An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- News Archive

New potential therapeutic target identified for Crohn’s disease
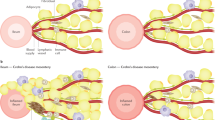
New research has shed light on how known genetic risk factors can contribute to Crohn’s disease and treatment response, opening the door to new treatment approaches. Crohn’s disease is a form of inflammatory bowel disease in which the digestive tract is marked by lesions of damaging inflammation. It can start at any age, causing lifelong episodes of cramping, diarrhea, and malnutrition. Medications that block a major component of the inflammatory response called tumor necrosis factor (TNF) are effective for many people, but in some cases the disease does not respond to these drugs. Among the scores of genetic variations that have been linked to a higher risk for developing Crohn’s disease, changes in a gene called NOD2 that impair its function have been found to be a major risk factor. Exactly how these NOD2 genetic variations could contribute to Crohn’s disease has been unclear, however, which has been a major roadblock for developing new therapies.
Researchers set out to answer this question by analyzing intestinal samples from a well-characterized group of male and female children with Crohn’s disease. They found that genetic variations inhibiting NOD2 function were linked to changes in fibroblasts (cells that make up connective tissue) and immune cells in Crohn’s disease lesions. Specifically, these cell types showed signs that they were “activated” and producing factors involved in inflammation. Importantly, activated immune cells and fibroblasts have also been found in lesions from people with refractory Crohn’s disease that is resistant to anti-TNF therapy, suggesting that these activated cells provide an additional route to inflammation that is independent of TNF-mediated inflammation. Using cultured cells and a zebrafish model that effectively mimics human Crohn’s disease, the researchers identified a protein known as gp130 that plays a critical role in activating these cells when NOD2 is impaired. Data from women and men with Crohn’s disease that did not respond well to anti-TNF therapy showed high levels of intestinal proteins in the cellular pathway used by gp130. Additionally, the researchers found that treating zebrafish or cultured cells with a gp130-blocking drug inhibits activation of inflammatory cells. More research is needed to determine if blocking gp130 will similarly reduce cellular activation in human intestinal lesions. However, this study suggests that drugs targeting gp130, when used in conjunction with anti-TNF therapy, might be effective treatments for people with Crohn’s disease resulting from NOD2 risk variants.
Nayar S, Morrison JK, Giri M,…Cho JH. A myeloid-stromal niche and gp130 rescue in NOD2-driven Crohn's disease . Nature 593: 275-281, 2021.
FDA Approves First Pill to Treat Moderate-to-Severe Crohn's Disease
FDA Approves First Pill to Treat Moderate-to-Severe Crohn's Disease
By Cara Murez HealthDay Reporter

FRIDAY, May 19, 2023 (HealthDay News) -- Patients with Crohn’s disease have a new treatment option, following U.S. Food and Drug Administration approval of a pill called Rinvoq (upadacitinib).
Rinvoq is meant to treat adults with moderately to severely active Crohn’s disease who have not had success with TNF (tumor necrosis factor) blockers. The daily pill is the first oral treatment for this group of patients.
Crohn's is a chronic inflammatory bowel disease. It causes inflammation in any part of the digestive tract, typically affecting the small intestine and the beginning of the large intestine. Common symptoms include diarrhea, cramping, stomach pain and weight loss.
The medication was previously approved for several other conditions, including eczema, rheumatoid arthritis, psoriatic arthritis and ulcerative colitis, according to the website of pharmaceutical company AbbVie.
U.S. Cities With the Most Homelessness

Researchers evaluated its safety and effectiveness in two randomized trials in 857 patients with the disease. Participants received either 45 mg of Rinvoq or a placebo daily for 12 weeks.
More patients treated with the medication achieved remission than those treated with the placebo, the FDA said in a news release. Also, more people treated with the medication had improvement in intestinal inflammation, which was assessed with a colonoscopy.
The FDA also assessed Rinvoq as a maintenance treatment, evaluating 343 patients who had responded to the 12 weeks of medication. This group received 15 mg or 30 mg once daily or a placebo for a year. More of those on the maintenance treatment achieved remission and reduced intestinal inflammation than those on the placebo.
Side effects of the medication were upper respiratory tract infections, anemia, fever, acne, herpes zoster and headache.
The drug is not recommended for use with other Janus kinase (JAK) inhibitors, biological therapies for Crohn’s disease or with strong immunosuppressants including azathioprine and cyclosporine.
Among the risks are serious infections, death, cancer, major adverse cardiovascular events and thrombosis (blood clot).
Patients should take 45 mg of Rinvoq once daily for 12 weeks and then start a 15 mg maintenance dose. A higher 30 mg maintenance dose can be considered for patients with refractory, severe or extensive Crohn’s disease, according to the FDA.
More information
The U.S. National Institutes of Health has more on Crohn’s disease .
SOURCE: U.S. Food and Drug Administration, news release, May 18, 2023
Copyright © 2023 HealthDay . All rights reserved.
Join the Conversation
Tags: prescription drugs , digestive disorders , drugs , FDA
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

The Week in Cartoons April 8-12
April 8, 2024, at 12:38 p.m.

Trump Abortion Quotes Over the Years
Cecelia Smith-Schoenwalder April 8, 2024

Trump Lays Out Stance on Abortion
Lauren Camera April 8, 2024

EXPLAINER: The Total Solar Eclipse

Inflation Back in the Picture
Tim Smart April 8, 2024

RFK Jr.’s Mixed-Up Messaging on Jan. 6
Susan Milligan April 5, 2024

- AGA Journals
- AGA University
- AGA Research Foundation
- AGA Community
- AGA Job Board
- Create Account

Clinical Guidance
Our clinical guidelines and updates help you make the best evidence-based decisions for your patients.
- Library AGA’s guidelines, practice updates and care pathways in one place.
- Guideline Toolkits Comprehensive resources for managing diseases – Crohn’s disease now available.

Journals & Publications
Latest research and ideas from the GI field.
- Gastroenterology The premier journal in GI.
- Clinical Gastroenterology and Hepatology (CGH) The go-to resource in clinical GI.
- Cellular and Molecular Gastroenterology and Hepatology (CMGH) Impactful digestive biology research.
- Techniques and Innovations in Gastrointestinal Endoscopy (TIGE) Cutting-edge advances in GI endoscopy.
- Gastro Hep Advances Open access GI and hepatology journal.
- GI & Hepatology News AGA’s official newspaper.
- The New Gastroenterologist Insights for fellows and early career GIs.

Meetings & Learning
Earn CME, MOC and improve your skills.
- AGA University Your hub for the best in GI education – AGA Postgraduate Course, Tech Summit and more.
- Digestive Disease Week® The most prestigious GI meeting.
- Crohn's & Colitis Congress® The premier meeting on IBD.
- Maintenance of Certification Resources for maintaining certification.
- DDSEP® The leading self-assessment tool for GI.
- Inside Scope Podcast An AGA podcast with bite-sized education.

More than 16,000 professionals worldwide call AGA their professional home.
- Join AGA Join our diverse mix of professionals.
- Renew Membership Continue to receive exclusive benefits and discounts.
- Benefits Unrivaled by any other GI organization.
- Membership Directory Contact other AGA members.
- Recognition Awards We honor our esteemed members.
- Initiatives & Programs Advancing the science and practice of GI.
- Get Involved with AGA Help us achieve a world free from digestive diseases.
- Advocacy & AGA PAC Advancing public policies that support gastroenterology.

Practice Resources
Tools to maximize efficiency and help you deliver high-quality care.
- Practice Tools Cutting-edge resources to improve your patient care.
- New Technology & Techniques The latest innovations in GI.
- Quality & Performance Measures Support to meet reporting requirements.
- Reimbursement Tools to understand policies and advocate for reimbursement.
- GI Patient Center By specialists, for patients.

Research & Awards
Funding opportunities and other initiatives advancing discovery.
- Research Awards More than $2 million in annual research funding.
- Registries & Studies Data to support new techs and treatments.
- Gut Microbiome One of GI’s most promising areas of research.
- AGA Research Foundation Funding the future of gastroenterology.

Fellows & Early Career
Resources designed for early career gastroenterologists.
- Resources Resources for every stage of your career.
- Fellowship Match Information for programs and candidates.
- AGA GTE® The first training exam for GI programs and fellows.
- Mentoring Connect with prospective mentors.
- Job Board Find your next opportunity.
- November 17, 2023
First comprehensive guideline on using biomarkers for monitoring Crohn’s disease
Bethesda, MD (Nov. 17, 2023) — The American Gastroenterological Association (AGA) released a new evidence-based guideline recommending the use of blood and stool-based biomarkers to help manage Crohn’s disease, a type of inflammatory bowel disease (IBD). IBD is estimated to affect 2.74 million people in the U.S . The guideline was published today in Gastroenterology.
Biomarkers are blood or stool tests that can give more information on an underlying disease process. In the context of IBD, biomarkers such as C-reactive protein (CRP) in blood and fecal calprotectin (FCP) in stool, can measure levels of inflammation. These levels can help doctors assess whether a patient’s Crohn’s disease is active or in remission.
AGA recommends the use of biomarkers in addition to colonoscopy and imaging studies.
“Patients’ symptoms do not always match endoscopic findings, so biomarkers are a useful tool to understand and monitor the status of inflammation and guide decision making in patients with Crohn’s disease,” says guideline author Siddarth Singh, MD, MS, University of California, San Diego.
For patients in remission:
- Check CRP and FCP every six to 12 months.
- These tests work best if CRP and FCP levels have previously matched with disease activity seen on endoscopic assessment.
For patients experiencing active symptoms:
- Check CRP and FCP every two to four months for patients experiencing an increase in symptoms (diarrhea and abdominal pain) to guide treatment adjustments.
- Before making any major treatment plan changes, consider repeating endoscopic or radiologic assessments.
For patients after surgery:
- FCP may be useful to monitor patients at low risk for disease recurrence.
- However, radiologic or endoscopic assessment should be performed when a post-operative recurrence is suspected rather than relying on biomarkers.
“Based on this guideline, biomarkers are no longer considered experimental and should be an integral part of IBD care,” says guideline author Ashwin Ananthakrishnan, MBBS, MPH, Massachusetts General Hospital.
This is a win for Crohn’s disease patients. Biomarkers are usually easier to obtain, less invasive, more cost-effective than frequent colonoscopies and can be assessed more frequently for tighter disease control and better long-term outcomes in Crohn’s disease.”
About Crohn’s disease
Crohn’s disease is a chronic inflammatory condition that can affect any part of the digestive tract, from the mouth to the anus. It causes inflammation and damage to the digestive system, leading to symptoms such as abdominal pain, diarrhea, weight loss and fatigue and complications such as strictures and fistulas. Crohn’s disease is a lifelong condition with periods of active symptoms (flare-ups) and periods of remission when symptoms are less severe or absent. It can be diagnosed at any age but is most often diagnosed between ages 13 and 30. It can vary in severity and usually requires ongoing medical management to control symptoms and improve quality of life. Learn more in the AGA GI Patient Center.
Resources
Guideline:
https://www.gastrojournal.org/article/S0016-5085(23)05064-3/fulltext
Clinical decision support tool:
https://www.gastrojournal.org/article/S0016-5085(23)05153-3/fulltext
Spotlight (infographic):
https://www.gastrojournal.org/article/S0016-5085(23)05154-5/fulltext
Media contact: Mara Shapiro, [email protected]
About the AGA Institute
The American Gastroenterological Association is the trusted voice of the GI community. Founded in 1897, the AGA has grown to more than 16,000 members from around the globe who are involved in all aspects of the science, practice and advancement of gastroenterology. The AGA Institute administers the practice, research and educational programs of the organization. www.gastro.org .
About Gastroenterology
Gastroenterology is the most prominent journal in the field of gastrointestinal disease. As the official journal of the AGA Institute, Gastroenterology delivers up-to-date and authoritative coverage of both basic and clinical gastroenterology. Regular features include articles by leading authorities and reports on the latest treatments for diseases. Original research is organized by clinical and basic-translational content, as well as by alimentary tract, liver, pancreas, and biliary content. www.gastrojournal.org/
AGA is now on Instagram . Like AGA and Gastroenterology on Facebook. Follow us on Twitter @AmerGastroAssn and @AGA_Gastro . Check out our videos on YouTube . Follow AGA on LinkedIn .
AGA clinical guidance
Find the latest evidence-based recommendations for treating your patients.

4930 Del Ray Avenue, Bethesda, MD 20814 301-654-2055
Connect with aga.
© American Gastroenterological Association

- Privacy Overview
- Strictly Necessary Cookies
- Cookie Policy
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
More information about our Cookie Policy
- skip to Cookie Notice
- skip to Main Navigation
- skip to Main Content
- skip to Footer
- Find a Doctor
- Find a Location
- Appointments & Referrals
- Patient Gateway
- Español
- Leadership Team
- Quality & Safety
- Equity & Inclusion
- Community Health
- Education & Training
- Centers & Departments
- Browse Treatments
- Browse Conditions A-Z
- View All Centers & Departments
- Clinical Trials
- Cancer Clinical Trials
- Cancer Center
- Digestive Healthcare Center
- Heart Center
- Mass General for Children
- Neuroscience
- Orthopaedic Surgery
- Information for Visitors
- Maps & Directions
- Parking & Shuttles
- Services & Amenities
- Accessibility
- Visiting Boston
- International Patients
- Medical Records
- Billing, Insurance & Financial Assistance
- Privacy & Security
- Patient Experience
- Explore Our Laboratories
- Industry Collaborations
- Research & Innovation News
- About the Research Institute
- Innovation Programs
- Education & Community Outreach
- Support Our Research
- Find a Researcher
- News & Events
- Ways to Give
- Patient Rights & Advocacy
- Website Terms of Use
- Apollo (Intranet)
- Like us on Facebook
- Follow us on Twitter
- See us on LinkedIn
- Print this page
Press Release Aug | 22 | 2022
New Insights into the Mechanisms Behind Crohn's Disease Point to Potential Therapeutic Target
Key takeaways.
- Mutations within Speckled Protein 140 (SP140) are associated with an increased risk of Crohn’s disease
- New research reveals that SP140 loss results in unleashed activity of a particular enzyme
- Inhibitors of this enzyme can reverse intestinal abnormalities in mice with inflammation characteristic of Crohn’s disease
BOSTON – The structure of chromatin—the mixture of DNA and proteins that form chromosomes—can affect gene expression, and certain chromatin “readers” are important for monitoring this structure often in response to environmental cues.
Mutations within one such reader, called Speckled Protein 140 (SP140), are associated with an increased risk of certain immune diseases, including Crohn’s disease, a type of inflammatory bowel disease.
New research led by investigators at Massachusetts General Hospital (MGH) and published in Cell provides insights into the mechanisms behind this link, pointing to potential therapeutic targets.
SP140 expression is uniquely restricted to immune cells such as macrophages, which surround and kill microorganisms, remove dead cells, and stimulate the action of other immune cells.
Protein analyses by Kate L. Jeffrey, PhD, a principal investigator of immunology at MGH and an associate professor of medicine at Harvard Medical School, and her colleagues revealed that SP140 represses topoisomerases (TOP), which are enzymes that help DNA untangle during replication.
The team also found that in humans and mice, SP140 loss resulted in unleashed TOP activity, ultimately leading to defective gene expression and bacterial killing by macrophages that caused intestinal abnormalities. Inhibiting TOP rescued these defects in mice with inflammation characteristic of Crohn’s disease, a condition that remains incurable by surgical or therapeutic interventions.
“Applying a combination of human genetics, proteomics, biochemistry, utilization of primary immune cells from Crohn’s disease individuals, and in vivo animal studies, our study highlights the power of examining human disease associated genetic mutations to advance mechanistic understanding of disease,” says Jeffrey. “The work broadens our understanding of epigenetics in health—or the physical changes in cells’ DNA structure that affect the expression of genes in response to environmental cues. Importantly though, it revealed how dysregulation of epigenetic factors drive diseases such as Crohn’s that are rising in incidence because of the complex interplay of genes plus environment.”
Several TOP inhibitors are approved for the treatment of certain cancers, and many in the drug class are being tested in ongoing cancer clinical trials. These latest findings indicate that clinical trials should also test their effectiveness against Crohn’s disease.
Additional co-authors include Hajera Amatullah, Isabella Fraschilla, Sreehaas Digumarthi, Julie Huang, Fatemeh Adiliaghdam, Gracia Bonilla, Lai Ping Wong, Marie-Eve Rivard, Claudine Beauchamp, Virginie Mercier, Philippe Goyette, Ruslan I. Sadreyev, Robert M. Anthony, and John Rioux.
This study was supported by the Canadian Institutes of Health Research, the Canada Foundation for Innovation, the National Institutes of Health and the MGH Research Scholar Program. Dr. Jeffrey is a John Lawrence MGH Research Scholar 2020-2025 .
About the Massachusetts General Hospital
Massachusetts General Hospital, founded in 1811, is the original and largest teaching hospital of Harvard Medical School. The Mass General Research Institute conducts the largest hospital-based research program in the nation, with annual research operations of more than $1 billion and comprises more than 9,500 researchers working across more than 30 institutes, centers and departments. In July 2022, Mass General was named #8 in the U.S. News & World Report list of "America’s Best Hospitals." MGH is a founding member of the Mass General Brigham healthcare system.
- Press Release
Centers and Departments
- Research Institute
- Allergy & Immunology
- Center for Immunology and Inflammatory Diseases
- Digestive Health
Check out the Mass General Research Institute blog
Bench Press highlights the groundbreaking research and boundary-pushing scientists working to improve human health and fight disease.
Support Research at Mass General
Your gift helps fund groundbreaking research aimed at understanding, treating and preventing human disease.
Inspiring Canadians to live better.
Advertisement
The future of crohn's disease: breakthrough research reveals cause and new treatment options, the genetic, environmental and microbial (gem) project, launched in 2008 by crohn’s and colitis canada and its research partners, has revealed that years before the development of crohn’s disease, the gut bacteria of those who go on to develop it is different from those who remain healthy..
by Karen Hawthorne
Share This Story

Faber’s sister in nearby Nanaimo, Jessica McCloskey, was diagnosed with Crohn ’s disease soon after she had her son, who is now 19, finally putting a label on her debilitating symptoms and moving her forward to treatment.
Identifying symptoms and diagnosing Crohn’s disease
Crohn’s disease and ulcerative colitis are the two main forms of inflammatory bowel disease (IBD) , a lifelong autoimmune condition where your immune system mistakenly attacks the lining of your gastrointestinal (GI) tract and interferes with your ability to digest food, absorb nutrients and excrete waste in a healthy way. Crohn’s can strike anywhere from mouth to anus, but it’s usually located in the lower part of the small bowel or the colon.
Related Stories
Crohn's and colitis canada: inflammatory bowel disease also comes with stigma, cancer risk and mental health issues.
Crohn's disease: What it feels like

Crohn’s disease: Do you know the signs?
ADVERTISEMENT
Signs to watch for include diarrhea, constipation, rectal bleeding, blood in stool, weight loss, joint pain and fatigue, according to Crohn’s and Colitis Canada . People can experience periods of acute symptom flare ups and also periods when symptoms go into remission – but there is no cure.
While the exact cause of the disease isn’t known, factors have been linked to the environment and the gut microbiome, and a genetic predisposition, so people with family members with the disease are at greater risk. And the prevalence is rising, with 322,600 Canadians estimated to be living with IBD in 2023 and a jump to 470,000 expected by 2035 (1.1 per cent of the population). Crohn’s and Colitis Canada recently issued the 2023 Impact of Inflammatory Bowel Disease in Canada report to flag the rapidly increasing number of people with IBD and improve access to care.
The Genetic, Environmental, and Microbial (GEM) Project: Uncovering new paths in Crohn’s disease research
The Genetic, Environmental and Microbial (GEM) Project , launched in 2008 by Crohn’s and Colitis Canada and its research partners, has revealed that years before the development of Crohn’s disease, the gut bacteria of those who go on to develop it is different from those who remain healthy.
Gastroenterologist Dr. Ken Croitoru of Mount Sinai Hospital in Toronto , part of Sinai Health, is the GEM project architect and lead investigator on the study. He says the discovery of a pattern of gut bacteria as a trigger for Crohn’s disease will benefit patients through specific treatments focused on the gut bacteria or diet modification that affects the gut.

The complex nature of Crohn’s disease and the role of gut bacteria
Complex chronic diseases like Crohn’s disease, can’t be traced to a single bacteria. BBC Science describes the human gut microbiome as where “thousands of native bacteria live in partnership with us. They survive a hostile environment of darkness, high acidity and low oxygen, in what is a tumultuous river flushing through the stomach and intestines.”
Raising awareness and understanding the signs of Crohn’s disease
Share story.

Digestive Diseases
- Advances in the treatment of Crohn's disease and ulcerative colitis
Feb. 09, 2019
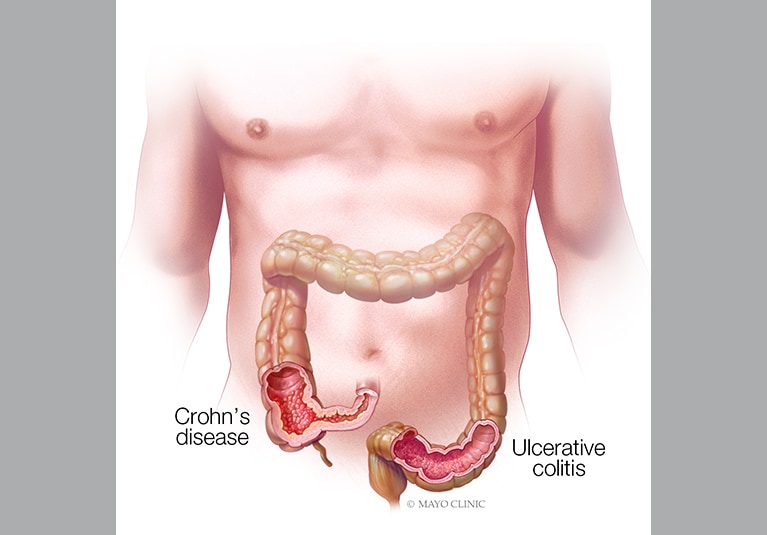
Inflammatory bowel disease (IBD) is a chronic inflammatory disease that can cause progressive functional and structural damage to the gastrointestinal tract. IBD is a global disease with increasing prevalence. In this article, Mayo gastroenterologists provide an overview of new treatment approaches for Crohn's disease and ulcerative colitis.
Treat-to-target approach
Medical therapies for IBD have traditionally focused on symptom control. While the use of oral aminosalicylates and corticosteroids can be effective in suppressing the inflammatory process and inducing symptomatic remission, this approach has not been shown to alter the natural history of IBD, reduce incidence of long-term complications or improve long-term patient outcomes. This fact, combined with the availability of other therapeutic approaches that can induce mucosal healing, has led clinicians and researchers to question whether symptom control is the most appropriate therapeutic goal in the treatment of IBD.
According to Edward V. Loftus Jr., M.D. , a gastroenterologist specializing in IBD at Mayo Clinic's campus in Rochester, Minnesota, many experts are now advocating for a paradigm shift that emphasizes mucosal healing, rather than clinical remission, as the primary treatment objective. "Administering therapies directed at mucosal healing that may favorably modify the natural history of IBD when used in a treat-to-target approach is gaining acceptance," says Dr. Loftus.
Disease severity assessment tools
Dr. Loftus notes that it's important to recognize that accurate assessment of disease activity and severity in IBD continues to be challenging. Two widely used tools, the symptom-based Crohn's disease activity index (CDAI) and the Crohn's disease endoscopic index of severity (CDEIS), often paint very different pictures of disease activity.
"In patients with Crohn's disease undergoing treatment with prednisolone, research data shows a complete lack of correlation between the CDAI and the CDEIS," explains Dr. Loftus. "This suggests that focusing on severity of symptoms alone may be an inappropriate measure of therapeutic efficacy because Crohn's disease symptoms are insensitive and nonspecific for bowel inflammation."
The presence of both inflammation and structural bowel damage in asymptomatic patients also underscores the utility of obtaining endoscopic evidence for mucosal healing, or other objective markers of inflammation, to guide therapeutic decisions and to evaluate their efficacy in IBD treatment trials. For patients with small bowel Crohn's disease, cross-sectional imaging such as computerized tomography enterography or magnetic resonance enterography may be a more appropriate modality to assess inflammation, extent and complications.
"A focus on mucosal or radiographic healing reduces the need for steroids, risk of hospitalization and surgery," says Dr. Loftus, "so treatment algorithms that incorporate endoscopy or enterography results into decision-making may do a better job of achieving long-term remission and reducing complications."
In addition to a treat-to-target approach, Talha A. Malik, M.D., M.P.H., notes that Mayo IBD specialists are also implementing and encouraging the development of a standardized approach to the management of IBD for Crohn's disease and ulcerative colitis that is systematic and evidence-based across all specialized IBD practices, clinics and centers. Dr. Malik is a gastroenterologist specializing in IBD at Mayo Clinic's campus in Arizona. "Our goal is to provide in-depth interpretation of test results, improved understanding of disease phenotype and severity, as well as management options that are consistent and driven by best research evidence."
New medications
The immunology of IBD is very complex, and drug targeting is complex. Within the United States, four anti-TNF agents are currently approved for the treatment of IBD — infliximab, adalimumab, certolizumab pegol and golimumab. Biosimilars to these anti-TNF agents have also been developed for use within the United States. Two anti-integrin biologics (natalizumab and vedolizumab) have been approved for use in IBD treatment. And ustekinumab, a biologic that targets cytokines interleukin-12 and interleukin-23 (IL-12 and IL-23), has been approved for Crohn's disease treatment.
According to Michael F. Picco, M.D., Ph.D. , the approach to maximizing the effectiveness of these medications often includes therapeutic drug monitoring. Dr. Picco is a gastroenterologist specializing in IBD at Mayo Clinic's campus in Florida. "Among patients with an incomplete response to a particular medication, measuring serum levels of a drug can help gain insight into whether a dosing adjustment or a switch to another agent is needed," says Dr. Picco.
Vedolizumab
Approved in 2014 for both ulcerative colitis and Crohn's disease, vedolizumab blocks migration of leukocytes into the gut via a blockade of α4β7 integrin (the ligand of which is mucosal vascular addressin cell adhesion molecule 1) and can be considered as a first line agent. Due to its gut-selective approach, this drug may be a good choice for older patients or those with a history of immunosuppression or malignancy.
Ustekinumab
Approved in 2016 for Crohn's disease only, ustekinumab blocks inflammation produced through IL-12 and IL-23. This molecule, too, can be considered a first line biologic agent, for older adults in particular, and for those with a history of immunosuppression or malignancy and those who have already undergone treatment with anti-TNF agents.
Tofacitinib
Approved in 2018, tofacitinib is a selective Janus kinase (JAK) inhibitor and the first oral medication approved for treatment of moderate to severe ulcerative colitis. The Food and Drug Administration examined results from three controlled clinical trials to assess tofacitinib's efficacy and safety. In two placebo-controlled induction trials — OCTAVE Induction 1 and 2 — tofacitinib outperformed the placebo in achieving remission in patients with UC. Published results for these two eight-week placebo-controlled trials demonstrated that 10 mg of tofacitinib given twice daily induces remission in 17 to 18 percent of patients by week eight. Among responders at week eight, tofacitinib maintained remission in 34 to 41 percent of patients at the end of one year, with steroid-free remission rates that were 30 to 42 percent better than those seen with placebo.
According to Dr. Loftus, multiple other potential drugs are now in the development pipeline, including several molecules that are beginning or more than halfway through phase III trials. JAK inhibitors such as upadacitinib and filgotinib are being studied in phase II and III trials of ulcerative colitis and Crohn's disease. Both of these molecules are selective JAK1 inhibitors, and data from phase II trials demonstrated their effectiveness as treatments for moderate to severe Crohn's disease.
Etrolizumab, an anti-β7 integrin, was effective in moderate to severe ulcerative colitis in a phase II trial and so far in an open-label induction trial in phase III. It also looks promising in induction of moderate to severe Crohn's disease.
The anti-mucosal addressin cell adhesion molecule (MAdCAM) monoclonal antibody (renamed SHP647) was effective in ulcerative colitis but not in Crohn's disease in phase II trials.
The anti-p19 (anti-IL-23) antibodies, brazikumab and risankizumab, were effective in moderate to severe Crohn's disease in phase II trials. Another p19 antibody, mirikizumab, was recently shown in a phase II trial to be effective for moderate to severe ulcerative colitis.
The oral sphingosine 1-phosphate (S1P) receptor modulator, ozanimod, was effective for moderate to severe ulcerative colitis in a phase II trial and appeared promising for moderate to severe Crohn's disease in an open-label induction trial. Another S1P receptor modulator, etrasimod, was recently shown to be effective for clinical response, clinical remission and endoscopic response in a phase II trial of moderate to severe ulcerative colitis.
"With multiple agents with different mechanisms of action for our patients with IBD under study, the future looks bright," says Dr. Loftus.
Receive Mayo Clinic news in your inbox.
Related content.

- Medical Professionals
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 02 April 2020
Crohn’s disease
- Giulia Roda 1 ,
- Siew Chien Ng 2 ,
- Paulo Gustavo Kotze 3 ,
- Marjorie Argollo 1 ,
- Remo Panaccione 4 ,
- Antonino Spinelli 5 , 6 ,
- Arthur Kaser 7 ,
- Laurent Peyrin-Biroulet 8 &
- Silvio Danese 1 , 6
Nature Reviews Disease Primers volume 6 , Article number: 22 ( 2020 ) Cite this article
31k Accesses
386 Citations
130 Altmetric
Metrics details
- Crohn's disease
- Gastrointestinal diseases
An Author Correction to this article was published on 19 June 2020
An Author Correction to this article was published on 20 May 2020
A Publisher Correction to this article was published on 06 April 2020
This article has been updated
Crohn’s disease is an inflammatory bowel disease that is characterized by chronic inflammation of any part of the gastrointestinal tract, has a progressive and destructive course and is increasing in incidence worldwide. Several factors have been implicated in the cause of Crohn’s disease, including a dysregulated immune system, an altered microbiota, genetic susceptibility and environmental factors, but the cause of the disease remains unknown. The onset of the disease at a young age in most cases necessitates prompt but long-term treatment to prevent disease flares and disease progression with intestinal complications. Thus, earlier, more aggressive treatment with biologic therapies or novel small molecules could profoundly change the natural history of the disease and decrease complications and the need for hospitalization and surgery. Although less invasive biomarkers are in development, diagnosis still relies on endoscopy and histological assessment of biopsy specimens. Crohn’s disease is a complex disease, and treatment should be personalized to address the underlying pathogenetic mechanism. In the future, disease management might rely on severity scores that incorporate prognostic factors, bowel damage assessment and non-invasive close monitoring of disease activity to reduce the severity of complications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Location is important: differentiation between ileal and colonic Crohn’s disease
Raja Atreya & Britta Siegmund
Microscopic colitis
Kristin E. Burke, Mauro D’Amato, … Hamed Khalili
Ulcerative colitis
Taku Kobayashi, Britta Siegmund, … Toshifumi Hibi
Change history
19 june 2020.
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
20 May 2020
06 april 2020.
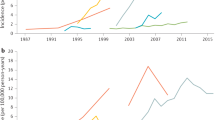
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390 , 2769–2778 (2018). This study provides a comprehensive analysis of the global IBD epidemiology .
Article Google Scholar
Torres, J., Mehandru, S., Colombel, J.-F. & Peyrin-Biroulet, L. Crohn’s disease. Lancet 389 , 1741–1755 (2017).
Article PubMed Google Scholar
Thia, K. T., Sandborn, W. J., Harmsen, W. S., Zinsmeister, A. R. & Loftus, E. V. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 139 , 1147–1155 (2010).
Fiorino, G., Bonifacio, C., Peyrin-Biroulet, L. & Danese, S. Preventing collateral damage in Crohn’s disease: the Lémann index. J. Crohns Colitis 10 , 495–500 (2016). This study clearly shows the importance of assessing bowel damage in a very early inflammatory stage of CD. The authors demonstrate that the presence of bowel damage in early CD is associated with a worse outcome .
Article PubMed PubMed Central Google Scholar
Zeng, Z. et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong province, China: a prospective population-based study. J. Gastroenterol. Hepatol. 28 , 1148–1153 (2013).
Zhao, J. et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of ‘western’ disease. Inflamm. Bowel Dis. 19 , 1839–1845 (2013).
PubMed Google Scholar
Ng, S. C. et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology 145 , 158–165.e2 (2013).
Kim, H. J. et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm. Bowel Dis. 21 , 623–630 (2015).
Park, S. H. et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong district of Seoul, Korea in 1986–2015. J. Crohns Colitis 13 , 1410–1417 (2019).
Ananthakrishnan, A. N. et al. Environmental triggers in IBD: a review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 15 , 39–49 (2018).
Bernstein, C. N. et al. Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm. Bowel Dis. 25 , 360–368 (2019).
Moradkhani, A., Beckman, L. J. & Tabibian, J. H. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J. Crohns Colitis 7 , 467–473 (2013).
Shah, S. C., Colombel, J.-F., Sands, B. E. & Narula, N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment. Pharmacol. Ther. 43 , 317–333 (2016).
Article CAS PubMed Google Scholar
Kaplan, G. G. & Ng, S. C. Globalisation of inflammatory bowel disease: perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 1 , 307–316 (2016).
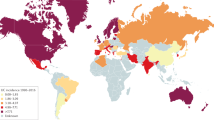
Ng, S. C. et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 62 , 630–649 (2013).
Yen, H.-H. et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide population-based study. Intest. Res. 17 , 54–62 (2019).
Ng, S. C. et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm. Bowel Dis. 22 , 1954–1960 (2016).
Mansour-Ghanaei, F. et al. Epidemiologic features of inflammatory bowel disease in Guilan province, north of Iran, during 2002-2012. Middle East. J. Dig. Dis. 7 , 69–74 (2015).
PubMed PubMed Central Google Scholar
Linares de la Cal, J. A., Cantón, C., Hermida, C., Pérez-Miranda, M. & Maté-Jiménez, J. Estimated incidence of inflammatory bowel disease in Argentina and Panama (1987–1993). Rev. Esp. Enferm. Dig. 91 , 277–286 (1999).
CAS PubMed Google Scholar
Piovani, D. et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 157 , 647–659.e4 (2019).
Lakatos, P. L. et al. Is current smoking still an important environmental factor in inflammatory bowel diseases? Results from a population-based incident cohort. Inflamm. Bowel Dis. 19 , 1010–1017 (2013).
Kondo, K. et al. The association between environmental factors and the development of Crohn’s disease with focusing on passive smoking: a multicenter case-control study in Japan. PLoS One 14 , e0216429 (2019).
Article CAS PubMed PubMed Central Google Scholar
Ng, S. C. et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut 64 , 1063–1071 (2015).
Levine, A., Sigall Boneh, R. & Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 67 , 1726–1738 (2018).
Khalili, H. et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: results from two large prospective cohort studies. Gut https://doi.org/10.1136/gutjnl-2019-319505 (2020).
Ortizo, R. et al. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: a meta-analysis of case-controlled and cohort studies. Eur. J. Gastroenterol. Hepatol. 29 , 1064–1070 (2017).
Ananthakrishnan, A. N. et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann. Intern. Med. 156 , 350–359 (2012).
Moninuola, O. O., Milligan, W., Lochhead, P. & Khalili, H. Systematic review with meta-analysis: association between acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) and risk of Crohn’s disease and ulcerative colitis exacerbation. Aliment. Pharmacol. Ther. 47 , 1428–1439 (2018).
Ungaro, R. et al. Statins associated with decreased risk of new onset inflammatory bowel disease. Am. J. Gastroenterol. 111 , 1416–1423 (2016).
Green, N., Miller, T., Suskind, D. & Lee, D. A review of dietary therapy for IBD and a vision for the future. Nutrients 11 , E947 (2019).
Article PubMed CAS Google Scholar
Halfvarson, J., Bodin, L., Tysk, C., Lindberg, E. & Järnerot, G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology 124 , 1767–1773 (2003).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411 , 599–603 (2001).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411 , 603–606 (2001).
Yamazaki, K. et al. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn’s disease. Hum. Mol. Genet. 14 , 3499–3506 (2005).
Huang, H. et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 547 , 173–178 (2017).
Ellinghaus, D. et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 48 , 510–518 (2016).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491 , 119–124 (2012).
Ogura, Y. et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut 52 , 1591–1597 (2003).
Sidiq, T., Yoshihama, S., Downs, I. & Kobayashi, K. S. Nod2: a critical regulator of ileal microbiota and Crohn’s disease. Front. Immunol. 7 , 367 (2016).
Article PubMed PubMed Central CAS Google Scholar
Hampe, J. et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39 , 207–211 (2007).
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47 , 979–986 (2015).
Hong, M. et al. Immunochip meta-analysis of inflammatory bowel disease identifies three novel loci and four novel associations in previously reported loci. J. Crohns Colitis 12 , 730–741 (2018).
Zhu, L. et al. IL-10 and IL-10 receptor mutations in very early onset inflammatory bowel disease. Gastroenterology Res. 10 , 65–69 (2017).
Uniken Venema, W. T., Voskuil, M. D., Dijkstra, G., Weersma, R. K. & Festen, E. A. The genetic background of inflammatory bowel disease: from correlation to causality. J. Pathol. 241 , 146–158 (2017).
Cleynen, I. et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet 387 , 156–167 (2016).
Peterson, L. W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14 , 141–153 (2014).
Zeissig, S. et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56 , 61–72 (2007).
Weber, C. R., Nalle, S. C., Tretiakova, M., Rubin, D. T. & Turner, J. R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Invest. 88 , 1110–1120 (2008).
Odenwald, M. A. & Turner, J. R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 14 , 9–21 (2017).
Wehkamp, J. et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 53 , 1658–1664 (2004).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456 , 259–263 (2008).
Thachil, E. et al. Abnormal activation of autophagy-induced crinophagy in Paneth cells from patients with Crohn’s disease. Gastroenterology 142 , 1097–1099.e4 (2012).
Zhang, Q. et al. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat. Immunol. 16 , 918–926 (2015).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134 , 743–756 (2008).
Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503 , 272–276 (2013).
Tschurtschenthaler, M. et al. Defective ATG16L1-mediated removal of IRE1α drives Crohn’s disease-like ileitis. J. Exp. Med. 214 , 401–422 (2017).
Willson, T. A., Jurickova, I., Collins, M. & Denson, L. A. Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm. Bowel Dis. 19 , 512–525 (2013).
Diamanti, M. A. et al. IKKα controls ATG16L1 degradation to prevent ER stress during inflammation. J. Exp. Med. 214 , 423–437 (2017).
Zhou, C., Qiu, Y. & Yang, H. CD4CD8αα IELs: they have something to say. Front. Immunol. 10 , 2269 (2019).
Regner, E. H. et al. Functional intraepithelial lymphocyte changes in inflammatory bowel disease and spondyloarthritis have disease specific correlations with intestinal microbiota. Arthritis Res. Ther. 20 , 149 (2018).
Catalan-Serra, I., Sandvik, A. K., Bruland, T. & Andreu-Ballester, J. C. Gammadelta T cells in Crohn’s disease: a new player in the disease pathogenesis? J. Crohns Colitis 11 , 1135–1145 (2017).
Hosomi, S. et al. Intestinal epithelial cell endoplasmic reticulum stress promotes MULT1 up-regulation and NKG2D-mediated inflammation. J. Exp. Med. 214 , 2985–2997 (2017).
Allez, M., Skolnick, B. E., Wisniewska-Jarosinska, M., Petryka, R. & Overgaard, R. V. Anti-NKG2D monoclonal antibody (NNC0142-0002) in active Crohn’s disease: a randomised controlled trial. Gut 66 , 1918–1925 (2017).
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28 , 573–621 (2010).
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J. Med. 361 , 2066–2078 (2009).
Ouellette, A. J. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroenterol. 26 , 547–553 (2010).
de Souza, H. S. P. & Fiocchi, C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13 , 13–27 (2016).
Uhlig, H. H. & Powrie, F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu. Rev. Immunol. 36 , 755–781 (2018).
Pazmandi, J., Kalinichenko, A., Ardy, R. C. & Boztug, K. Early-onset inflammatory bowel disease as a model disease to identify key regulators of immune homeostasis mechanisms. Immunol. Rev. 287 , 162–185 (2019).
Cooney, R. et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 16 , 90–97 (2010).
Travassos, L. H. et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11 , 55–62 (2010).
Segal, A. W. The role of neutrophils in the pathogenesis of Crohn’s disease. Eur. J. Clin. Invest. 48 , e12983 (2018).
Geremia, A. & Arancibia-Cárcamo, C. V. Innate lymphoid cells in intestinal inflammation. Front. Immunol. 8 , 1296 (2017).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of CD127 + group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43 , 146–160 (2015).
van der Gracht, E., Zahner, S. & Kronenberg, M. When insult is added to injury: cross talk between ILCs and intestinal epithelium in IBD. Mediators Inflamm. 2016 , 9765238 (2016).
Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25 , 309–318 (2006).
Feagan, B. G. et al. Ustekinumab as Induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375 , 1946–1960 (2016).
Feagan, B. G. et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 389 , 1699–1709 (2017).
Sands, B. E. et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 153 , 77–86.e6 (2017).
Sarin, R., Wu, X. & Abraham, C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc. Natl Acad. Sci. USA 108 , 9560–9565 (2011).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314 , 1461–1463 (2006).
Fantini, M. C. et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology 136 , 1308–1316 (2009).
Lo Presti, A. et al. Fecal and mucosal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease. Front. Microbiol. 10 , 1655 (2019).
Vich Vila, A. et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl Med. 10 , eaap8914 (2018).
Pascal, V. et al. A microbial signature for Crohn’s disease. Gut 66 , 813–822 (2017).
Palmela, C. et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 67 , 574–587 (2018).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105 , 16731–16736 (2008).
Barnich, N. & Darfeuille-Michaud, A. Adherent-invasive Escherichia coli and Crohn’s disease. Curr. Opin. Gastroenterol. 23 , 16–20 (2007).
Simpson, K. W. et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect. Immun. 74 , 4778–4792 (2006).
Yilmaz, B. et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat. Med. 25 , 323–336 (2019).
Libertucci, J. et al. Inflammation-related differences in mucosa-associated microbiota and intestinal barrier function in colonic Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 315 , G420–G431 (2018).
Vieira-Silva, S. et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat. Microbiol. 4 , 1826–1831 (2019).
Norman, J. M. et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160 , 447–460 (2015).
Pérez-Brocal, V. et al. Study of the viral and microbial communities associated with Crohn’s disease: a metagenomic approach. Clin. Transl. Gastroenterol. 4 , e36 (2013).
Imai, T. et al. Characterization of fungal dysbiosis in Japanese patients with inflammatory bowel disease. J. Gastroenterol. 54 , 149–159 (2019).
Feuerstein, J. D. & Cheifetz, A. S. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin. Proc. 92 , 1088–1103 (2017).
Gomollón, F. et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J. Crohns Colitis 11 , 3–25 (2017).
Kuriyama, M. et al. Specific gastroduodenoscopic findings in Crohn’s disease: comparison with findings in patients with ulcerative colitis and gastroesophageal reflux disease. Dig. Liver Dis. 40 , 468–475 (2008).
Sawczenko, A. & Sandhu, B. K. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch. Dis. Child. 88 , 995–1000 (2003).
Peyrin-Biroulet, L., Loftus, E. V., Colombel, J.-F. & Sandborn, W. J. The natural history of adult Crohn’s disease in population-based cohorts. Am. J. Gastroenterol. 105 , 289–297 (2010). This comprehensive article describes the natural history of CD .
Fiorino, G. et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn’s disease and its impact on disease outcome. J. Crohns Colitis 11 , 274–280 (2017).
Safroneeva, E. et al. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Aliment. Pharmacol. Ther. 42 , 977–989 (2015).
Peyrin-Biroulet, L. et al. Perianal Crohn’s disease findings other than fistulas in a population-based cohort. Inflamm. Bowel Dis. 18 , 43–48 (2012).
Ott, C. & Schölmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 10 , 585–595 (2013).
Park, S. H. et al. Update on the natural course of fistulizing perianal Crohn’s disease in a population-based cohort. Inflamm. Bowel Dis. 25 , 1054–1060 (2019).
Freeman, H. J. Natural history and long-term clinical course of Crohn’s disease. World J. Gastroenterol. 20 , 31–36 (2014).
Danese, S. et al. Development of red flags index for early referral of adults with symptoms and signs suggestive of Crohn’s disease: an IOIBD initiative. J. Crohns Colitis 9 , 601–606 (2015).
Vavricka, S. R. et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am. J. Gastroenterol. 106 , 110–119 (2011).
Jang, H.-J., Kang, B. & Choe, B.-H. The difference in extraintestinal manifestations of inflammatory bowel disease for children and adults. Transl. Pediatr. 8 , 4–15 (2019).
Peyrin-Biroulet, L., Loftus, E. V., Colombel, J.-F. & Sandborn, W. J. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn’s disease in population-based cohorts. Inflamm. Bowel Dis. 17 , 471–478 (2011). This comprehensive article describes long-term outcomes in patients with CD .
Pennazio, M. et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 47 , 352–376 (2015).
Koulaouzidis, A., Rondonotti, E. & Karargyris, A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J. Gastroenterol. 19 , 3726–3746 (2013).
Dionisio, P. M. et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am. J. Gastroenterol. 105 , 1240–1248 (2010).
Magro, F. et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohns Colitis 7 , 827–851 (2013).
Annese, V. et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohns Colitis 7 , 982–1018 (2013).
Tontini, G. E., Vecchi, M., Neurath, M. F. & Neumann, H. Advanced endoscopic imaging techniques in Crohn’s disease. J. Crohns Colitis 8 , 261–269 (2014).
Allocca, M., Fiorino, G. & Danese, S. Cross-sectional imaging modalities in Crohn’s disease. Dig. Dis. 31 , 199–201 (2013).
Chatu, S., Subramanian, V. & Pollok, R. C. G. Meta-analysis: diagnostic medical radiation exposure in inflammatory bowel disease. Aliment. Pharmacol. Ther. 35 , 529–539 (2012).
Horsthuis, K., Bipat, S., Bennink, R. J. & Stoker, J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 247 , 64–79 (2008).
Panés, J. et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment. Pharmacol. Ther. 34 , 125–145 (2011).
Sahni, V. A., Ahmad, R. & Burling, D. Which method is best for imaging of perianal fistula? Abdom. Imaging 33 , 26–30 (2008).
Allocca, M. et al. Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing Crohn’s disease and guiding clinical decision-making. J. Crohns Colitis 12 , 1280–1287 (2018).
Magro, F. et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohns Colitis 11 , 649–670 (2017).
Vermeire, S., Schreiber, S., Sandborn, W. J., Dubois, C. & Rutgeerts, P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin. Gastroenterol. Hepatol. 8 , 357–363 (2010).
Best, W. R. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw index. Inflamm. Bowel Dis. 12 , 304–310 (2006).
Mitsuyama, K. et al. Antibody markers in the diagnosis of inflammatory bowel disease. World J. Gastroenterol. 22 , 1304–1310 (2016).
Gu, P. et al. Serological, genetic and clinical associations with increased health-care resource utilization in inflammatory bowel disease. J. Dig. Dis. 19 , 15–23 (2018).
Plevy, S. et al. Combined serological, genetic, and inflammatory markers differentiate non-IBD, Crohn’s disease, and ulcerative colitis patients. Inflamm. Bowel Dis. 19 , 1139–1148 (2013).
Maaser, C. et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 13 , 144–164 (2019).
Vermeire, S., Van Assche, G. & Rutgeerts, P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm. Bowel Dis. 10 , 661–665 (2004).
Vermeire, S., Van Assche, G. & Rutgeerts, P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 55 , 426–431 (2006).
Solem, C. A. et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm. Bowel Dis. 11 , 707–712 (2005).
Cellier, C. et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 35 , 231–235 (1994).
Lakatos, P. L. et al. Serum lipopolysaccharide-binding protein and soluble CD14 are markers of disease activity in patients with Crohn’s disease. Inflamm. Bowel Dis. 17 , 767–777 (2011).
Kwon, J. H. et al. Disease phenotype, activity and clinical course prediction based on C-reactive protein levels at diagnosis in patients with Crohn’s disease: results from the CONNECT study. Gut Liver 10 , 595–603 (2016).
Carroccio, A. et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin. Chem. 49 , 861–867 (2003).
Diamanti, A. et al. Diagnostic work-up of inflammatory bowel disease in children: the role of calprotectin assay. Inflamm. Bowel Dis. 16 , 1926–1930 (2010).
Goutorbe, F. et al. Endoscopic factors influencing fecal calprotectin value in Crohn’s disease. J. Crohns Colitis 9 , 1113–1119 (2015).
van Rheenen, P. F., Van de Vijver, E. & Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 341 , c3369 (2010).
Suray, N. de et al. Close monitoring of CRP and fecal calprotectin is able to predict clinical relapse in patients with Crohn’s disease in remission after infliximab withdrawal. a sub-analysis of the Stori study. Gastroenterology 142 , S-149 (2012).
Orlando, A. et al. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn’s disease: a comparison with ultrasound. Eur. Rev. Med. Pharmacol. Sci. 10 , 17–22 (2006).
Guo, S. et al. A simple fecal bacterial marker panel for the diagnosis of Crohn’s disease. Front. Microbiol. 10 , 1306 (2019).
Marlicz, W., Skonieczna-Żydecka, K., Dabos, K. J., Łoniewski, I. & Koulaouzidis, A. Emerging concepts in non-invasive monitoring of Crohn’s disease. Ther. Adv. Gastroenterol. 11 , 1756284818769076 (2018).
Somineni, H. K. et al. Blood-derived DNA methylation signatures of Crohn’s disease and severity of intestinal inflammation. Gastroenterology 156 , 2254–2265.e3 (2019).
Leong, R. W. et al. Full-spectrum endoscopy improves surveillance for dysplasia in patients with inflammatory bowel diseases. Gastroenterology 152 , 1337–1344.e3 (2017).
Stidham, R. W. & Higgins, P. D. R. Colorectal cancer in inflammatory bowel disease. Clin. Colon. Rectal Surg. 31 , 168–178 (2018).
Tontini, G. E., Vecchi, M., Pastorelli, L., Neurath, M. F. & Neumann, H. Differential diagnosis in inflammatory bowel disease colitis: state of the art and future perspectives. World J. Gastroenterol. 21 , 21–46 (2015).
He, Y. et al. Development and validation of a novel diagnostic nomogram to differentiate between intestinal tuberculosis and Crohn’s disease: a 6-year prospective multicenter study. Am. J. Gastroenterol. 114 , 490–499 (2019).
Bae, J. H. et al. Development and validation of a novel prediction model for differential diagnosis between Crohn’s disease and intestinal tuberculosis. Inflamm. Bowel Dis. 23 , 1614–1623 (2017).
Lee, S. K., Kim, B. K., Kim, T. I. & Kim, W. H. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy 41 , 9–16 (2009).
Valenti, S., Gallizzi, R., De Vivo, D. & Romano, C. Intestinal Behçet and Crohn’s disease: two sides of the same coin. Pediatr. Rheumatol. Online J. 15 , 33 (2017).
Kedia, S. et al. Differentiating Crohn’s disease from intestinal tuberculosis. World J. Gastroenterol. 25 , 418–432 (2019).
Oliveira, S. B. & Monteiro, I. M. Diagnosis and management of inflammatory bowel disease in children. BMJ 357 , j2083 (2017).
Amre, D. K., Lu, S.-E., Costea, F. & Seidman, E. G. Utility of serological markers in predicting the early occurrence of complications and surgery in pediatric Crohn’s disease patients. Am. J. Gastroenterol. 101 , 645–652 (2006).
Gisbert, J. P., Marín, A. C. & Chaparro, M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment. Pharmacol. Ther. 42 , 391–405 (2015).
Peyrin-Biroulet, L. et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am. J. Gastroenterol. 110 , 1324–1338 (2015).
van Deen, W. K. et al. Value redefined for inflammatory bowel disease patients: a choice-based conjoint analysis of patients’ preferences. Qual. Life Res. 26 , 455–465 (2017).
Loy, L. et al. Detection and management of early stage inflammatory bowel disease: an update for clinicians. Expert Rev. Gastroenterol. Hepatol. 13 , 547–555 (2019).
Bewtra, M. et al. Inflammatory bowel disease patients’ willingness to accept medication risk to avoid future disease relapse. Am. J. Gastroenterol. 110 , 1675–1681 (2015).
Torres, J. et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J. Crohns Colitis 10 , 1385–1394 (2016).
Beaugerie, L., Seksik, P., Nion-Larmurier, I., Gendre, J.-P. & Cosnes, J. Predictors of Crohn’s disease. Gastroenterology 130 , 650–656 (2006).
Loly, C., Belaiche, J. & Louis, E. Predictors of severe Crohn’s disease. Scand. J. Gastroenterol. 43 , 948–954 (2008).
Beaugerie, L. & Sokol, H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J. Gastroenterol. 18 , 3806–3813 (2012).
Mao, R. et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm. Bowel Dis. 18 , 1894–1899 (2012).
Ghaly, S. et al. High vitamin D-binding protein concentration, low albumin, and mode of remission predict relapse in Crohn’s disease. Inflamm. Bowel Dis. 22 , 2456–2464 (2016).
Qin, G. et al. Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn’s Disease activity. Med. Sci. Monit. 22 , 4393–4400 (2016).
Allez, M. et al. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am. J. Gastroenterol. 97 , 947–953 (2002).
Nahon, S. et al. Diagnostic delay in a French cohort of Crohn’s disease patients. J. Crohns Colitis 8 , 964–969 (2014).
Maconi, G. et al. The impact of symptoms, irritable bowel syndrome pattern and diagnostic investigations on the diagnostic delay of Crohn’s disease: a prospective study. Dig. Liver Dis. 47 , 646–651 (2015).
Vavricka, S. R. et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm. Bowel Dis. 18 , 496–505 (2012).
Schoepfer, A. M. et al. Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am. J. Gastroenterol. 108 , 1744–1753 (2013).
Peyrin-Biroulet, L. et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: results of an international expert opinion process. Am. J. Gastroenterol. 107 , 1770–1776 (2012). This is the first description of early CD, a category of the disease defined by prognostic factors that predict a favourable response to early aggressive treatment .
Danese, S., Fiorino, G., Fernandes, C. & Peyrin-Biroulet, L. Catching the therapeutic window of opportunity in early Crohn’s disease. Curr. Drug Targets 15 , 1056–1063 (2014).
Høivik, M. L. et al. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN study. Gut 62 , 368–375 (2013).
Frøslie, K. F., Jahnsen, J., Moum, B. A., Vatn, M. H. & IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 133 , 412–422 (2007).
Colombel, J.-F. et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 390 , 2779–2789 (2018).
Peyrin-Biroulet, L. et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 63 , 88–95 (2014).
Louis, E. et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 142 , 63–70.e5 (2012).
Doherty, G. et al. European Crohn’s and Colitis Organisation topical review on treatment withdrawal [‘exit strategies’] in inflammatory bowel disease. J. Crohns Colitis 12 , 17–31 (2018).
Munkholm, P., Langholz, E., Davidsen, M. & Binder, V. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut 35 , 360–362 (1994).
Modigliani, R. et al. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Gastroenterology 98 , 811–818 (1990).
Lamb, C. A. et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68 , s1–s106 (2019).
Panés, J. et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology 145 , 766–774.e1 (2013).
Beaugerie, L. et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut 63 , 1416–1423 (2014).
Cosnes, J. et al. Early administration of azathioprine vs conventional management of Crohn’s disease: a randomized controlled trial. Gastroenterology 145 , 758–765.e2 (2013).
Chande, N., Townsend, C. M., Parker, C. E. & MacDonald, J. K. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 10 , CD000545 (2016).
Chatu, S., Subramanian, V., Saxena, S. & Pollok, R. C. G. The role of thiopurines in reducing the need for surgical resection in Crohn’s disease: a systematic review and meta-analysis. Am. J. Gastroenterol. 109 , 23–34 (2014).
Herfarth, H. H., Kappelman, M. D., Long, M. D. & Isaacs, K. L. Use of methotrexate in the treatment of inflammatory bowel diseases. Inflamm. Bowel Dis. 22 , 224–233 (2016).
Colombel, J. F. et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 362 , 1383–1395 (2010).
Dulai, P. S. et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am. J. Gastroenterol. 111 , 1147–1155 (2016).
Kariburyo, M. F., Xie, L., Teeple, A., Tan, H. & Ingham, M. Predicting pre-emptive discussions of biologic treatment: results from an openness and preference survey of inflammatory bowel disease patients and their prescribers. Adv. Ther. 34 , 1398–1410 (2017).
Sands, B. E. et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 381 , 1215–1226 (2019).
Vande Casteele, N. et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 64 , 1539–1545 (2015).
Nanda, K. S., Cheifetz, A. S. & Moss, A. C. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am. J. Gastroenterol. 108 , 40–47; quiz 48 (2013).
Seinen, M. L., De Boer, N. K. & van Bodegraven, A. A. Key insights from therapeutic drug monitoring in Crohn’s disease patients. Expert Opin. Drug Metab. Toxicol. 15 , 399–406 (2019).
Restellini, S., Khanna, R. & Afif, W. Therapeutic drug monitoring with ustekinumab and vedolizumab in inflammatory bowel disease. Inflamm. Bowel Dis. 24 , 2165–2172 (2018).
D’Amico, F., Fiorino, G., Furfaro, F., Allocca, M. & Danese, S. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin. Investig. Drugs 27 , 595–599 (2018).
Peyrin-Biroulet, L., Christopher, R., Behan, D. & Lassen, C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun. Rev. 16 , 495–503 (2017).
Ma, C., Jairath, V., Khanna, R. & Feagan, B. G. Investigational drugs in phase I and phase II clinical trials targeting interleukin 23 (IL23) for the treatment of Crohn’s disease. Expert Opin. Investig. Drugs 27 , 649–660 (2018).
Prideaux, L., Kamm, M. A., De Cruz, P. P., Chan, F. K. L. & Ng, S. C. Inflammatory bowel disease in Asia: a systematic review. J. Gastroenterol. Hepatol. 27 , 1266–1280 (2012).
Prideaux, L. et al. Comparison of clinical characteristics and management of inflammatory bowel disease in Hong Kong versus Melbourne. J. Gastroenterol. Hepatol. 27 , 919–927 (2012).
Gálvez, J. Role of Th17 cells in the pathogenesis of human IBD. ISRN Inflamm. 2014 , 928461 (2014).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61 , 1693–1700 (2012).
van der Giessen, J. et al. Modulation of cytokine patterns and microbiome during pregnancy in IBD. Gut 69 , 473–486 (2020).
van der Giessen, J., Huang, V. W., van der Woude, C. J. & Fuhler, G. M. Modulatory effects of pregnancy on inflammatory bowel disease. Clin. Transl. Gastroenterol. 10 , e00009 (2019).
Maunder, R. G., Cohen, Z., McLeod, R. S. & Greenberg, G. R. Effect of intervention in inflammatory bowel disease on health-related quality of life: a critical review. Dis. Colon Rectum 38 , 1147–1161 (1995).
Chen, X.-L. et al. Inflammatory bowel disease-specific health-related quality of life instruments: a systematic review of measurement properties. Health Qual. Life Outcomes 15 , 177 (2017).
Kaplan, G. G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12 , 720–727 (2015).
Cohen, R. D. The quality of life in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 16 , 1603–1609 (2002).
López Blanco, B., Moreno-Jiménez, B., Devesa Múgica, J. M. & Rodríguez Muñoz, A. Relationship between socio-demographic and clinical variables, and health-related quality of life in patients with inflammatory bowel disease. Rev. Esp. Enferm. Dig. 97 , 887–898 (2005). This study reveals the effect of IBD on QOL, which needs to be considered in clinical practice .
Blondel-Kucharski, F. et al. Health-related quality of life in Crohn’s disease: a prospective longitudinal study in 231 patients. Am. J. Gastroenterol. 96 , 2915–2920 (2001).
Andersson, P., Olaison, G., Bendtsen, P., Myrelid, P. & Sjödahl, R. Health related quality of life in Crohn’s proctocolitis does not differ from a general population when in remission. Colorectal Dis. 5 , 56–62 (2003).
Bernklev, T. et al. Course of disease, drug treatment and health-related quality of life in patients with inflammatory bowel disease 5 years after initial diagnosis. Eur. J. Gastroenterol. Hepatol. 17 , 1037–1045 (2005).
Casellas, F., López-Vivancos, J., Badia, X., Vilaseca, J. & Malagelada, J. R. Impact of surgery for Crohn’s disease on health-related quality of life. Am. J. Gastroenterol. 95 , 177–182 (2000).
Romberg-Camps, M. J. L. et al. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm. Bowel Dis. 16 , 2137–2147 (2010).
Schirbel, A. et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J. Gastroenterol. 16 , 3168–3177 (2010).
Katz, L. et al. Mechanisms of quality of life and social support in inflammatory bowel disease. J. Clin. Psychol. Med. Settings 23 , 88–98 (2016).
Reinink, A. R., Lee, T. C. & Higgins, P. D. R. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: a meta-analysis. Inflamm. Bowel Dis. 22 , 1859–1869 (2016).
Peyrin-Biroulet, L. et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin. Gastroenterol. Hepatol. 14 , 348–354.e17 (2016).
Pariente, B. et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm. Bowel Dis. 17 , 1415–1422 (2011).
Siegel, C. A. et al. Development of an index to define overall disease severity in IBD. Gut 67 , 244–254 (2018). This study discusses the development of severity indices to allow assessment of disease severity and bowel damage progression in the future .
Rieder, F. & Fiocchi, C. Intestinal fibrosis in inflammatory bowel disease - current knowledge and future perspectives. J. Crohns Colitis 2 , 279–290 (2008).
Burke, J. P. et al. Fibrogenesis in Crohn’s disease. Am. J. Gastroenterol. 102 , 439–448 (2007).
Allen, P. B., Gower-Rousseau, C., Danese, S. & Peyrin-Biroulet, L. Preventing disability in inflammatory bowel disease. Ther. Adv. Gastroenterol. 10 , 865–876 (2017).
McGovern, D. Personalized medicine in inflammatory bowel disease. Gastroenterol. Hepatol. 10 , 662–664 (2014).
Google Scholar
Siegel, C. A. et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment. Pharmacol. Ther. 43 , 262–271 (2016).
Download references
Acknowledgements
Work in the laboratory of A.K. is supported by the Wellcome Trust (Senior Investigator Award 106260/Z/14/Z) and the European Research Council (Consolidator Grant 648889).
Author information
Authors and affiliations.
IBD Center, Department of Gastroenterology, Humanitas Clinical and Research Center – IRCCS –, Rozzano, Italy
Giulia Roda, Marjorie Argollo & Silvio Danese
Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Science, Chinese University of Hong Kong, Hong Kong, China
Siew Chien Ng
Colorectal Surgery Unit, Catholic University of Paraná, Curitiba, Brazil
Paulo Gustavo Kotze
Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, University of Calgary, Calgary, Alberta, Canada
Remo Panaccione
Humanitas Clinical and Research Center – IRCCS –, Rozzano, Italy
Antonino Spinelli
Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy
Antonino Spinelli & Silvio Danese
Division of Gastroenterology and Hepatology, Department of Medicine, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK
Arthur Kaser
Department of Gastroenterology, University Hospital of Nancy, Nancy, France
Laurent Peyrin-Biroulet
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (G.R., L.P.-B. and S.D.); Epidemiology (S.C.N.); Mechanisms/pathophysiology (A.K.); Diagnosis, screening and prevention (P.G.K. and M.A.); Management (R.P.); Quality of life (G.R., A.S., L.P.-B. and S.D.); Outlook (G.R., A.S., L.P.-B. and S.D.).
Corresponding author
Correspondence to Silvio Danese .
Ethics declarations
Competing interests.
S.D. has served as a speaker, consultant and advisory board member for Schering-Plough, AbbVie, Merck Sharp & Dohme, UCB Pharma, Ferring, Cellerix, Takeda Pharmaceutical Company, Nycomed, Pharmacosmos, Actelion, Alpha Wasserman, Genentech, Grünenthal, Pfizer, AstraZeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor, Johnson & Johnson and Nikkiso Europe GmbH. L.P.-B. has received consulting fees from AbbVie, Amgen, Biogaran, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Ferring, Genentech, HAC Pharma, Hospira, Index Pharmaceuticals, Janssen, Lilly, Merck, Mitsubishi, Norgine, Pfizer, Pharmacosmos, Pilege, Sandoz, Takeda, Therakos, Tillotts, UCB Pharma and Vifor and lecture fees from AbbVie, Ferring, HAC Pharma, Janssen, Merck, Mitsubishi, Norgine, Takeda, Therakos, Tillotts and Vifor. A.S. has acted as a consultant or speaker for Ethicon, Olympus, Frankenman, Transenterix (not active), Tigenyx, Pfizer, Takeda and Sandoz. P.G.K has been a lecturer for AbbVie, Janssen, Pfizer and Takeda and is a member of the advisory board of AbbVie, Pfizer and Takeda. A.K. has served as an adviser to Boehringer Ingelheim, Ferring, Genentech, GlaxoSmithKline, Gilead, Hospira, Janssen, Pfizer, and VHSquared. R.P. has received consultant and/or lecture fees from AbbVie, Amgen, AstraZeneca, Axcan Pharma (now Aptalis), Biogen Idec, Bristol-Myers Squibb, Centocor, ChemoCentryx, Eisai Medical Research Inc., Elan Pharmaceuticals, Ferring, Genentech, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Millennium Pharmaceuticals (now Takeda Oncology), Ocera Therapeutics Inc., Otsuka America Pharmaceutical, Pfizer, Shire Pharmaceuticals, Prometheus Laboratories, Schering-Plough Corporation, Synta Pharmaceuticals Corp., Teva, UCB Pharma and Warner Chilcott. S.C.N. has received consulting and speaker fees from AbbVie, Ferring, Janssen, Menarini and Takeda, has served as a scientific advisory board member for AbbVie, Ferring and Takeda and has received research grants from AbbVie, Ferring and Janssen. The other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks B.D. Ye, A. Forbes, J. Gisbert, G. Monteleone, A. Regueiro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Roda, G., Chien Ng, S., Kotze, P.G. et al. Crohn’s disease. Nat Rev Dis Primers 6 , 22 (2020). https://doi.org/10.1038/s41572-020-0156-2
Download citation
Accepted : 14 February 2020
Published : 02 April 2020
DOI : https://doi.org/10.1038/s41572-020-0156-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Efficacy and safety of stem cell therapy for crohn’s disease: a meta-analysis of randomized controlled trials.
- Yunfeng Qiu
- Changfeng Li
- Shihou Sheng
Stem Cell Research & Therapy (2024)
Increased CpG methylation at the CDH1 locus in inflamed ileal mucosa of patients with Crohn disease
- Charles de Ponthaud
- Solafah Abdalla
- Pierre Bougnères
Clinical Epigenetics (2024)
YAP represses intestinal inflammation through epigenetic silencing of JMJD3
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials.
- Liuting Zeng
- Kailin Yang
- Lingyun Sun
BMC Medicine (2024)
Etrolizumab-s fails to control E-Cadherin-dependent co-stimulation of highly activated cytotoxic T cells
- Maximilian Wiendl
- Mark Dedden
- Sebastian Zundler
Nature Communications (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Connect with others who understand.

- Resources Ask the Expert: Your Crohn’s Questions Answered
New Crohn’s Disease Medications and Other Treatment Research

- There’s promising research on new treatment options for Crohn’s disease.
- Potential treatments now in clinical trials include fecal transplant and new classes of medications.
- The U.S. Food and Drug Administration (FDA) recently approved several new biologic drugs and the first oral drug to treat Crohn’s disease.

When you live with inflammatory bowel disease (IBD) like Crohn’s disease, it can feel like you’ve tried everything to manage your condition. As one MyCrohnsAndColitisTeam member shared, “I’m running out of treatment options.”
Keeping up with research and the latest advances in treatments can help you and your doctor find new options for the treatment of Crohn’s and to improve your quality of life. Research on IBD is ongoing in several areas, from understanding the causes of IBD to running clinical trials that test new treatments.

Promising Animal Studies
Developing new treatments often starts with scientists using lab-grown cells or animals to study genetic factors or environmental triggers that may influence why someone develops Crohn’s. They might also begin research after noticing something common among people living with a particular condition.
For example, a recent study looked at nine families of people with Crohn’s disease. The findings showed that a fungus called Candida tropicalis was more abundant in the people with Crohn’s compared with their relatives who didn’t have the condition. Scientists then examined mice with C. tropicalis infections and found differences in their gastrointestinal bacteria and immune systems that made the infected mice likely to develop intestinal inflammation. More studies will be needed to assess the strength of the association between C. tropicalis fungal infection and Crohn’s disease.
Another new area of research involves zebrafish with mutations (changes) in a gene called NOD2. Around 10 percent of people with Crohn’s disease have changes in the NOD2 gene and are more likely to be diagnosed with Crohn’s before age 18. Scientists studied zebrafish with the same genetic change and found that a medication called bazedoxifene (Conbriza) calmed overactive immune cells in the fish. Bazedoxifene is already approved to treat menopausal symptoms in humans. Scientists are hopeful that this drug could someday help people with Crohn’s disease and NOD2 gene mutations.
Active Clinical Trials
Before being used in a medical clinic, treatments must go through clinical trials to evaluate possible side effects and adverse events and show that the drugs are safe and effective. Although taking a drug that hasn’t been approved by the FDA comes with certain risks, clinical trials offer the earliest opportunity for people to receive a new medication.
Following are just a few of the recent and ongoing clinical trials for new treatments for Crohn’s disease.
Fecal Transplants
In a fecal transplant, a person with Crohn’s disease is given “good” bacteria from someone else’s digestive system to help improve symptoms. The transplant can be given orally or performed using an endoscope, a thin, tubelike instrument.
One team member asked, “I want to know if anyone has done a fecal transplant and how it worked for them. Was it helpful? Did it decrease your symptoms?”
In reply, a member said that they’d had one for a Clostridioides difficile infection and saw an ”immediate improvement.” ”I’ve been C. difficile-free for nine months, and I feel like it also helped my Crohn’s symptoms beyond my regular treatments,” they wrote.
Another member added, “Studies are looking at the fecal microbiome transplant as a treatment for colitis itself (not just for C. difficile). I’m interested to see where that goes.”

Several studies are or will be recruiting volunteers for further study of fecal transplants for Crohn’s disease.
Medications
Most of these clinical trials are investigating medications. For example, the Cultivate trial is studying etrasimod, a once-a-day pill that is a selective sphingosine 1-phosphate (S1P) receptor modulator. S1P receptor modulators are medications that reduce immune reactions and are currently used to treat multiple sclerosis, which, like Crohn’s, is an autoimmune disease. Cultivate is looking into whether etrasimod may help people with active Crohn’s disease who are resistant to other types of Crohn’s medications.
Are you interested in participating in clinical trials? ClinicalTrials.gov has more than 200 listings of clinical trials for Crohn’s disease that are currently recruiting. You can search using your location, age, gender, the type of trial, and more to find trials that may be a good fit for you. Sometimes, your care team may suggest you enroll in a clinical trial. Always follow up with your health care providers if you are looking for other treatment options, including clinical trials.
New Treatment Strategies and Drug Approvals
Several new treatments and related management strategies are available now.
Lifestyle Changes
Following special diets as a strategy for managing Crohn’s disease is a common topic, and there are many eating plans to choose from.
For example, one team member asked, “SCD, IBD-AID, FODMAP, anti-inflammatory, GAPS, paleo — I’m wondering whether anyone with IBD has tried these diets, and if so, what happened?”
A review of seven studies involving the Crohn’s disease exclusion diet (CDED) was recently published. CDED focuses on fresh, whole foods and excludes foods that can negatively affect the intestines, such as gluten, dairy, animal fat, and processed products. Although the studies involving the CDED differed to some extent, the review found that, in general, the diet was associated with remission from Crohn’s disease symptoms.
Read more about what foods to eat — and to avoid — with Crohn’s disease .
The FDA recently approved new drugs in various categories to treat Crohn’s disease. Drugs are approved based on the safety and effectiveness they demonstrated in clinical trials.

Until recently, the biologic infliximab-dyyb (sold under the brand name Inflectra ) needed to be administered via an intravenous infusion at a clinic. In October 2023, the FDA approved a version of infliximab sold under the brand name Zymfentra for subcutaneous (under the skin) injection. Both versions work by blocking an inflammatory molecule called tumor necrosis factor, so infliximab is known as an anti-TNF drug.
One newer biologic, risankizumab-rzaa ( Skyrizi ), is an inhibitor of a cytokine called interleukin (IL)-23 — the drug blocks this inflammatory molecule. It was approved in June 2022 to treat Crohn’s disease. IL-23 inhibitors now in clinical trials include guselkumab and mirikizumab.
A related treatment, ustekinumab ( Stelara ), also reduces disease activity by lowering inflammation. Ustekinumab is a human monoclonal antibody (a laboratory-made protein) that blocks signals from both IL-23 and IL-12. It’s FDA-approved for adults with Crohn’s disease. Now clinical trials are seeing if it’s safe for children with Crohn’s disease.
Kinase inhibitor
In May 2023, upadacitinib ( Rinvoq ) became the first drug in a class called Janus kinase (JAK) inhibitors to be FDA-approved for treatment of moderately to severely active Crohn’s disease. It’s also the first oral drug to receive approval to treat Crohn’s. JAK inhibitors are known as “small molecules” and are available to your body when taken by mouth, whereas biologics must be injected.
Speak With Your Treatment Team
Always speak with your gastroenterology care team before you adjust your treatment plan. Be honest about your symptoms and concerns so that your doctor can discuss your options and find the most effective treatment to help you reach remission from Crohn’s . Your gastroenterologist should also be aware of clinical trials for Crohn’s disease treatments. They can advise you when a clinical trial may be the best option for your treatment.
Get tips for making the most out of your appointment with your Crohn’s specialist.

Talk With Others Who Understand
On MyCrohnsAndColitisTeam , you’ll meet other people with inflammatory bowel disease, including Crohn’s disease and ulcerative colitis. Here, more than 178,000 members who understand life with IBD come together to share support, advice, and stories from their daily lives.
Are you interested in recent advances in the treatment of Crohn’s disease? Have you asked your health care provider about clinical trials? Share your experiences and thoughts in the comments below or by posting on MyCrohnsAndColitisTeam.
- Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease — American Society for Microbiology
- Candida Tropicalis Infection Modulates the Gut Microbiome and Confers Enhanced Susceptibility to Colitis in Mice — Cellular and Molecular Gastroenterology and Hepatology
- Mutation Spectrum of NOD2 Reveals Recessive Inheritance as a Main Driver of Early Onset Crohn’s Disease — Scientific Reports
- A Myeloid–Stromal Niche and GP130 Rescue in NOD2-Driven Crohn’s Disease — Nature
- Gp130 Blockade To NOD Off Crohn’s Disease — Trends in Immunology
- Fecal Microbiota Transplantation for Induction of Remission in Crohn’s Disease: A Systematic Review and Meta-Analysis — International Journal of Colorectal Disease
- A Study Evaluating the Efficacy and Safety of Oral Etrasimod in the Treatment of Adult Participants With Moderately to Severely Active Crohn’s Disease (Cultivate) — ClinicalTrials.gov
- Clinical Trial Resources — Crohn’s & Colitis Foundation
- Effects of Crohn’s Disease Exclusion Diet on Remission: A Systematic Review — Therapeutic Advances in Gastroenterology
- IBD-AID Diet — UMass Chan Medical School
- Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients With Crohn’s Disease — Gastroenterology
- FDA Approves Risankizumab-Rzaa for Moderately to Severely Active Crohn Disease — Pharmacy Times
- Investigational Drugs in Phase I and Phase II Clinical Trials Targeting Interleukin 23 (IL23) for the Treatment of Crohn’s Disease — Expert Opinion on Investigational Drugs
- A Study of Guselkumab in Participants With Moderately to Severely Active Crohn’s Disease — ClinicalTrials.gov
- A Study of Mirikizumab (LY3074828) in Participants With Crohn’s Disease (VIVID-1) — ClinicalTrials.gov
- A Long-Term Extension Study of Ustekinumab in Pediatric Participants (United) — ClinicalTrials.gov
- A Study of the Efficacy and Safety of Upadacitinib (ABT-494) in Participants With Moderately to Severely Active Crohn’s Disease Who Have Inadequately Responded to or Are Intolerant to Conventional and/or Biologic Therapies (U-Excel) — ClinicalTrials.gov
- Janus Kinase Inhibitors (JAK Inhibitors) — Crohn’s & Colitis Foundation
- AGA Recommends Early Use of Biologics in Patients With Moderate-to-Severe Crohn’s Disease — American Gastroenterological Association
- FDA Approves Subcutaneous Infliximab for IBD — Gastroenterology & Endoscopy News
- FDA Approves First Oral Treatment for Moderately to Severely Active Crohn’s Disease — U.S. Food and Drug Administration
- Will Biologics Surpass Small Molecules in the Pharmaceutical Race? — BioPharmaTrend.com
Get medically-reviewed Crohn's or colitis resources delivered to your inbox
Don’t miss the latest news, tips and treatment options.
Thank you for subscribing!
Become a member to get even more:.

sign up for free
Attach a gif
Click on a gif to attach it
Become a Subscriber
Get the latest articles about Crohn's and colitis sent to your inbox.

5 Crohn’s Flare-Up Questions Answered: Causes, Length, Food, and More

Can You Eat White Rice With Crohn’s Disease? Best Starches for Crohn’s
Related articles, proctosigmoiditis: 6 facts to know for ulcerative colitis.

Proctitis vs. Ulcerative Colitis: 5 Facts To Know

Hair Loss and IBD: Causes and Treatment

How Long Is C. Diff Contagious and What Can It Lead To?

Types of Crohn’s and Colitis and Their Differences

4 Facts To Know About Gastroduodenal Crohn’s Disease

Recent Articles
Fistulas and crohn’s disease: what you need to know.

Stem Cell Therapy for Crohn’s Disease: 5 Facts To Know

6 Ways Sarah Takes Control of Her Life With Crohn’s Disease

9 Low-Cost Items That Make Living With Crohn’s or Colitis Easier (VIDEO)

Molly’s Journey: How Finding Community Helped Her Face Life With UC

2 Spices To Avoid and 5 To Use With Crohn’s and Colitis

Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
When an antibiotic fails: MIT scientists are using AI to target “sleeper” bacteria
Press contact :.
Previous image Next image
Since the 1970s, modern antibiotic discovery has been experiencing a lull. Now the World Health Organization has declared the antimicrobial resistance crisis as one of the top 10 global public health threats.
When an infection is treated repeatedly, clinicians run the risk of bacteria becoming resistant to the antibiotics. But why would an infection return after proper antibiotic treatment? One well-documented possibility is that the bacteria are becoming metabolically inert, escaping detection of traditional antibiotics that only respond to metabolic activity. When the danger has passed, the bacteria return to life and the infection reappears.
“Resistance is happening more over time, and recurring infections are due to this dormancy,” says Jackie Valeri, a former MIT-Takeda Fellow (centered within the MIT Abdul Latif Jameel Clinic for Machine Learning in Health) who recently earned her PhD in biological engineering from the Collins Lab. Valeri is the first author of a new paper published in this month’s print issue of Cell Chemical Biology that demonstrates how machine learning could help screen compounds that are lethal to dormant bacteria.
Tales of bacterial “sleeper-like” resilience are hardly news to the scientific community — ancient bacterial strains dating back to 100 million years ago have been discovered in recent years alive in an energy-saving state on the seafloor of the Pacific Ocean.
MIT Jameel Clinic's Life Sciences faculty lead James J. Collins, a Termeer Professor of Medical Engineering and Science in MIT’s Institute for Medical Engineering and Science and Department of Biological Engineering, recently made headlines for using AI to discover a new class of antibiotics, which is part of the group’s larger mission to use AI to dramatically expand the existing antibiotics available.
According to a paper published by The Lancet , in 2019, 1.27 million deaths could have been prevented had the infections been susceptible to drugs, and one of many challenges researchers are up against is finding antibiotics that are able to target metabolically dormant bacteria.
In this case, researchers in the Collins Lab employed AI to speed up the process of finding antibiotic properties in known drug compounds. With millions of molecules, the process can take years, but researchers were able to identify a compound called semapimod over a weekend, thanks to AI's ability to perform high-throughput screening.
An anti-inflammatory drug typically used for Crohn’s disease, researchers discovered that semapimod was also effective against stationary-phase Escherichia coli and Acinetobacter baumannii .
Another revelation was semapimod's ability to disrupt the membranes of so-called “Gram-negative” bacteria, which are known for their high intrinsic resistance to antibiotics due to their thicker, less-penetrable outer membrane.
Examples of Gram-negative bacteria include E. coli , A. baumannii , Salmonella , and Pseudomonis , all of which are challenging to find new antibiotics for.
“One of the ways we figured out the mechanism of sema [sic] was that its structure was really big, and it reminded us of other things that target the outer membrane,” Valeri explains. “When you start working with a lot of small molecules ... to our eyes, it’s a pretty unique structure.”
By disrupting a component of the outer membrane, semapimod sensitizes Gram-negative bacteria to drugs that are typically only active against Gram-positive bacteria.
Valeri recalls a quote from a 2013 paper published in Trends Biotechnology : “For Gram-positive infections, we need better drugs, but for Gram-negative infections we need any drugs.”
Share this news article on:
Related links.
- Collins Lab
- Abdul Latif Jameel Clinic for Machine Learning in Health
- Institute for Medical Engineering and Science
- Department of Biological Engineering
Related Topics
- Biological engineering
- Institute for Medical Engineering and Science (IMES)
- Jameel Clinic
- Artificial intelligence
- Health sciences and technology
- Drug discovery
- Antibiotics
Related Articles

Using AI, MIT researchers identify a new class of antibiotic candidates

Using AI, scientists find a drug that could combat drug-resistant infections
Artificial intelligence yields new antibiotic
Previous item Next item
More MIT News

MIT community members gather on campus to witness 93 percent totality
Read full story →

Extracting hydrogen from rocks
MIT engineers design flexible “skeletons” for soft, muscle-powered robots
This 3D printer can figure out how to print with an unknown material

For Julie Greenberg, a career of research, mentoring, and advocacy
Reevaluating an approach to functional brain imaging
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
- Clinical Trials
A Study of Vedolizumab in Pediatric Participants With Ulcerative Colitis (UC) or Crohn's Disease (CD)
- Print details
Tab Title Description
- Observational study — observes people and measures outcomes without affecting results.
- Interventional study (clinical trial) — studies new tests, treatments, drugs, surgical procedures or devices.
- Medical records research — uses historical information collected from medical records of large groups of people to study how diseases progress and which treatments and surgeries work best.
Study phase
During the early phases (phases 1 and 2), researchers assess safety, side effects, optimal dosages and risks/benefits. In the later phase (phase 3), researchers study whether the treatment works better than the current standard therapy. They also compare the safety of the new treatment with that of current treatments. Phase 3 trials include large numbers of people to make sure that the result is valid. There are also less common very early (phase 0) and later (phase 4) phases. Phase 0 trials are small trials that help researchers decide if a new agent should be tested in a phase 1 trial. Phase 4 trials look at long-term safety and effectiveness, after a new treatment has been approved and is on the market.
- Rochester, Minnesota: 23-006900
About this study
The purpose of this study is to determine the long-term safety of vedolizumab intravenous (IV) treatment in pediatric participants with Ulcerative Colitis (UC) or Crohn's Disease (CD).
Participation eligibility
Participant eligibility includes age, gender, type and stage of disease, and previous treatments or health concerns. Guidelines differ from study to study, and identify who can or cannot participate. There is no guarantee that every individual who qualifies and wants to participate in a trial will be enrolled. Contact the study team to discuss study eligibility and potential participation.
Main Inclusion Criteria: For Treatment Cohort: 1. The participant should have completed Study MLN0002-3024 or Study MLN0002-3025 and achieved corticosteroid-free clinical response at Week 54 (and has tapered off of steroids, as applicable, at least 12 weeks before Week 54) as defined by a reduction of partial Mayo score of ≥2 points and ≥25% from baseline for participants with UC, or by a decrease of pediatric Crohn's disease activity index (PCDAI) of ≥15 points for participants with CD. 2. A male participant who is sexually active with a female partner of childbearing potential agrees to use barrier method of contraception (e.g., condom with or without spermicide) from signing of informed consent throughout the duration of the study and for 18 weeks after last dose. The female partner of a male participant should also be advised to use a highly effective method of contraception. 3. A female participant of childbearing potential who is sexually active with a nonsterilized male partner agrees to use a highly effective method of contraception from signing of informed consent throughout the duration of the study and 18 weeks after the last dose. For Observational Cohort: 1. The participant has received at least 1 dose of vedolizumab during Study MLN0002-3024 or Study MLN0002-3025 and early terminated OR completed the Week 54 visit of Study MLN0002-3024 or Study MLN0002-3025 but was not eligible to enroll in the treatment cohort of this study. Main Exclusion Criteria: For Treatment Cohort only: 1. The participant currently requires major surgical intervention for UC or CD (e.g., bowel resection), or is anticipated to require major surgical intervention for UC or CD during the study. 2. The participant has developed any new unstable or uncontrolled cardiovascular, heart failure moderate to severe (New York Class Association III or IV), pulmonary, hepatic, renal, gastrointestinal (GI), genitourinary, hematological, coagulation, immunological, endocrine/metabolic, neurological, or other medical disorder that, in the opinion of the investigator, would confound the study results or compromise participant safety. 3. The participant has other serious comorbidities that will limit their ability to complete the study. 4. The participant is unable to comply with all study assessments. 5. The participant has hypersensitivity or allergies to any of the vedolizumab excipients. 6. The participant is lactating or pregnant.
Note: Other protocol defined Inclusion/Exclusion criteria may apply.
Eligibility last updated 7/7/23. Questions regarding updates should be directed to the study team contact.
Participating Mayo Clinic locations
Study statuses change often. Please contact the study team for the most up-to-date information regarding possible participation.
More information
- Publications
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.

Bristol Myers' (BMY) Zeposia Fails Crohn's Disease Study
Bristol Myers BMY announced that a late-stage study on orally-administered Zeposia (ozanimod) in Crohn’s disease (CD) indication failed to achieve its primary endpoint.
This late-stage study was the first of the two induction studies in the phase III YELLOWSTONE clinical program, which evaluated Zeposia in 600 adult patients with moderate to severe active CD over a 12-week treatment period. Initial results from the study showed that treatment with Zeposia failed to meet the primary endpoint of clinical remission at week 12.
However, Bristol Myers did not report any numerical data/figures from the study, nor did it provide an update on its plans for the drug in CD indication. Management plans to share the full results from this study at a future medical meeting.
Apart from the induction studies, the YELLOWSTONE clinical program also includes a 52-week maintenance study and an optional 264-week, open-label extension (OLE) study.
CD is a chronic inflammatory bowel disease that causes inflammation in the digestive tract. Common disease symptoms include diarrhea, abdominal pain, fatigue and weight loss.
Year to date, Bristol Myers’ shares have risen 5.7% compared with the industry’s 0.5% growth.
Image Source: Zacks Investment Research
A sphingosine 1-phosphate (S1P) receptor modulator,Zeposia is currently approved in the United States and Europe for two indications, namely multiple sclerosis (MS) and ulcerative colitis (UC).
The Bristol Myers drug faces stiff competition from Pfizer ’s PFE S1P receptor modulator, Velsipity (etrasimod), which was approved by the FDA last year in UC indication. etrasimod is being evaluated in multiple clinical stages for immuno-inflammatory indications. Apart from UC, Pfizer is also evaluating etrasimod in separate mid-stage studies across multiple gastroenterology and dermatology indications, including alopecia areata, atopic dermatitis, CD and eosinophilic esophagitis.
The FDA granted label expansion to AbbVie ’s ABBV blockbuster JAK inhibitor Rinvoq last year for use in certain adult patients with moderately to severely active CD. Following this approval, the AbbVie drug became the first approved oral product to treat this indication.
S1P receptor modulators like Zeposia and Pfizer’s Velsipity represent a different mechanism of action than available UC therapies (like AbbVie’s Rinvoq and J&J’s Stelara) and represent a new treatment option that can provide symptom relief and remission to patients. Per BMY, an S1P modulator is yet to achieve success in a late-stage study for CD indication.
In a separate press release, Bristol Myers also announced results from the pivotal late-stage KRYSTAL-12 study, evaluating Krazati (adagrasib) in pre-treated patients with KRASG12C-mutated non-small cell lung cancer (NSCLC). This study met its primary endpoint of progression-free survival (PFS) and the key secondary endpoint of overall response rate (ORR) compared with standard-of-care chemotherapy.
We remind investors that Krazati was granted accelerated approval by the FDA to treat adult patients with KRASG12C-mutated locally advanced or metastatic NSCLC. The KRYSTAL-12 study is intended to serve as a confirmatory study seeking to convert this accelerated approval to a full one.
Bristol Myers Squibb Company Price
Bristol Myers Squibb Company price | Bristol Myers Squibb Company Quote
Zacks Rank & A Key Pick
Bristol Myers currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the overall healthcare sector include ADMA Biologics ADMA, which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here .
In the past 60 days, estimates for ADMA Biologics’ 2024 earnings per share (EPS) have risen from 22 cents to 30 cents. During the same period, EPS estimates for 2025 have improved from 32 cents to 50 cents. Year to date, shares of ADMA have risen 46.0%.
Earnings of ADMA Biologics beat estimates in three of the last four quarters while meeting the same on one occasion. ADMA delivered a four-quarter average earnings surprise of 85.00%.
To read this article on Zacks.com click here.
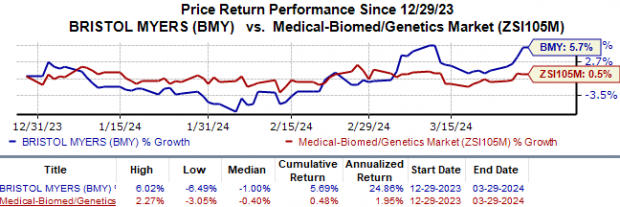
- Search Menu
- Advance articles
- Open Access
- Editor's Choice
- Supplements
- ECCO Guideline/Consensus Papers
- ECCO Topical Reviews
- ECCO Scientific Workshop Papers
- ECCO Position Statements
- Why Publish with JCC
- Author Guidelines
- Submission Site
- Order Offprints
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Journal of Crohn's and Colitis
- About the European Crohn's and Colitis Organisation
- Editorial Board
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
New surgery and hospital-diagnosed infections in elderly patients with inflammatory bowel disease undergoing surgery - a nationwide cohort study.
- Article contents
- Figures & tables
- Supplementary Data
Bente Mertz Nørgård, Olav Sivertsen Garvik, Floor Dijkstra Zegers, Jan Nielsen, Ken Lund, Torben Knudsen, Jens Kjeldsen, New surgery and hospital-diagnosed infections in elderly patients with inflammatory bowel disease undergoing surgery - a nationwide cohort study, Journal of Crohn's and Colitis , 2024;, jjae047, https://doi.org/10.1093/ecco-jcc/jjae047
- Permissions Icon Permissions
Elderly patients with inflammatory bowel disease (IBD) are fragile in many aspects. Therefore, in these patients, we studied post-operative complications (new abdominal surgery and serious infections after the first IBD surgery).
This is a nationwide cohort study based on Danish health registries and included patients with IBD undergoing surgery. The study population was split into ulcerative colitis (UC) and Crohn’s disease (CD). The exposed cohort (elderly) constituted those at an age of ≥ 60 years at first IBD surgery, and the unexposed (adults) those with surgery at the age of 18-59 years. We estimated adjusted Hazard Ratios (aHR) of a) new abdominal surgery within 2 years, and b) serious (hospital-diagnosed) infections within 6 and 12 months. We adjusted for several confounders including type of index surgery (laparoscopic or open).
The aHR for a new surgery among elderly with UC and CD were 0.69 (95% CI 0.58-0.83) and 0.98 (95% CI 0.83-1.15), respectively. In elderly with UC, the aHRs of infections within 6 and 12 months after surgery were 1.07 (95% CI 0.81- 1.40) and 0.85 (95% CI 0.67-1.08), respectively. In the elderly with CD, the aHRs of infections within 6 and 12 months were 1.45 (95% CI 1.12-1.88) and 1.26 (95% CI 1.00-1.59), respectively.
The elderly with IBD did not have an increased risk of new abdominal surgery within two years of the first surgery. Elderly with CD, but not UC, had an increased risk of serious infections within 6 months of surgery.

Email alerts
Citing articles via.
- About Journal of Crohn's and Colitis
- Recommend to your Library
Affiliations
- Online ISSN 1876-4479
- Print ISSN 1873-9946
- Copyright © 2024 European Crohn's and Colitis Organisation (ECCO) Published by Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 4, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Researchers announce findings from landmark clinical trial for pediatric Crohn's disease
by University of North Carolina Health Care
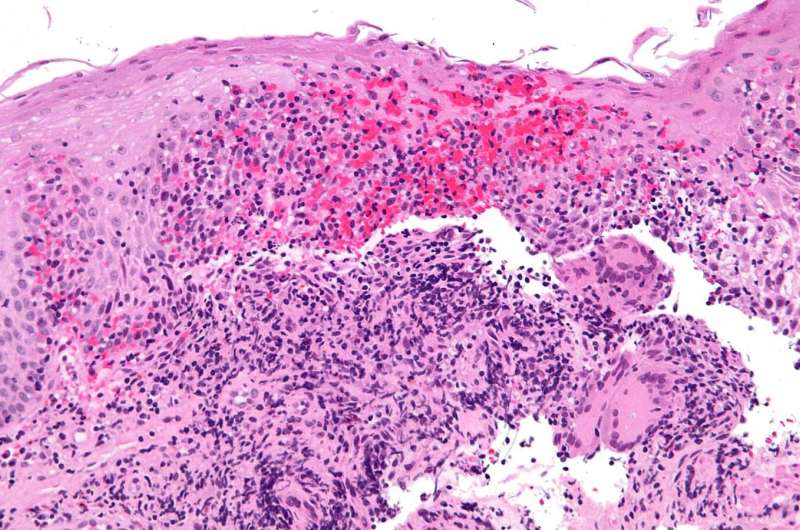
Crohn's disease is a lifelong inflammatory bowel disease (IBD) that is characterized by swelling in the lining of the digestive tract, causing severe diarrhea, abdominal pain, and rectal bleeding. For pediatric patients, the disease is harder to treat. If untreated, it can impair growth, psychosocial development, and puberty.
A significant clinical trial under the direction of Michael Kappelman, MD, MPH, professor of pediatrics at UNC School of Medicine, found that patients receiving the tumor necrosis factor inhibitor adalimumab combined with a low dose of methotrexate, a second immunosuppressant, did better than those treated with adalimumab alone. However, patients initiating infliximab, another tumor necrosis factor inhibitor, had similar outcomes with or without methotrexate.
Their findings were published in Gastroenterology , the leading journal in the field of gastrointestinal disease .
Adalimumab and infliximab, the key drugs of interest, belong to a class of medications called anti-inflammatory tumor necrosis factor (TNF) inhibitors. These medications suppress the body's natural response to TNF, a protein which is produced by white blood cells during inflammatory events. Adalimumab was approved by the Food and Drug Administration in 2014 for treatment in pediatric patients with Crohn's disease, whereas infliximab was approved in 2006.
"We know that both adalimumab and infliximab have proven to be effective and safe," said Kappelman. "We also know that they that they don't work for all patients and don't work forever. This pivotal trial comparing anti-TNF alone or in combination with methotrexate has provided clear results that can immediately impact patient care ."
The clinical trial had been in the works for a decade. Starting with the important decision of which research question was the most urgent and pressing need, Kappelman then engaged key stakeholder groups through a collaboration with ImproveCareNow , a national pediatric community that brings together medical professionals , patients, and families to advance care through research and quality improvement , support for psychosocial functioning and mental health, and escalating initiatives to address disparities, equity, and inclusivity.
David Wohl, MD, professor of medicine in the Division of Infectious Diseases in UNC's Department of Medicine, joined ImproveCareNow soon after his son was diagnosed with pediatric Crohn's disease at nine years old. He was surprised at how little evidence there was behind treatment decisions.
"I became vocal about the 'data desert' parents like me have to wander through to find answers," said Wohl. "I immediately joined as a parent representative and became active in the organization and at our UNC site. My superpower was not only being a parent of a child with IBD, but also a clinician-researcher."
After Wohl's son started seeing Kappelman for clinical care, they spoke about a need for better clinical trial data. Kappelman then approached Wohl with an idea, which would later become the landmark clinical trial. Subsequently, Wohl assumed a lead role in patient and parent engagement and enrollment, along with Lisa Pitch from Nevada and other parents through ImproveCareNow.
"To see the study now provide us with a clear answer about what medicines work best in kids with Crohn's is incredibly gratifying," said Wohl. "It is truly a game-changer."
Kappelman and his team, with UNC-Chapel Hill acting as the lead site, were successful in obtaining an $8-million funding award from the Patient-Centered Outcomes Research Institute (PCORI) and substantial co-funding from the Helmsley Charitable Trust. They recruited and followed patients at over thirty centers across the country, rigorously collecting, quality checking, and analyzing data, and synthesizing results. Two of the sites were located in North Carolina—one at UNC Children's Hospital and another at Levine Children's Hospital in Charlotte.
"We are grateful to our funders and to the hundreds of physicians and staff at sites across the country who worked hard to make this project a success," said Kappelman. "Most importantly, we would like to thank all of the patients and families who bravely and generously volunteered to be part of this study."
The results will also be presented at Digestive Disease Week, a prestigious meeting the hosted by the American Gastroenterological Association.
Explore further
Feedback to editors
Heart disease and depression may be genetically linked by inflammation
6 hours ago

Low cardiorespiratory fitness in youth associated with decreased work ability throughout adulthood, finds 45-year study
Long COVID leaves telltale traces in the blood, finds new study
7 hours ago
Preventive percutaneous coronary intervention for high-risk coronary plaques found to reduce cardiac events
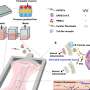
Heart-on-a-chip model used to glean insights into COVID-19-induced heart inflammation

Biomedical engineers use AI to build new tool for studying and diagnosing heart function

Everyday social interactions predict language development in infants
8 hours ago

Research shows pregnancy accelerates biological aging in a healthy, young adult population

Researchers uncover underlying mechanism driving membranous nephropathy, a chronic kidney disease in children
9 hours ago

Researchers find more action is needed to prevent arthritis after knee reconstruction surgery
Related stories.
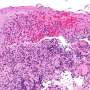
Two new treatments for Crohn's disease equally effective
Jul 18, 2022
Ustekinumab more effective than TNF-alpha inhibitors in psoriasis
Apr 22, 2016

Study finds TNF inhibitor more effective with regular serum assessment to adjust dose
Nov 2, 2021

New drug expands treatment opportunities for rheumatoid arthritis
Aug 25, 2022
Crohn's disease study identifies genetic variant with potential to personalize treatment
Oct 7, 2019

Intensive Crohn's treatment is safe compared to the current standard of care
Mar 2, 2022
Recommended for you
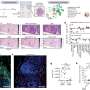
New study reveals how T cells gain and maintain tolerance to gut bacteria
Apr 4, 2024

New study targets major risk factor for gastric cancer
Apr 3, 2024

Scientists link certain gut bacteria to lower heart disease risk
Apr 2, 2024

Probiotics in kombucha found to mimic fasting and reduce fat stores in C. elegans model
Mar 28, 2024

Synthetic material could improve ease and cut cost of gut microbiome research
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
New research has shed light on how known genetic risk factors can contribute to Crohn's disease and treatment response, opening the door to new treatment approaches. Crohn's disease is a form of inflammatory bowel disease in which the digestive tract is marked by lesions of damaging inflammation.
Crohn s disease is a chronic inflammatory disease of the gastrointestinal tract, mostly affecting the ileum. ... Important studies published in 2023 outlined new agents and strategies for the ...
Methods. In two phase 3 induction trials (U-EXCEL and U-EXCEED), we randomly assigned patients with moderate-to-severe Crohn's disease to receive 45 mg of upadacitinib or placebo (2:1 ratio ...
Crohn's disease. Read the latest research on Crohn's disease: risk factors, reducing flare ups, and new treatment options.
The PROFILE trial, led by researchers at the University of Cambridge, involved 386 patients with newly-diagnosed active Crohn's disease. Recruiting from 40 hospitals across the UK, and supported ...
FRIDAY, May 19, 2023 (HealthDay News) -- Patients with Crohn's disease have a new treatment option, following U.S. Food and Drug Administration approval of a pill called Rinvoq (upadacitinib).
Bethesda, MD (Nov. 17, 2023) — The American Gastroenterological Association (AGA) released a new evidence-based guideline recommending the use of blood and stool-based biomarkers to help manage Crohn's disease, a type of inflammatory bowel disease (IBD). IBD is estimated to affect 2.74 million people in the U.S.The guideline was published today in Gastroenterology.
Patients with Crohn's disease have a new treatment option, following U.S. Food and Drug Administration approval of a pill called Rinvoq (upadacitinib). Rinvoq is meant to treat adults with ...
Crosstalk between activated myeloid and stromal cells is critical in the pathogenicity of Crohn's disease 1, 2, and increases in intravasating monocytes are correlated with a lack of response to ...
Mutations within Speckled Protein 140 (SP140) are associated with an increased risk of Crohn's disease; New research reveals that SP140 loss results in unleashed activity of a particular enzyme; Inhibitors of this enzyme can reverse intestinal abnormalities in mice with inflammation characteristic of Crohn's disease
The Genetic, Environmental, and Microbial (GEM) Project: Uncovering new paths in Crohn's disease research. Now Faber's love and concern for her little sister have helped move the Crohn's and colitis research community to breakthrough findings that experts say will improve treatment and even prevent Crohn's disease. Faber is one of the ...
Two new treatments for Crohn's disease showed roughly equal performance in a clinical trial, according to findings published in The Lancet . This allows clinicians and patients to make treatment ...
New treatment options for Crohn's disease patients may be on the horizon thanks to the research linking a common fungal pathogen to inflammatory bowel disease. ... This new research from the Case ...
Latest Crohn's Research. Crohn's disease can be a frustrating cycle of diarrhea, belly cramps, and constipation. There's still a lot we don't know about this long-lasting inflammatory disease. But ...
"In patients with Crohn's disease undergoing treatment with prednisolone, research data shows a complete lack of correlation between the CDAI and the CDEIS," explains Dr. Loftus. ... as well as management options that are consistent and driven by best research evidence." New medications. The immunology of IBD is very complex, and drug targeting ...
A stem cell therapy for Crohn's disease developed by UC Davis Health researchers has shown promising results in mouse studies. The research, published in npj Regenerative Medicine, showed that ...
About Crohn's Disease Crohn's disease is a chronic, systemic disease that manifests as inflammation within the gastrointestinal tract, causing persistent diarrhea and abdominal pain. 1,2 It is a progressive disease, meaning it gets worse over time in a substantial proportion of patients or may develop complications that require urgent medical ...
Reviewed. Reviewed by Emily Henderson, B.Sc. Oct 5 2022. A new study may have solved a mystery surrounding Crohn's disease, a type of inflammatory bowel disease in which immune defenses meant to ...
The purpose of this study is to determine if taking an increased sampling of mesentery (fatty tissue next to the intestine) and lymph nodes at the time of the subject's ileocolic resection prevents a 4-6 month recurrence of Crohn's disease at the site of the new connection.
Crohn's disease is a progressive, destructive inflammatory bowel disease of unclear cause and involves chronic inflammation of any part of the gastrointestinal tract. This Primer reviews the ...
There's promising research on new treatment options for Crohn's disease. Potential treatments now in clinical trials include fecal transplant and new classes of medications. The U.S. Food and Drug Administration (FDA) recently approved several new biologic drugs and the first oral drug to treat Crohn's disease.
Tales of bacterial "sleeper-like" resilience are hardly news to the scientific community — ancient bacterial strains dating back to 100 million years ago have been discovered in recent years alive in an energy-saving state on the ... An anti-inflammatory drug typically used for Crohn's disease, ... a career of research, mentoring, and ...
New research reveals broccoli sprouts may alleviate Crohn's disease symptoms in youth. In a recent study published in the American Society for Microbiology's journal MSystems, a team of ...
The purpose of this study is to determine the long-term safety of vedolizumab intravenous (IV) treatment in pediatric participants with Ulcerative Colitis (UC) or Crohn's Disease (CD). Participation eligibility. Participant eligibility includes age, gender, type and stage of disease, and previous treatments or health concerns.
Bristol Myers BMY announced that a late-stage study on orally-administered Zeposia (ozanimod) in Crohn's disease (CD) indication failed to achieve its primary endpoint. This late-stage study was ...
The study population was split into ulcerative colitis (UC) and Crohn's disease (CD). The exposed cohort (elderly) constituted those at an age of ≥ 60 years at first IBD surgery, and the unexposed (adults) those with surgery at the age of 18-59 years.
Feedback to editors. Crohn's disease is a lifelong inflammatory bowel disease (IBD) that is characterized by swelling in the lining of the digestive tract, causing severe diarrhea, abdominal pain ...
A total of 3260 patients were assigned to receive empagliflozin and 3262 to receive placebo. During a median follow-up of 17.9 months, a first hospitalization for heart failure or death from any ...
Cancer is becoming increasingly prevalent among younger people. New evidence presented at AACR suggests why that may be the case: On a cellular level, younger generations seem to be aging faster ...
Scientists have successfully grown 'mini kidneys' in the lab and grafted them into live mice, revealing new insights into the metabolic defects and a potential therapy for polycystic kidney disease.