
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

Boyle’s Law – Definition, Formula, Example

Boyle’s law or Mariotte’s law states that pressure of an ideal gas is inversely proportional to volume under conditions of constant mass and temperature. When the gas volume increases, pressure decreases. When the volume decreases, pressure increases. Boyle’s law takes its name from chemist and physicist Robert Boyle , who published the law in 1862.
Boyle’s law states that the absolute pressure of an ideal gas is inversely proportional to its volume under conditions of constant mass and temperature.
Boyle’s Law Formula
There are three common formulas for Boyle’s law:
P ∝ 1/V PV = k P 1 V 1 = P 2 V 2
P is absolute pressure, V is volume, and k is a constant.
Graphing Boyle’s Law

The graph of volume versus pressure has a characteristic downward curved shape that shows the inverse relationship between pressure and volume. Boyle used the graph of experimental data to establish the relationship between the two variables.
Richard Towneley and Henry Power described the relationship between the pressure and volume of a gas in the 17th century. Robert Boyle experimentally confirmed their results using a device constructed by his assistant, Robert Hooke. The apparatus consisted of a closed J-shaped tube. Boyle poured mercury into the tube, decreasing the air volume and increasing its pressure. He used different amounts of mercury, recording air pressure and volume measurements, and graphed the data. Boyle published his results in 1662. Sometimes the gas law is called the Boyle-Mariotte law or Mariotte’s law because French physicist Edme Mariotte independently discovered the law in 1670.
Examples of Boyle’s Law in Everyday Life
There are examples of Boyle’s law in everyday life:
- The bends : A diver ascends to the water surface slowly to avoid the bends. As a diver rises to the surface, the pressure from the water decreases, which increases the volume of gases in the blood and joints. Ascending too quickly allows these gases to form bubbles, blocking blood flow and damaging joints and even teeth.
- Air bubbles : Similarly, air bubbles expand as they rise up a column of water. If you have a tall glass, you can watch bubble expand in volume as pressure decreases. One theory about why ships disappear in the Bermuda Triangle relates to Boyle’s law. Gases released from the seafloor rise and expand so much that they essentially become a gigantic bubble by the time they reach the surface. Small boats fall into the bubbles and are engulfed by the sea.
- Deep-sea fish : Deep-sea fish die if you bring them up to the surface. As outside pressure drops, the volume of gas within their swim bladder increases. Essentially, the fish blow up or pop.
- Syringe : Depressing the plunger on a sealed syringe decreases the air volume inside it and increases its pressure. Similarly, if you have a syringe containing a small amount of water and pull back on the plunger, the volume of air increases, but it’s pressure decreases. The pressure drop is enough to boil the water within the syringe at room temperature.
- Breathing: The diaphragm expands the volume of the lungs, causing a pressure drop that allows outside air to rush into the lungs (inhalation). Relaxing the diaphragm reduces the volume of the lungs, increasing the gas pressure within them. Exhaling occurs naturally to equalize pressure.
Boyle’s Law Example Problem
For example, calculate the final volume of a balloon if it has a volume of 2.0 L and pressure of 2 atmospheres and the pressure is reduced to 1 atmosphere. Assume temperature remains constant.
P 1 V 1 = P 2 V 2 (2 atm)(2.0 L) = (1 atm)V 2 V 2 = (2 atm)(2.0 L)/(1 atm) V 2 = 4.0 L
It’s a good idea to check your work to make sure the answer makes sense. In this example, the balloon pressure decreased by a factor of two (halved). The volume increased and doubled. This is what you expect from an inverse proportion relationship.
Most of the time, homework and test questions require reasoning rather than math. For example, if volume increases by a factor of 10, what happens to pressure? You know increasing volume decreases pressure by the same amount. Pressure decreases by a factor of 10.
See another Boyle’s law example problem .
- Fullick, P. (1994). Physics . Heinemann. ISBN 978-0-435-57078-1.
- Holton, Gerald James (2001). Physics, The Human Adventure: From Copernicus to Einstein and Beyond . Rutgers University Press. ISBN 978-0-8135-2908-0.
- Tortora, Gerald J.; Dickinson, Bryan (2006). ‘Pulmonary Ventilation’ in Principles of Anatomy and Physiology (11th ed.). Hoboken: John Wiley & Sons, Inc. pp. 863–867.
- Walsh, C.; Stride, E.; Cheema, U.; Ovenden, N. (2017). “A combined three-dimensional in vitro–in silico approach to modelling bubble dynamics in decompression sickness.” Journal of the Royal Society Interface . 14(137). doi: 10.1098/rsif.2017.0653
- Webster, Charles (1965). “The discovery of Boyle’s law, and the concept of the elasticity of air in seventeenth century”. Archive for the History of Exact Sciences . 2(6) : 441–502.
Related Posts
Chemistry Learner
It's all about chemistry.
- Chemical Bonds
- Chemical Reactions
- Materials Chemistry
- Organic Chemistry
- Periodic Trends
- Periodic Table Groups
- How to Read Periodic Table
- Naming Covalent Compounds Worksheets
- Net Ionic Equation Worksheets
- Types of Chemical Reactions Worksheets
- Word Equations Worksheets
- Valence Electrons Worksheets
- Graphing Periodic Trends Worksheets
- Periodic Trends Ionization Energy Worksheets
- Atomic Structure And Isotopes Worksheets
Boyle’s Law
Problems and solutions.
Boyle’s law is an experimental gas law that explains the relationship between pressure and volume. According to Boyle’s law, the pressure and volume are inversely proportional, provided the temperature and mass remain unchanged [1-4] .

The law was named after Anglo-Irish physicist and chemist Robert Boyle, who published the law in 1662.
Suppose P is the pressure and V is the volume of the gas. Mathematically, Boyle’s law is given by [1-6]
Or, P = k/V
P : Pressure
k : Proportionality constant
This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. A graphical representation of the above equation is shown below.

Boyle’s law can be used to establish a relationship between two states of a gas. Suppose the gas with pressure P 1 and volume V 1 expands or shrinks to pressure P 2 and volume V 2 . Then, using Boyle’s law equation,
P 1 V 1 = k and P 2 V 2 = k
From the above two equations
P 1 V 1 = P 2 V 2
This equation shows that as the pressure increases, the volume decreases and vice versa. For example, when the pressure doubles, the volume is decreased by half. Also, the units of pressure and volume must be consistent. P 1 and P 2 must be expressed in Pa or atm. V 1 and V 2 must be expressed in m 3 or L.

Here are some examples of Boyle’s law in real life [3] .
Respiration and Breathing : When we inhale, our lungs expand. As a result, the volume increases, and pressure decreases. The air pressure inside the lungs is less than the environmental pressure. Hence, oxygen from the environment fills up the lungs. The reverse happens during exhalation. When we exhale, the lungs shrink. Its volume decreases, and pressure increases. The air pressure inside the lungs is higher than the environmental pressure. Hence, the air is expelled out of the lungs.
Syringe : A syringe draws liquid from a small vial and injects it into a body. It consists of a barrel, needle, and plunger. When the plunger is pulled, the volume inside the barrel increases, resulting in a decrease in pressure. Any fluid flows from high pressure to a low-pressure region. Since the pressure outside the syringe, near the needle, is higher than the pressure inside it, fluid will flow into the syringe. The reverse also holds. When the plunger is pushed, it creates a low volume and high pressure inside the syringe. As the pressure inside is higher than outside, the fluid will flow out of the syringe.
Balloon : When a balloon filled with air is squeezed, its volume decreases, and pressure increases. If the balloon is squeezed further, the pressure inside the balloon is so high that it causes the balloon to burst.
Problem 1 : A gas confined to a volume of 2 L at a pressure of 10 atm. It can flow into a 10 L container by opening the valve that connects the two containers. What is the final pressure of the gas?
P 1 = 10 atm
From Boyle’s law,
Or, P 2 = P 1 V 1 / V 2
Or, P 2 = 10 atm x 2 L/ 10 L
Or, P 2 = 2 atm
Problem 2 : A gas exerts a pressure of 5 kPa on the walls of a container. When the container is emptied into a 12 L container, the pressure exerted by the gas increases to 8 kPa. Find the volume of container 1.
P 1 = 5 kPa
P 2 = 8 kPa
Or, V 1 = P 2 V 2 /P 1
Or, V 1 = 8 kPa x 12 L/5 kPa
Or, V 1 = 19.2 L
Ans. The variables involved in Boyle’s law are pressure and volume. On the other hand, temperature and the number of moles of gas are the constants.
- Grc.nasa.gov
- Courses.lumenlearning.com
- Thoughtco.com
- Chem.libretexts.org
- Cpanhd.sitehost.iu.edu
- Ch301.cm.utexas.edu
Related Articles

Sublimation

Graham’s Law

Crystal Field Theory

Van der Waals Equation
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Trending Topics
© 2024 ( Chemistry Learner )
Boyle's Law: Worked Chemistry Problems
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
If you trap a sample of air and measure its volume at different pressures (constant temperature ), then you can determine a relation between volume and pressure. If you do this experiment, you will find that as the pressure of a gas sample increases, its volume decreases. In other words, the volume of a gas sample at constant temperature is inversely proportional to its pressure. The product of the pressure multiplied by the volume is a constant:
PV = k or V = k/P or P = k/V
where P is pressure, V is volume, k is a constant, and the temperature and quantity of gas are held constant. This relationship is called Boyle's Law , after Robert Boyle , who discovered it in 1660.
Key Takeaways: Boyle's Law Chemistry Problems
- Simply put, Boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. The equation for this is PV = k, where k is a constant.
- At a constant temperature, if you increase the pressure of a gas, its volume decreases. If you increase its volume, the pressure decreases.
- The volume of a gas is inversely proportional to its pressure.
- Boyle's law is a form of the Ideal Gas Law. At normal temperatures and pressures, it works well for real gases. However, at high temperature or pressure, it is not a valid approximation.
Worked Example Problem
The sections on the General Properties of Gases and Ideal Gas Law Problems may also be helpful when attempting to work Boyle's Law problems .
A sample of helium gas at 25°C is compressed from 200 cm 3 to 0.240 cm 3 . Its pressure is now 3.00 cm Hg. What was the original pressure of the helium?
It's always a good idea to write down the values of all known variables, indicating whether the values are for initial or final states. Boyle's Law problems are essentially special cases of the Ideal Gas Law:
Initial: P 1 = ?; V 1 = 200 cm 3 ; n 1 = n; T 1 = T
Final: P 2 = 3.00 cm Hg; V 2 = 0.240 cm 3 ; n 2 = n; T 2 = T
P 1 V 1 = nRT ( Ideal Gas Law )
P 2 V 2 = nRT
so, P 1 V 1 = P 2 V 2
P 1 = P 2 V 2 /V 1
P 1 = 3.00 cm Hg x 0.240 cm 3 /200 cm 3
P 1 = 3.60 x 10 -3 cm Hg
Did you notice that the units for the pressure are in cm Hg? You may wish to convert this to a more common unit, such as millimeters of mercury, atmospheres, or pascals.
3.60 x 10 -3 Hg x 10mm/1 cm = 3.60 x 10 -2 mm Hg
3.60 x 10 -3 Hg x 1 atm/76.0 cm Hg = 4.74 x 10 -5 atm
- Levine, Ira N. (1978). Physical Chemistry . University of Brooklyn: McGraw-Hill.
- The Formula for Boyle's Law
- Boyle's Law Definition in Chemistry
- Gases - General Properties of Gases
- Boyle's Law Explained With Example Problem
- Ideal Gas Law: Worked Chemistry Problems
- Charles' Law Example Problem
- The Major Laws of Chemistry
- Avogadro's Law Example Problem
- Gases Study Guide
- The Combined Gas Law in Chemistry
- Gay-Lussac's Gas Law Examples
- What Is Avogadro's Law? Definition and Example
- Inverse Proportion Definition
- What Is the Formula for Charles' Law?
- Gay-Lussac's Law Definition
- Specific Volume
- States of Matter
Boyle’s Law
What is boyle’s law.
Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. Boyle’s law was put forward by the Anglo-Irish chemist Robert Boyle in the year 1662.
Table of Contents
Recommended videos, formula and derivation.
Examples of Boyle’s Law
Solved Exercises on Boyle’s Law
Frequently asked questions – faqs.

For a gas, the relationship between volume and pressure (at constant mass and temperature) can be expressed mathematically as follows.
Where P is the pressure exerted by the gas and V is the volume occupied by it. This proportionality can be converted into an equation by adding a constant, k.
P = k*(1/V) ⇒ PV = k
The pressure v/s volume curve for a fixed amount of gas kept at constant temperature is illustrated below.

Boyle’s law
It can be observed that a straight line is obtained when the pressure exerted by the gas (P) is taken on the Y-axis and the inverse of the volume occupied by the gas (1/V) is taken on the X-axis.
As per Boyle’s law, any change in the volume occupied by a gas (at constant quantity and temperature) will result in a change in the pressure exerted by it. In other words, the product of the initial pressure and the initial volume of a gas is equal to the product of its final pressure and final volume (at constant temperature and number of moles). This law can be expressed mathematically as follows:
P 1 V 1 = P 2 V 2
- P 1 is the initial pressure exerted by the gas
- V 1 is the initial volume occupied by the gas
- P 2 is the final pressure exerted by the gas
- V 2 is the final volume occupied by the gas
This expression can be obtained from the pressure-volume relationship suggested by Boyle’s law. For a fixed amount of gas kept at a constant temperature, PV = k. Therefore,
P 1 V 1 = k (initial pressure * initial volume)
P 2 V 2 = k (final pressure * final volume)
∴ P 1 V 1 = P 2 V 2
This equation can be used to predict the increase in the pressure exerted by a gas on the walls of its container when the volume of its container is decreased (and its quantity and absolute temperature remain unchanged).
When a filled balloon is squeezed, the volume occupied by the air inside the balloon decreases. This is accompanied by an increase in the pressure exerted by the air on the balloon, as a consequence of Boyle’s law. As the balloon is squeezed further, the increasing pressure eventually pops it. An illustration describing the increase in pressure that accompanies a decrease in the volume of a gas is provided below.

If a scuba diver rapidly ascends from a deep zone towards the surface of the water, the decrease in the pressure can cause the gas molecules in his/her body to expand. These gas bubbles can go on to cause damage to the diver’s organs and can also result in death. This expansion of the gas caused by the ascension of the scuba diver is another example of Boyle’s law. Another similar example can be observed in the deep-sea fish that die after reaching the surface of the water (due to the expansion of dissolved gasses in their blood).
A fixed amount of a gas occupies a volume of 1L and exerts a pressure of 400 kPa on the walls of its container. What would be the pressure exerted by the gas if it is completely transferred into a new container having a volume of 3 liters (assuming the temperature and quantity of gas remains constant)?
Initial volume (V 1 ) = 1L
Initial pressure (P 1 ) = 400 kPa
Final volume (V 2 ) = 3L
As per Boyle’s law, P 1 V 1 = P 2 V 2 ⇒ P 2 = (P 1 V 1 )/V 2
P 2 = (1L * 400 kPa)/3L = 133.33 kPa
Therefore, the gas exerts a pressure of 133.33 kPa on the walls of the 3-liter container.
A gas exerts a pressure of 3 kPa on the walls of container 1. When container 1 is emptied into a 10-liter container, the pressure exerted by the gas increases to 6 kPa. Find the volume of container 1. Assume that the temperature and quantity of the gas remain constant.
Initial pressure, P 1 = 3kPa
Final pressure, P 2 = 6kPa
Final volume, V 2 = 10L
According to Boyle’s law, V 1 = (P 2 V 2 )/P 1
V 1 = (6 kPa * 10 L)/3 kPa = 20 L
Therefore, the volume of container 1 is 20 L.
How does Boyle’s law work?
Boyle’s law is a gas law that states that a gas’s pressure and volume are inversely proportional. When the temperature is kept constant, as volume increases, pressure falls and vice versa.
Why is Boyle law important?
Boyle’s law is significant because it explains how gases behave. It proves beyond a shadow of a doubt that gas pressure and volume are inversely proportional. When you apply pressure on a gas, the volume shrinks and the pressure rises.
What is the formula for Boyle’s gas law?
The empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; i.e., pv = k, a constant, as proposed by physicist Robert Boyle in 1662.
What is a good example of Boyle’s Law?
A balloon is a good example of Boyle’s law in action. The balloon is inflated by blowing air into it; the pressure of the air pulls on the rubber, causing the balloon to expand. When one end of the balloon is compressed, the pressure within rises, causing the un-squeezed section of the balloon to expand outward.
Can Boyle’s law be experimentally proven?
Boyle’s law is a connection between pressure and volume. It asserts that under constant temperature, the pressure of a specific quantity of gas is inversely proportional to its volume. It is possible to prove the law empirically. The paper discusses a syringe-based experimental approach for verifying the law.
What is Boyle’s law?
What is the relationship between pressure and volume, why does volume decrease when pressure is increased, what happens to pressure if the volume is doubled, why boyle’s law is not applicable at high pressure.
To learn more about Boyle’s law and other important gas laws, such as Charles’ law , register with BYJU’S, and download the mobile application on your smartphone.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
I really enjoyed your explanation on boyl’s law.
Well explained. Thank you
perfectly explained, thank u very much
cool i learned so much thank you
I really love it Thank you.
Wow I wasn’t understanding this part of chemistry class until I found this site. It was very helpful. Looking forward to visit again!!.
Thankyou I loved learning it
it’s nice it really helped I might keep visiting
well explained, I really like it.
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


HIGH SCHOOL
- ACT Tutoring
- SAT Tutoring
- PSAT Tutoring
- ASPIRE Tutoring
- SHSAT Tutoring
- STAAR Tutoring
GRADUATE SCHOOL
- MCAT Tutoring
- GRE Tutoring
- LSAT Tutoring
- GMAT Tutoring
- AIMS Tutoring
- HSPT Tutoring
- ISAT Tutoring
- SSAT Tutoring
Search 50+ Tests
Loading Page
math tutoring
- Elementary Math
- Pre-Calculus
- Trigonometry
science tutoring
Foreign languages.
- Mandarin Chinese
elementary tutoring
- Computer Science
Search 350+ Subjects
- Video Overview
- Tutor Selection Process
- Online Tutoring
- Mobile Tutoring
- Instant Tutoring
- How We Operate
- Our Guarantee
- Impact of Tutoring
- Reviews & Testimonials
- Media Coverage
- About Varsity Tutors
High School Chemistry : Using Boyle's Law
Study concepts, example questions & explanations for high school chemistry, all high school chemistry resources, example questions, example question #11 : gases and gas laws.

Since the volume of the gas is the only variable that has changed, we can use Boyle's law in order to find the final pressure. Since pressure and volume are on the same side of the ideal gas law, they are inversely proportional to one another. In other words, as one increases, the other will decrease, and vice versa.
Boyle's law can be written as follows:

Use the given volumes and the initial pressure to solve for the final pressure.

Example Question #1 : Using Boyle's Law
What law is the following formula?

Charles's law
Boyle's law
Combined gas law
Ideal gas law
Gay-Lussac's law
Boyle's law relates the pressure and volume of a system, which are inversely proportional to one another. When the parameters of a system change, Boyle's law helps us anticipate the effect the changes have on pressure and volume.

Example Question #13 : Gases And Gas Laws

To solve this question we will need to use Boyle's law:

We are given the final pressure and volume, along with the initial volume. Using these values, we can calculate the initial pressure.

Example Question #14 : Gases And Gas Laws
The graph depicted here represents which of the gas laws?

The graph shows that there is an inverse relationship between the volume and pressure of a gas, when kept at a constant temperature. This was described by Robert Boyle and can be represented mathematically as Boyle's law:

Gay-Lussac's law shows the relationship between pressure and temperature. Charles's law shows the relationship between volume and temperature. Hund's rule (Hund's law) is not related to gases, and states that electron orbitals of an element will be filled with single electrons before any electrons will form pairs within a single orbital.

We are given the initial pressure and volume, along with the final pressure. Using these values, we can calculate the final volume.

A gas is initially in a 5L piston with a pressure of 1atm.
If pressure changes to 3.5atm by moving the piston down, what is new volume?

Use Boyle's Law:

Plug in known values and solve for final volume.

Use Boyle's law and plug in appropriate parameters:

Boyle's Law is:

Notice the answer has 3 significant figures.

Report an issue with this question
If you've found an issue with this question, please let us know. With the help of the community we can continue to improve our educational resources.
DMCA Complaint
If you believe that content available by means of the Website (as defined in our Terms of Service) infringes one or more of your copyrights, please notify us by providing a written notice (“Infringement Notice”) containing the information described below to the designated agent listed below. If Varsity Tutors takes action in response to an Infringement Notice, it will make a good faith attempt to contact the party that made such content available by means of the most recent email address, if any, provided by such party to Varsity Tutors.
Your Infringement Notice may be forwarded to the party that made the content available or to third parties such as ChillingEffects.org.
Please be advised that you will be liable for damages (including costs and attorneys’ fees) if you materially misrepresent that a product or activity is infringing your copyrights. Thus, if you are not sure content located on or linked-to by the Website infringes your copyright, you should consider first contacting an attorney.
Please follow these steps to file a notice:
You must include the following:
A physical or electronic signature of the copyright owner or a person authorized to act on their behalf; An identification of the copyright claimed to have been infringed; A description of the nature and exact location of the content that you claim to infringe your copyright, in \ sufficient detail to permit Varsity Tutors to find and positively identify that content; for example we require a link to the specific question (not just the name of the question) that contains the content and a description of which specific portion of the question – an image, a link, the text, etc – your complaint refers to; Your name, address, telephone number and email address; and A statement by you: (a) that you believe in good faith that the use of the content that you claim to infringe your copyright is not authorized by law, or by the copyright owner or such owner’s agent; (b) that all of the information contained in your Infringement Notice is accurate, and (c) under penalty of perjury, that you are either the copyright owner or a person authorized to act on their behalf.
Send your complaint to our designated agent at:
Charles Cohn Varsity Tutors LLC 101 S. Hanley Rd, Suite 300 St. Louis, MO 63105
Or fill out the form below:
Contact Information
Complaint details.


- Illustrations
- Problem Sets
- Calculators
You are here
Boyle's law calculations - example solutions.
- Boyle's Law
- Boyle's Law Concepts
Search form
Latest illustrations.
- Ocean Gyres and Geostrophic Flow
- Langmuir Circulation
- Test sensitivity - specificity calculator
- How earthquakes show us the inside of the Earth
- Surface currents, the Ekman spiral, and Ekman transport
© Andrew Staroscik 2011-2022
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History
... and beyond
- Socratic Meta
- Featured Answers

What is an example of a Boyle's law practice problem?

Related questions
- What is used to measure gas pressure in a closed container?
- What tool is used to measure gas pressure?
- What units are used to measure gas pressure?
- What is Boyle's law?
- What is an example of a gas pressure practice problem?
- What are some common mistakes students make when measuring gas pressure?
- How do Boyle's law and Charles' law differ?
- How can I measure gas pressure?
- How does Boyle's law affect the human body?
- How does Boyle's law affect hot air balloons?
Impact of this question


- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.3: The Simple Gas Laws- Boyle’s Law, Charles’s Law and Avogadro’s Law
- Last updated
- Save as PDF
- Page ID 37996
Learning Objectives
- To understand the relationships among pressure, temperature, volume, and the amount of a gas.
Early scientists explored the relationships among the pressure of a gas ( P ) and its temperature ( T ), volume ( V ), and amount ( n ) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). The history of their discoveries provides several excellent examples of the scientific method .
The Relationship between Pressure and Volume: Boyle's Law
As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Conversely, as the pressure on a gas decreases, the gas volume increases because the gas particles can now move farther apart. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; that is, the atmospheric gas exerts less pressure on the surface of the balloon, so the interior gas expands until the internal and external pressures are equal.
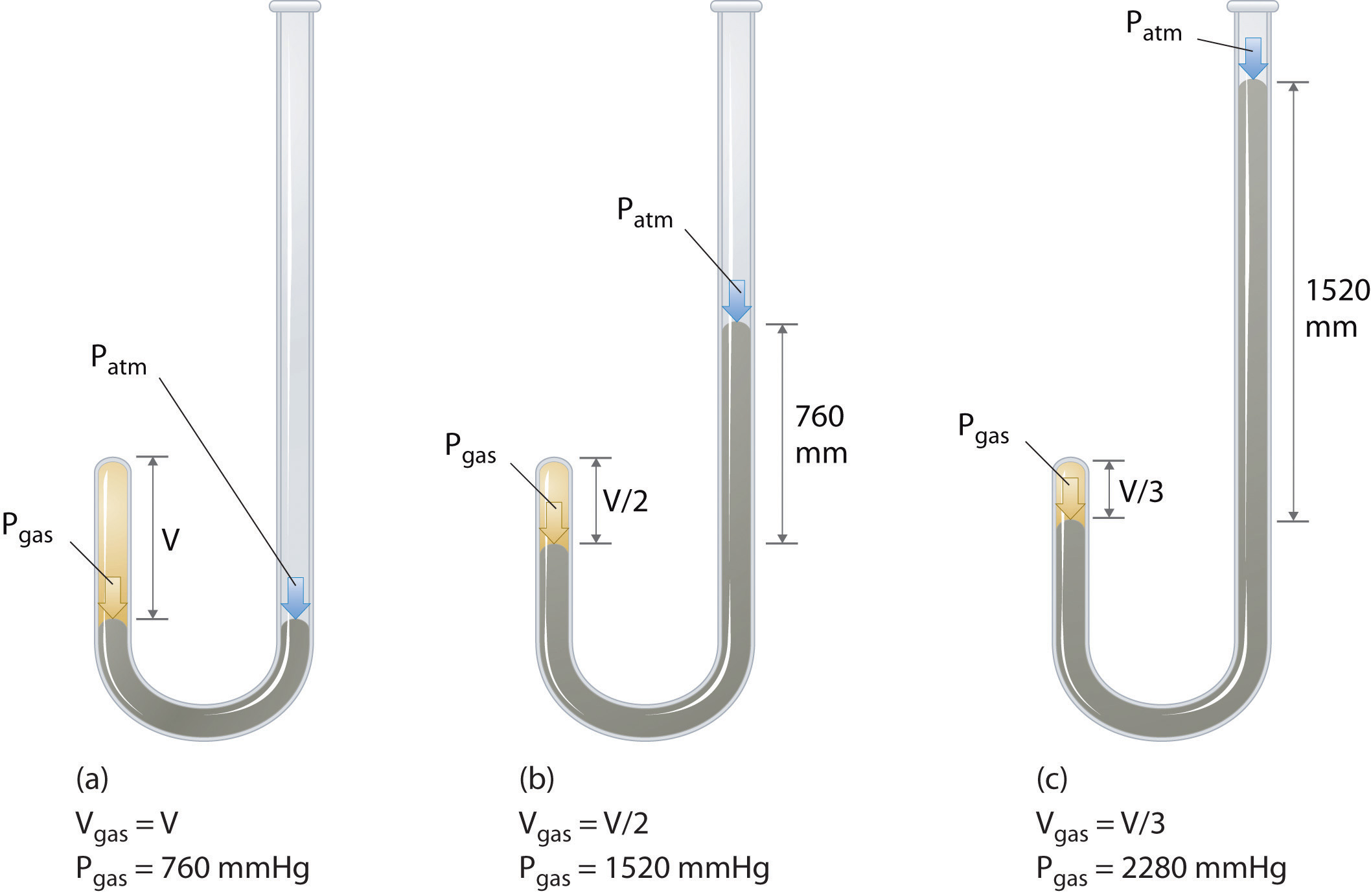
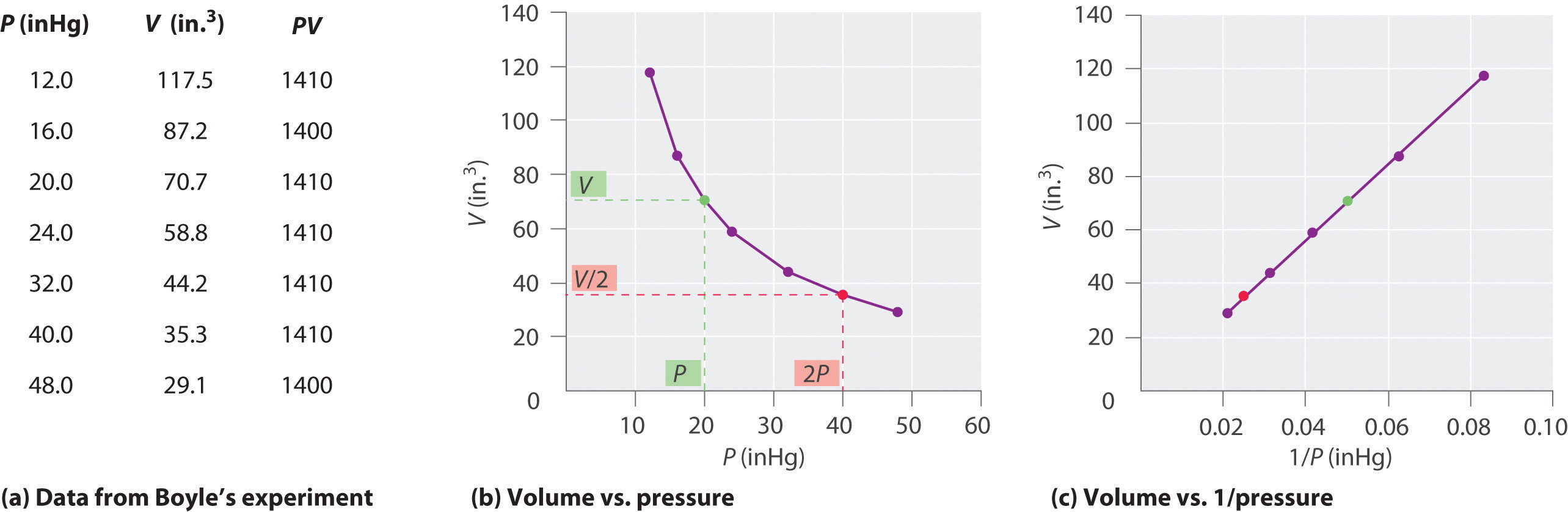
The Irish chemist Robert Boyle (1627–1691) carried out some of the earliest experiments that determined the quantitative relationship between the pressure and the volume of a gas. Boyle used a J-shaped tube partially filled with mercury, as shown in Figure \(\PageIndex{1}\). In these experiments, a small amount of a gas or air is trapped above the mercury column, and its volume is measured at atmospheric pressure and constant temperature. More mercury is then poured into the open arm to increase the pressure on the gas sample. The pressure on the gas is atmospheric pressure plus the difference in the heights of the mercury columns, and the resulting volume is measured. This process is repeated until either there is no more room in the open arm or the volume of the gas is too small to be measured accurately. Data such as those from one of Boyle’s own experiments may be plotted in several ways (Figure \(\PageIndex{2}\)). A simple plot of \(V\) versus \(P\) gives a curve called a hyperbola and reveals an inverse relationship between pressure and volume: as the pressure is doubled, the volume decreases by a factor of two. This relationship between the two quantities is described as follows:
\[PV = \rm constant \label{10.3.1} \]
Dividing both sides by \(P\) gives an equation illustrating the inverse relationship between \(P\) and \(V\):
\[V=\dfrac{\rm const.}{P} = {\rm const.}\left(\dfrac{1}{P}\right) \label{10.3.2} \]
\[V \propto \dfrac{1}{P} \label{10.3.3} \]
where the ∝ symbol is read “is proportional to.” A plot of V versus 1/ P is thus a straight line whose slope is equal to the constant in Equations \(\ref{10.3.1}\) and \(\ref{10.3.3}\). Dividing both sides of Equation \(\ref{10.3.1}\) by V instead of P gives a similar relationship between P and 1/ V . The numerical value of the constant depends on the amount of gas used in the experiment and on the temperature at which the experiments are carried out. This relationship between pressure and volume is known as Boyle’s law, after its discoverer, and can be stated as follows: At constant temperature, the volume of a fixed amount of a gas is inversely proportional to its pressure. This law in practice is shown in Figure \(\PageIndex{2}\).
At constant temperature, the volume of a fixed amount of a gas is inversely proportional to its pressure
The Relationship between Temperature and Volume: Charles's Law
Hot air rises, which is why hot-air balloons ascend through the atmosphere and why warm air collects near the ceiling and cooler air collects at ground level. Because of this behavior, heating registers are placed on or near the floor, and vents for air-conditioning are placed on or near the ceiling. The fundamental reason for this behavior is that gases expand when they are heated. Because the same amount of substance now occupies a greater volume, hot air is less dense than cold air. The substance with the lower density—in this case hot air—rises through the substance with the higher density, the cooler air.
The first experiments to quantify the relationship between the temperature and the volume of a gas were carried out in 1783 by an avid balloonist, the French chemist Jacques Alexandre César Charles (1746–1823). Charles’s initial experiments showed that a plot of the volume of a given sample of gas versus temperature (in degrees Celsius) at constant pressure is a straight line. Similar but more precise studies were carried out by another balloon enthusiast, the Frenchman Joseph-Louis Gay-Lussac (1778–1850), who showed that a plot of V versus T was a straight line that could be extrapolated to a point at zero volume, a theoretical condition now known to correspond to −273.15°C (Figure \(\PageIndex{3}\)).A sample of gas cannot really have a volume of zero because any sample of matter must have some volume. Furthermore, at 1 atm pressure all gases liquefy at temperatures well above −273.15°C. Note from part (a) in Figure \(\PageIndex{3}\) that the slope of the plot of V versus T varies for the same gas at different pressures but that the intercept remains constant at −273.15°C. Similarly, as shown in part (b) in Figure \(\PageIndex{3}\), plots of V versus T for different amounts of varied gases are straight lines with different slopes but the same intercept on the T axis.
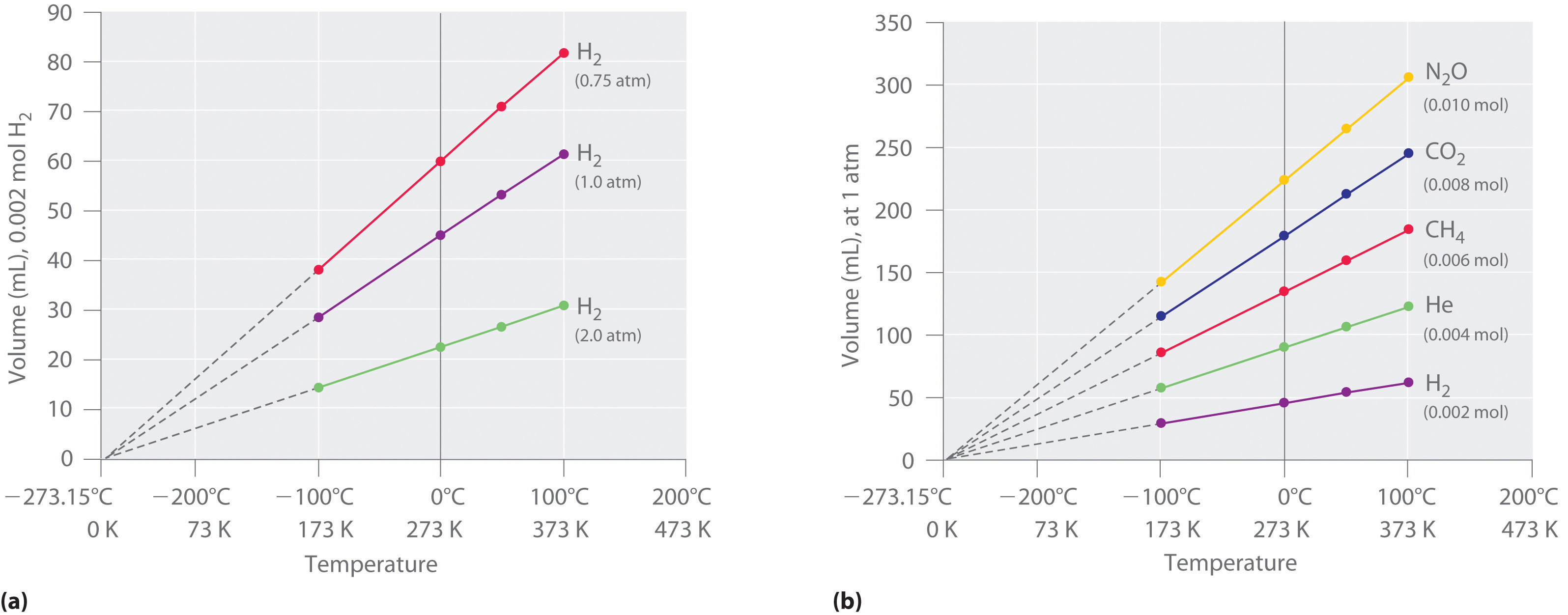
The significance of the invariant T intercept in plots of V versus T was recognized in 1848 by the British physicist William Thomson (1824–1907), later named Lord Kelvin. He postulated that −273.15°C was the lowest possible temperature that could theoretically be achieved, for which he coined the term absolute zero (0 K).
We can state Charles’s and Gay-Lussac’s findings in simple terms: At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in kelvins). This relationship, illustrated in part (b) in Figure \(\PageIndex{3}\) is often referred to as Charles’s law and is stated mathematically as
\[V ={\rm const.}\; T \label{10.3.4} \]
\[V \propto T \label{10.3.5} \]
with temperature expressed in kelvins, not in degrees Celsius. Charles’s law is valid for virtually all gases at temperatures well above their boiling points.
The Relationship between Amount and Volume: Avogadro's Law
We can demonstrate the relationship between the volume and the amount of a gas by filling a balloon; as we add more gas, the balloon gets larger. The specific quantitative relationship was discovered by the Italian chemist Amedeo Avogadro, who recognized the importance of Gay-Lussac’s work on combining volumes of gases. In 1811, Avogadro postulated that, at the same temperature and pressure, equal volumes of gases contain the same number of gaseous particles (Figure \(\PageIndex{4}\)). This is the historic “Avogadro’s hypothesis.”
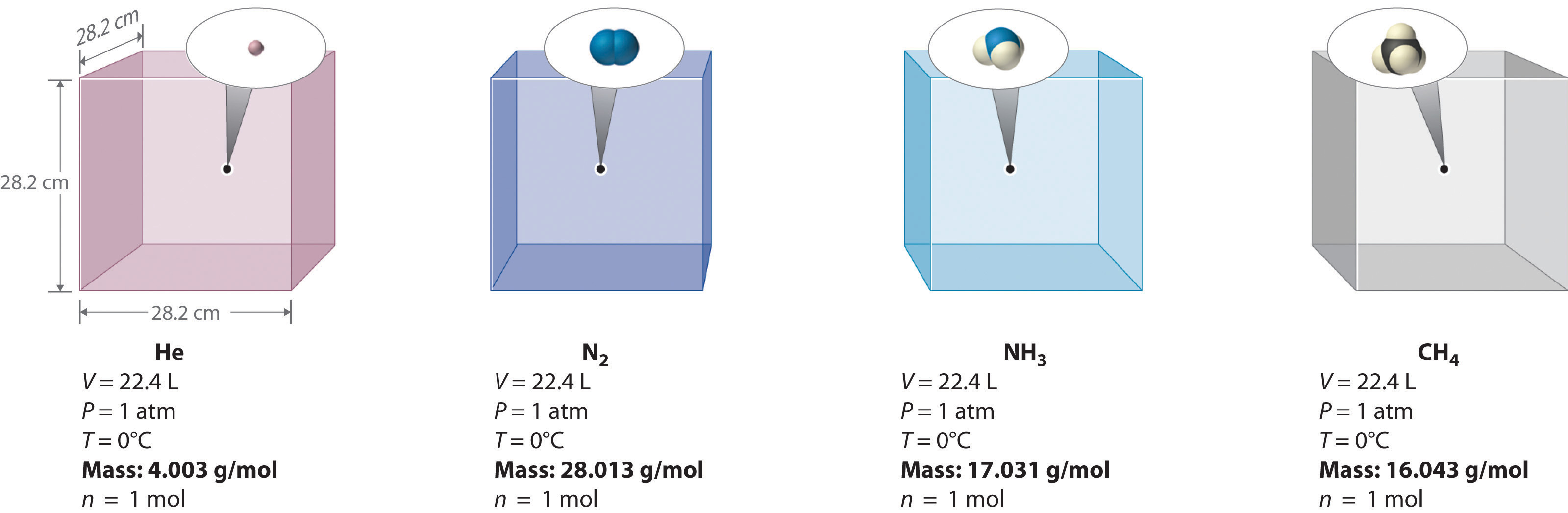
A logical corollary to Avogadro's hypothesis (sometimes called Avogadro’s law) describes the relationship between the volume and the amount of a gas: At constant temperature and pressure, the volume of a sample of gas is directly proportional to the number of moles of gas in the sample. Stated mathematically,
\[V ={\rm const.} \; (n) \label{10.3.6} \]
\[V \propto.n \text{@ constant T and P} \label{10.3.7} \]
This relationship is valid for most gases at relatively low pressures, but deviations from strict linearity are observed at elevated pressures.
For a sample of gas,
- V increases as P decreases (and vice versa)
- V increases as T increases (and vice versa)
- V increases as n increases (and vice versa)
The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in Figure \(\PageIndex{5}\). Volume increases with increasing temperature or amount, but decreases with increasing pressure.
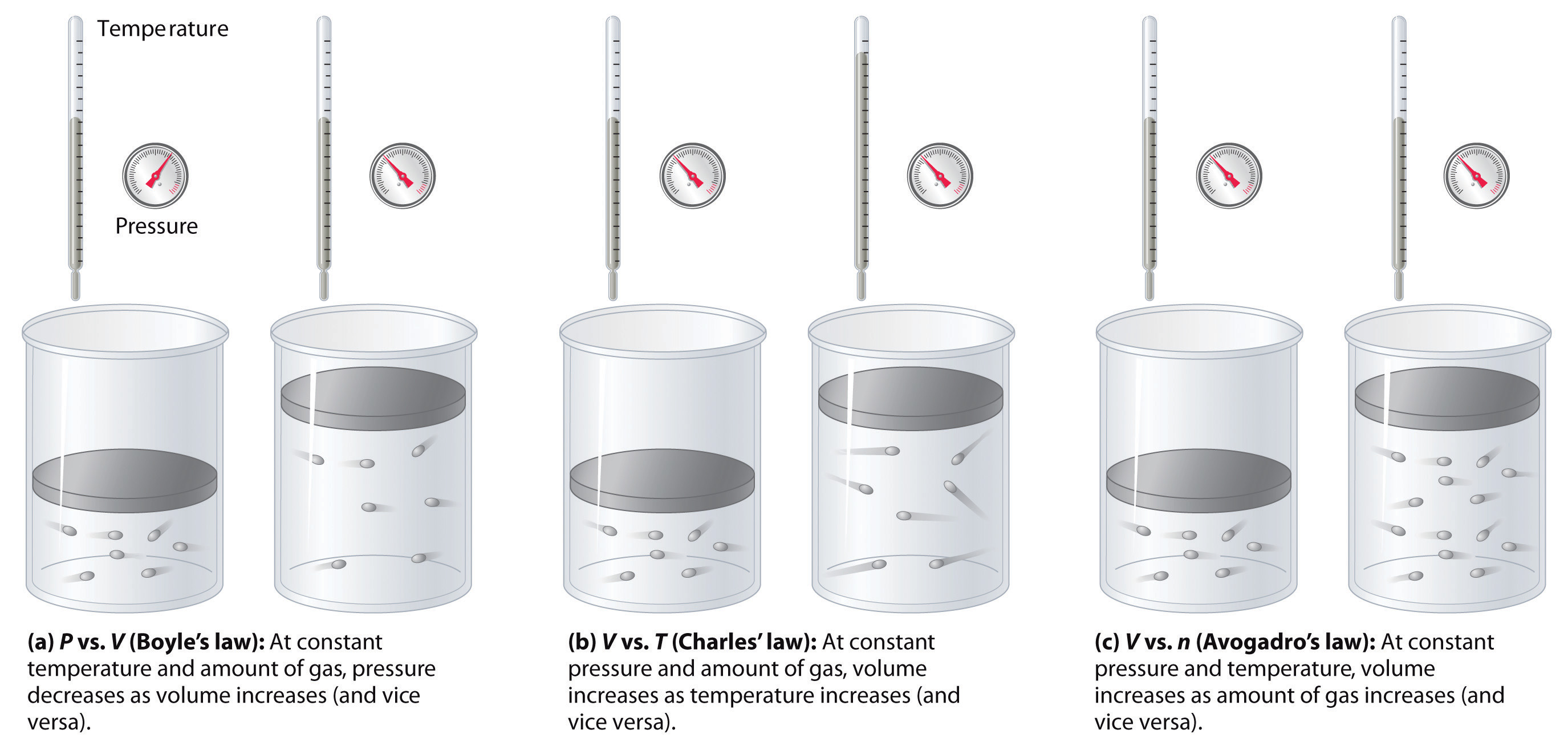
The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure ( Boyle’s law ), Charles and Gay-Lussac demonstrated that the volume of a gas is directly proportional to its temperature (in kelvins) at constant pressure ( Charles’s law ), and Avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas present ( Avogadro’s law ). Plots of the volume of gases versus temperature extrapolate to zero volume at −273.15°C, which is absolute zero (0 K) , the lowest temperature possible. Charles’s law implies that the volume of a gas is directly proportional to its absolute temperature.
V 1 / T 1 = V 2 / T 2
V 1 T 2 = V 2 T 1
(2.00 L) / 294.0 K) = (1.00 L) / (x) cross multiply to get: 2x = 293 x = 147.0 K Converting 147.0 K to Celsius, we find -126.0 °C, for a total decrease of 147.0 °C, from 21.0 °C to -126.0 °C.
(600.0 mL) / (293.0) = (x) / (333.0 K) x = 682 mL
(900.0 mL) / (300.0 K) = (x) / (405.0 K) x = 1215 mL
(60.0 mL) / (306.0 K) = (x) / (278.00 K) Cross multiply to get: 306x = 16680 x = 54.5 mL The volume decreases by 5.5 mL.
In cross-multiplied form, it is this: V 1 T 2 = V 2 T 1 V 2 = (V 1 T 2 ) / T 1 1 x = [(300.0 mL) (283.0 K)] / 290.0 K
In cross-multiplied form, it is this: V 1 T 2 = V 2 T 1 V 2 = (V 1 ) [T 2 / T 1 ] x = (1.00 L) [(606.0 K) / (273.0 K)] x = 2.22 L
(6.00 L) / (300.0 K) = (x) / (423.0 K) or (6.00 L) (423.0 K) = (x) (300.0 K)
V 2 = (V 1 ) [T 2 / T 1 ] x = (400.0 mL) [(400.0 K) / (498.0 K) x = 321 mL
(400.0 mL) / (498.0 K) = (x) / (400.0 K)
(8.00 L) / (483.0 K) = (x) / (250.0 K) Note how you can have a negative Celsius temperature, but not a negative Kelvin temperature.
V 1 / T 1 = V 2 / T 2 x / 588 K = 852 mL / 725 K (x) (725 K) = (852 mL) (588 K) x = 691 mL
2.05 L / 278 K = V 2 / 294 K Calculate V 2 . The volume that "escapes" is V 2 minus 2.05 L

IMAGES
VIDEO
COMMENTS
1) Let us use a ratio and proportion to estimate the pressure required for water to boil at 88 °C: 100 °C is to 101.3 kPa as 88 °C is to x. x = 89.144 kPa. 2) Now, we can solve the problem using Boyle's Law: P 1 V 1 = P 2 V 2. (101.3) (2.0) = (88.144) (x) x = 2.27 L. The balloon will not burst.
P final = 1/z x V initial. Boyle's Law describes the relationship between pressure and volume of a gas when mass and temperature are held constant. (NASA) Example Problem. For example, calculate the final volume of a gas if the pressure of a 4.0 L sample is changed from 2.5 atm to 5.0 atm. You calculate z = P final /P initial. z = 5.0 / 2.5.
Boyle's gas law states that the volume of a gas is inversely proportional to the pressure of the gas when the temperature is held constant. Anglo-Irish chemist Robert Boyle (1627-1691) discovered the law and for it he is considered the first modern chemist. This example problem uses Boyle's law to find the volume of gas when pressure changes.
Boyle's Law Example Problem. For example, calculate the final volume of a balloon if it has a volume of 2.0 L and pressure of 2 atmospheres and the pressure is reduced to 1 atmosphere. Assume temperature remains constant. P 1 V 1 = P 2 V 2. (2 atm) (2.0 L) = (1 atm)V 2.
FREE Online Course: https://www.socratica.com/courses/chemistryBUY Practice Tests: https://bookstore.socratica.com/?tags=chemistryJOIN Chemistry Club: https...
Figure 11.4.1 11.4. 1: Boyle's Law. A piston having a certain pressure and volume (left piston) will have half the volume when its pressure is twice as much (right piston). One can also plot P versus V for a given amount of gas at a certain temperature; such a plot will look like the graph on the right. Boyle's Law is an example of a second ...
Suppose the gas with pressure P 1 and volume V 1 expands or shrinks to pressure P 2 and volume V 2. Then, using Boyle's law equation, P 1 V 1 = k and P 2 V 2 = k. From the above two equations. P 1 V 1 = P 2 V 2. This equation shows that as the pressure increases, the volume decreases and vice versa. For example, when the pressure doubles, the ...
Worked Example Problem. The sections on the General Properties of Gases and Ideal Gas Law Problems may also be helpful when attempting to work Boyle's Law problems . Problem. A sample of helium gas at 25°C is compressed from 200 cm 3 to 0.240 cm 3. Its pressure is now 3.00 cm Hg.
Learn how to solve problems involving Boyle's law. Boyle's law states that as pressure increases then volume decreases and pressure decreases volume increase...
This chemistry video tutorial explains how to solve practice problems associated with boyle's law. it provides an example that illustrates the concept of bo...
Gas Mass and Gas Temperature. Answer: (d), In Boyle's law, the mass of the gas its temperature are kept constant. Q5. Boyle's law is valid only for. Ideal gases. Non-ideal gases. Light Gases. Heavy Gases. Answer: (a), Boyle's law is valid only for ideal gases.
This expression can be obtained from the pressure-volume relationship suggested by Boyle's law. For a fixed amount of gas kept at a constant temperature, PV = k. Therefore, P1V1 = k (initial pressure * initial volume) P2V2 = k (final pressure * final volume) ∴ P1V1 = P2V2. This equation can be used to predict the increase in the pressure ...
This equation is an example of a gas law. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. This particular gas law is called Boyle's law, after the English scientist Robert Boyle, who first announced it in 1662. Figure \(\PageIndex{1}\) shows two representations of how Boyle's law works.
Correct answer: Explanation: Since the volume of the gas is the only variable that has changed, we can use Boyle's law in order to find the final pressure. Since pressure and volume are on the same side of the ideal gas law, they are inversely proportional to one another. In other words, as one increases, the other will decrease, and vice versa.
Figure 11.4.1 11.4. 1: Boyle's Law. A piston having a certain pressure and volume (left piston) will have half the volume when its pressure is twice as much (right piston). One can also plot P versus V for a given amount of gas at a certain temperature; such a plot will look like the graph on the right. Boyle's Law is an example of a second ...
Back to the Boyle's Law calculation practice Related Content Illustrations Boyle's Law Problem Sets Boyle's Law Concepts
This problem is a relationship between pressure and volume. To solve for the volume we would use Boyle's Law, which is comparison of the inverse relationship between pressure and volume. (P i)(V i) = (P f)(V f) Rearrange algebraically to solve for x. xL = (1.1atm)(4.0L) 3.4atm. We get value of 1.29 L.
Figure 11.4.1 11.4. 1: Boyle's Law. A piston having a certain pressure and volume (left piston) will have half the volume when its pressure is twice as much (right piston). One can also plot P versus V for a given amount of gas at a certain temperature; such a plot will look like the graph on the right. Boyle's Law is an example of a second ...
This law in practice is shown in Figure \(\PageIndex{2}\). Figure \(\PageIndex{2}\): Plots of Boyle's Data. (a) Here are actual data from a typical experiment conducted by Boyle. Boyle used non-SI units to measure the volume (in. 3 rather than cm 3) and the pressure (in. Hg rather than mmHg). (b) This plot of pressure versus volume is a ...
Solution: Write Charles Law and substitute values in: V 1 / T 1 = V 2 / T 2. x / 588 K = 852 mL / 725 K. (x) (725 K) = (852 mL) (588 K) x = 691 mL. Note the large °C values, trying to get you to forget to add 273. Remember, only Kelvin temperatures are allowed in the calculations. Bonus Problem: An open "empty" 2 L plastic pop container, which ...