well to immunotherapies, according to a Dana-Farber Cancer Institute researcher. The results of an expanded phase 1 trial ...