
- Mastering Stoichiometry: Your Guide to Problem Solving
Welcome to Warren Institute! In this article, we will delve into the fascinating world of stoichiometry and learn how to solve its problems effectively. Stoichiometry is a fundamental concept in Chemistry that involves the calculation of quantities in chemical reactions. Whether you're a student struggling with stoichiometry or a teacher looking for innovative teaching methods, this guide is for you. We will explore step-by-step strategies, equation balancing techniques , and essential tips to crack stoichiometry problems like a pro. Get ready to enhance your problem-solving skills and gain a deeper understanding of this crucial topic.

Understanding Stoichiometry Problems
Steps to solve stoichiometry problems, common challenges in solving stoichiometry problems, strategies for success in stoichiometry problem solving, what is stoichiometry and why is it important in chemistry, how do you balance chemical equations and use stoichiometry to solve problems, what are the steps involved in solving stoichiometry problems, how do you convert between moles, grams, and molecules in stoichiometry calculations, can you provide examples of stoichiometry problems and step-by-step solutions.
In this section, we will dive into the concept of stoichiometry and its relevance in mathematics education . We will explore what stoichiometry problems are and why it is important to understand them.
Stoichiometry is a branch of chemistry that deals with the calculation of quantities of reactants and products involved in chemical reactions. It is based on the principle of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Stoichiometry problems involve determining the relationship between the amounts of substances involved in a chemical reaction. This often requires balancing chemical equations , understanding mole ratios, and converting between different units of measurement. These problems can be challenging for students but are crucial for understanding the fundamentals of chemistry and mathematics.
Key takeaway: Understanding stoichiometry problems is essential for mastering the mathematical aspects of chemistry and building a solid foundation in mathematics education.
In this section, we will outline a step-by-step approach to solving stoichiometry problems. By following these steps, students can simplify complex problems and arrive at accurate solutions.
Step 1: Balance the chemical equation - Begin by ensuring that the chemical equation is balanced, with an equal number of atoms on both sides. This step is crucial for accurately determining mole ratios and other calculations.
Step 2: Convert given quantities - Convert the given quantities of substances into moles using the molar mass. This step involves using conversion factors and dimensional analysis to establish the relationship between grams, moles, and other units.
Step 3: Determine the mole ratio - Using the balanced chemical equation, determine the mole ratio between the known and unknown substances. This ratio allows for the conversion of moles of one substance to moles of another.
Step 4: Calculate the desired quantity - Finally, use the mole ratio and the known quantity of one substance to calculate the desired quantity of another substance. This step often involves additional conversions between moles, grams, or other units.
Key takeaway: Following a systematic approach to solving stoichiometry problems can help students break down complex calculations into manageable steps and arrive at accurate solutions.
In this section, we will address some common challenges that students may encounter when solving stoichiometry problems. By understanding these challenges, educators can better support students in overcoming them.
Challenge 1: Balancing chemical equations - Balancing chemical equations requires a solid understanding of the concept and practice. Students may struggle with determining the appropriate coefficients to balance different elements correctly.
Challenge 2: Unit conversions - Converting between different units of measurement, such as grams to moles or moles to liters, can be confusing for students. Understanding conversion factors and practicing dimensional analysis is critical for successful problem-solving.
Challenge 3: Identifying limiting reactants - In complex stoichiometry problems, identifying the limiting reactant can be challenging. Students need to grasp the concept of limiting reactants and how they affect the overall reaction.
Key takeaway: Awareness of common challenges in solving stoichiometry problems can guide educators in providing targeted support and resources to help students overcome difficulties.
In this section, we will discuss effective strategies that students can employ to excel in solving stoichiometry problems.
Strategy 1: Practice, practice, practice - Stoichiometry problems require repetitive practice to build confidence and proficiency. Students should attempt a variety of problems to strengthen their understanding and problem-solving skills.
Strategy 2: Understand the underlying concepts - Rather than relying solely on memorizing formulas, students should strive to understand the underlying concepts of stoichiometry. This understanding will enable them to apply their knowledge flexibly to different problem scenarios.
Strategy 3: Seek support and resources - Students should not hesitate to seek help from educators, fellow students, or online resources when encountering difficulties. There are numerous educational websites, textbooks, and videos available that provide additional explanations and practice problems.
Key takeaway: By employing effective strategies, such as practicing regularly and seeking support when needed, students can enhance their problem-solving abilities and achieve success in solving stoichiometry problems.
frequently asked questions
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It involves using mathematical calculations to determine the amount of substances involved in a reaction, including the ratio of reactants and products. Stoichiometry is important in chemistry because it allows us to understand and predict the outcome of reactions, determine the amount of reactants needed for a desired product, and analyze the efficiency of chemical reactions.
In order to balance chemical equations and use stoichiometry to solve problems, you need to follow a systematic approach. First, you balance the equation by adjusting the coefficients in front of each compound so that the number of atoms on both sides of the equation is equal. This ensures the law of conservation of mass is satisfied. Then, using the balanced equation, you can determine the mole ratios between reactants and products. This allows you to calculate the amount of one substance given the amount of another using stoichiometry.
The steps involved in solving stoichiometry problems in Mathematics education are:
1. Write a balanced chemical equation: Start by writing a balanced chemical equation for the given reaction. This helps determine the mole ratios between the reactants and products.
2. Convert known quantities to moles: Convert the given quantities of reactants or products to moles using their respective molar masses.
3. Apply the mole ratio: Use the mole ratios from the balanced chemical equation to determine the moles of the desired substance.
4. Convert moles to desired units: Convert the moles of the desired substance to the desired units (grams, liters, etc.) using the appropriate conversion factors.
5. Check for consistency: Verify that the units and dimensions of the final answer are consistent with the problem statement.
Note: These steps may vary depending on the specific problem and the level of mathematics education.
In stoichiometry calculations, you can convert between moles, grams, and molecules using the concept of Avogadro's number. The conversion factors are as follows:
- To convert from moles to grams, you use the molar mass of the substance.
- To convert from grams to moles, you use the reciprocal of the molar mass.
- To convert from moles to molecules, you use Avogadro's number (6.022 x 10^23).
Problem: Calculate the amount of product formed when 25 grams of reactant A reacts with excess reactant B according to the following balanced equation: 2A + 3B → 4C
Solution: 1. Convert the given mass of reactant A to moles using its molar mass. 2. Use the stoichiometric coefficients from the balanced equation to set up a ratio between reactant A and product C. 3. Convert the moles of reactant A to moles of product C using the stoichiometric ratio. 4. Convert the moles of product C to grams using its molar mass.
Step-by-step calculation: 1. Moles of A = Mass of A / Molar mass of A Moles of A = 25 g / (molar mass of A)
2. Use the stoichiometric coefficients to set up the ratio: 2 moles A : 4 moles C
3. Moles of C = Moles of A * (4 moles C / 2 moles A)
4. Mass of C = Moles of C * Molar mass of C
This is just one example, but stoichiometry problems involve using the mole ratios from a balanced chemical equation to calculate the quantities of reactants and products involved in a chemical reaction.
In conclusion, mastering the art of solving stoichiometry problems is an essential skill for students studying Mathematics education. By understanding the concepts and principles behind stoichiometry, students can effectively calculate the quantities of reactants and products involved in chemical reactions. This not only strengthens their problem-solving abilities but also enhances their understanding of mathematical concepts within a real-world context. Stoichiometry, therefore, serves as a bridge between Mathematics and Chemistry, allowing students to apply mathematical skills to solve practical problems. By utilizing the step-by-step approach outlined in this article, students can confidently tackle stoichiometry problems and develop a deeper appreciation for the interplay between Mathematics and the sciences. So, let's embrace the power of stoichiometry and empower students to excel in their Mathematics education journey!
If you want to know other articles similar to Mastering Stoichiometry: Your Guide to Problem Solving you can visit the category General Education .

Michaell Miller
Michael Miller is a passionate blog writer and advanced mathematics teacher with a deep understanding of mathematical physics. With years of teaching experience, Michael combines his love of mathematics with an exceptional ability to communicate complex concepts in an accessible way. His blog posts offer a unique and enriching perspective on mathematical and physical topics, making learning fascinating and understandable for all.
- Unlocking the Science: Boiling Point Elevation and Colligative Properties
- Mastering Trigonometry: Graphing Functions & Formulas
Discovering Triangles: Exploring Segments' Potential for Triangle Formation
Exploring the 2-Methyl-1,3-Cyclohexadiene Reaction with HCl: A Comprehensive Study
If t is the Midpoint of su: Understanding the Relationship with st
Why Igor Felt Unhappy After Taking His Spelling Test: An Analysis
The Box Plot Reveals Home Run Distribution in Baseball
What Goes in a Toaster Riddle: Can You Solve This Kitchen Conundrum?

Chemistry Steps

General Chemistry
Stoichiometry.
This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts.
The links to the corresponding topics are given below.
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Practice Problems
Balance the following chemical equations:
a) HCl + O 2 → H 2 O + Cl 2
b) Al(NO 3 ) 3 + NaOH → Al(OH) 3 + NaNO 3
c) H 2 + N 2 → NH 3
d) PCl 5 + H 2 O → H 3 PO 4 + HCl
e) Fe + H 2 SO 4 → Fe 2 (SO 4 ) 3 + H 2
f) CaCl 2 + HNO 3 → Ca(NO 3 ) 2 + HCl
g) KO 2 + H 2 O → KOH + O 2 + H 2 O 2
h) Al + H 2 O → Al 2 O 3 + H 2
i) Fe + Br 2 → FeBr 3
j) Cu + HNO 3 → Cu(NO 3 ) 2 + NO 2 + H 2 O
k) Al(OH) 3 → Al 2 O 3 + H 2 O
l) NH 3 + O 2 → NO + H 2 O
m) Ca(AlO 2 ) 2 + HCl → AlCl 3 + CaCl 2 + H 2 O
n) C 5 H 12 + O 2 → CO 2 + H 2 O
o) P 4 O 10 + H 2 O → H 3 PO 4
p) Na 2 CrO 4 + Pb(NO 3 ) 2 → PbCrO 4 + NaNO 3
q) MgCl 2 + AgNO 3 → AgCl + Mg(NO 3 ) 2
r) KClO 3 → KClO 4 + KCl
s) Ca(OH) 2 + H 3 PO 4 → Ca 3 (PO 4 ) 2 + H 2 O
Consider the balanced equation:
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
Complete the table showing the appropriate number of moles of reactants and products.
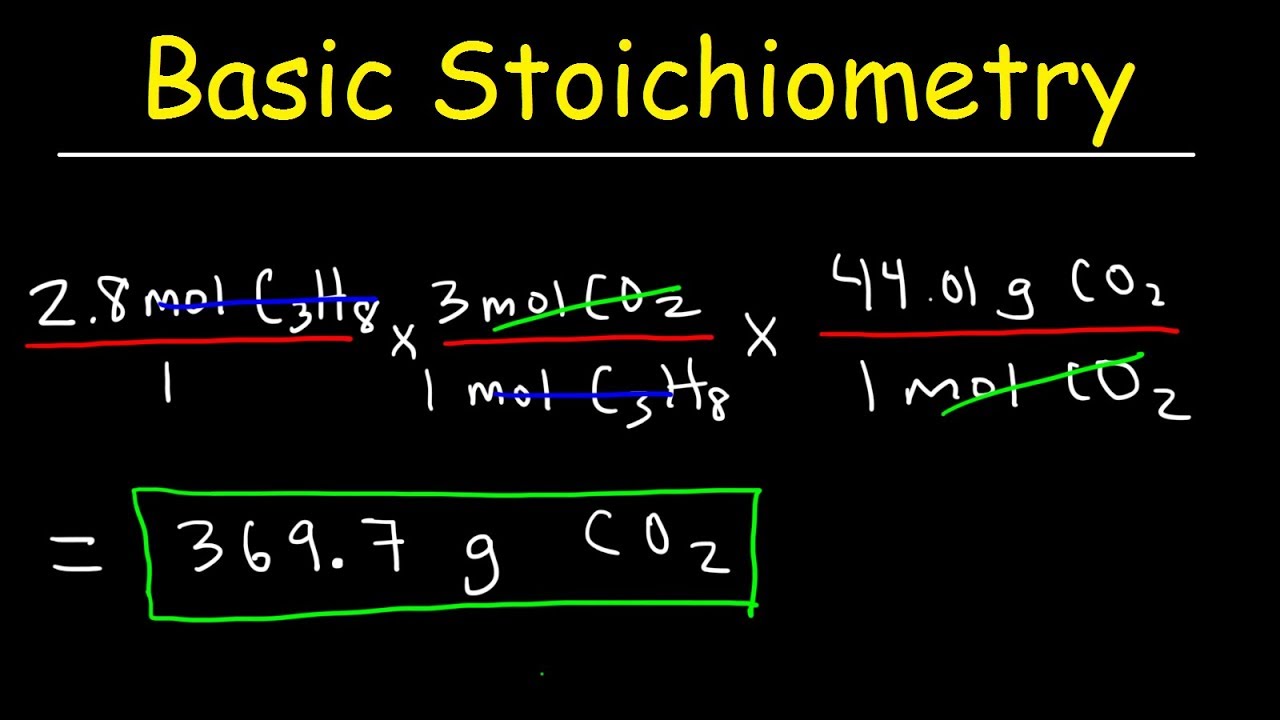
How many grams of CO 2 and H 2 O are produced from the combustion of 220. g of propane (C 3 H 8 )?
C 3 H 8 (g) + 5O 2 (g) → 3CO 2 (g) + 4H 2 O(g)
How many grams of CaCl 2 can be produced from 65.0 g of Ca(OH) 2 according to the following reaction,
Ca(OH) 2 + 2HCl → CaCl 2 + 2H 2 O
How many moles of oxygen are formed when 75.0 g of Cu(NO 3 ) 2 decomposes according to the following reaction?
2Cu(NO 3 ) 2 → 2CuO + 4NO 2 + O 2
How many grams of MnCl 2 can be prepared from 52.1 grams of MnO 2 ?
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
Determine the mass of oxygen that is formed when an 18.3-g sample of potassium chlorate is decomposed according to the following equation:
2KClO 3 (s) → 2KCl(s) + 3O 2 (g).
How many grams of H 2 O will be formed when 48.0 grams H 2 are mixed with excess hydrogen gas?
2H 2 + O 2 → 2H 2 O
Consider the chlorination reaction of methane (CH4):
CH 4 (g) + 4Cl 2 (g) → CCl 4 (g) + 4HCl(g)
How many moles of CH 4 were used in the reaction if 51.9 g of CCl4 were obtained?
How many grams of Ba(NO 3 ) 2 can be produced by reacting 16.5 g of HNO 3 with an excess of Ba(OH) 2 ?
Ethanol can be obtained by fermentation – a complex chemical process breaking down glucose to ethanol and carbon dioxide.
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 glucose ethanol
How many mL of ethanol (d =0.789 g/mL) can be obtained by this process starting with 286 g of glucose?
36.0 g of butane (C 4 H 10 ) was burned in an excess of oxygen and the resulting carbon dioxide (CO 2 ) was collected in a sealed vessel.
2C 4 H 10 + 13O 2 → 8CO 2 + 10H 2 O
How many grams of LiOH will be necessary to consume all the CO 2 from the first reaction?
2LiOH + CO 2 → Li 2 CO 3 + H 2 O
13. Which statement about limiting reactant is correct?
a) The limiting reactant is the one in a smaller quantity.
b) The limiting reactant is the one in greater quantity.
c) The limiting reactant is the one producing less product.
d) The limiting reactant is the one producing more product.
Find the limiting reactant for each initial amount of reactants.
4NH 3 + 5O 2 → 4NO + 6H 2 O
a) 2 mol of NH 3 and 2 mol of O 2
b) 2 mol of NH 3 and 3 mol of O 2
c) 3 mol of NH 3 and 3 mol of O 2
d) 3 mol of NH 3 and 2 mol of O 2
Note: This is not a multiple-choice question. Each row represents a separate question where you need to determine the limiting reactant.
How many g of hydrogen are left over in producing ammonia when 14.0 g of nitrogen is reacted with 8.0 g of hydrogen?
N 2 (g) + 3 H 2 (g) → 2 NH 3 (g)
How many grams of PCl 3 will be produced if 130.5 g Cl 2 is reacted with 56.4 g P 4 according to the following equation?
6Cl 2 (g) + P 4 (s) → 4PCl 3 (l)
How many grams of sulfur can be obtained if 12.6 g H 2 S is reacted with 14.6 g SO 2 according to the following equation?
2H 2 S(g) + SO 2 (g) → 3S(s) + 2H 2 O(g)
The following equation represents the combustion of octane, C 8 H 18 , a component of gasoline:
2C 8 H 18 (g) + 25O 2 (g) → 16CO 2 (g) + 18H 2 O(g)
Will 356 g of oxygen be enough for the complete combustion of 954 g of octane?
When 140.0 g of AgNO 3 was added to an aqueous solution of NaCl, 86.0 g of AgCl was collected as a white precipitate. Which salt was the limiting reactant in this reaction? How many grams of NaCl were present in the solution when AgNO 3 was added?
AgNO 3 (aq) + NaCl(aq) → AgCl(s) + NaNO 3 (aq)
Consider the reaction between MnO 2 and HCl:
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
What is the theoretical yield of MnCl 2 in grams when 165 g of MnO 2 is added to a solution containing 94.2 g of HCl?
Percent Yield
21. In a chemistry experiment, a student obtained 5.68 g of a product. What is the percent yield of the product if the theoretical yield was 7.12 g?
When 38.45 g CCl 4 is reacted with an excess of HF, 21.3 g CCl 2 F 2 is obtained. Calculate the theoretical and percent yields of this reaction.
CCl 4 + 2HF → CCl 2 F 2 + 2HCl
Iron(III) oxide reacts with carbon monoxide according to the equation:
Fe 2 O 3 ( s ) + 3CO( g ) → 2Fe( s ) + 3CO 2 ( g )
What is the percent yield of this reaction if 623 g of iron oxide produces 341 g of iron?
Determine the percent yield of the reaction if 77.0 g of CO 2 are formed from burning 2.00 moles of C 5 H 12 in 4.00 moles of O 2 .
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
The percent yield for the following reaction was determined to be 84%:
N 2 ( g ) + 2H 2 ( g ) → N 2 H 4 ( l )
How many grams of hydrazine (N 2 H 4 ) can be produced when 38.36 g of nitrogen reacts with 6.68 g of hydrogen?
Silver metal can be prepared by reducing its nitrate, AgNO 3 with copper according to the following equation:
Cu( s ) + 2AgNO 3 ( aq ) → Cu(NO 3 ) 2 ( aq ) + 2Ag( s )
What is the percent yield of the reaction if 71.5 grams of Ag was obtained from 132.5 grams of AgNO 3 ?
Industrially, nitric acid is produced from ammonia by the Ostwald process in a series of reactions:
4NH 3 ( g ) + 5O 2 ( g ) → 4NO( g ) + 6H 2 O( l )
2NO( g ) + O 2 ( g ) → 2NO 2 ( g )
2NO 2 ( g ) + H 2 O( l ) → HNO 3 ( aq ) + HNO 2 ( aq )
Considering that each reaction has an 85% percent yield, how many grams of NH 3 must be used to produce 25.0 kg of HNO 3 by the above procedure?
Aspirin (acetylsalicylic acid) is widely used to treat pain, fever, and inflammation. It is produced from the reaction of salicylic acid with acetic anhydride. The chemical equation for aspirin synthesis is shown below:
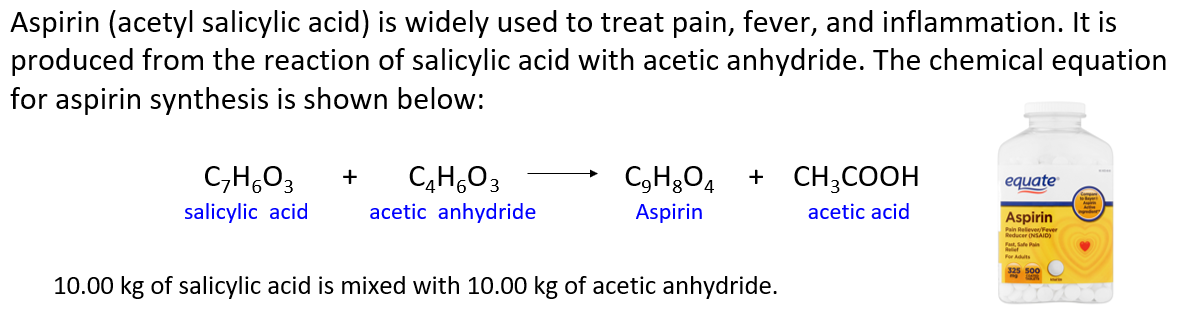
In one container, 10.00 kg of salicylic acid is mixed with 10.00 kg of acetic anhydride.
a) Which reactant is limiting? Which is in excess? b) What mass of excess reactant is left over? c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)? d) What mass of aspirin is formed if the reaction yield is 70.0% ? e) If the actual yield of aspirin is 11.2 kg, what is the percent yield? f) How many kg of salicylic acid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?

3 thoughts on “Stoichiometry Practice Problems”
You forgot the subscript 3 for O in the molecular formula for acetic anhydride and the reaction is not balanced as written. For part F) it’s 18.1 kg and not1.81 kg as written in the final line of the solution.
Thanks for letting me know! Fixed.
You’re welcome!
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.2.1: Practice Problems- Reaction Stoichiometry
- Last updated
- Save as PDF
- Page ID 217275
PROBLEM \(\PageIndex{1}\)
Write the balanced equation and determine the information requested. Don't worry about state symbols in these reactions.
- The number of moles and the mass (in grams) of chlorine, Cl 2 , required to react with 10.0 g of sodium metal, Na, to produce sodium chloride, NaCl.
- The number of moles and the mass (in milligrams) of diatomic oxygen formed by the decomposition of 1.252 g of mercury(II) oxide.
- The number of moles and the mass (in g) of sodium nitrate, NaNO 3 , required to decompose and produce 128 g of diatomic oxygen, where NaNO 2 is the other product.
- The number of moles and the mass (in kg) of carbon dioxide formed by the combustion of 20.0 kg of carbon in an excess of diatomic oxygen.
- The number of moles and the mass (in kg) of copper(II) carbonate needed to decompose in order to produce 1.500 kg of copper(II) oxide, where CO 2 is the other product.
- The number of moles and mass (in grams) of C 2 H 4 required to react with water to produce 9.55 g C 2 H 6 O.
\(\ce{2Na}+\ce{Cl2}\rightarrow \ce{2NaCl}\)
0.217 mol Cl 2
15.43 g Cl 2
\(\ce{2HgO}\rightarrow \ce{2Hg}+\ce{O2}\)
0.00289 mol O 2
\(\ce{2NaNO3}\rightarrow \ce{2NaNO3}+\ce{O2}\)
8 mol NaNO 3
680 g NaNO 3
\(\ce{C}+\ce{O2}\rightarrow \ce{CO2}\)
1666.67 mol CO 2
73.3 kg CO 2
\(\ce{CuCO3}\rightarrow \ce{CuO}+\ce{CO2}\)
18.87 mol CuCO 3
2.330 kg CuCO 3
\(\ce{C2H4}+\ce{H2O}\rightarrow \ce{C2H6O}\)
0.207 mol C 2 H 4
5.81 g C 2 H 4
*Apologies for the brief phone ringing*
PROBLEM \(\PageIndex{2}\)
I 2 is produced by the reaction of 0.4235 mol of CuCl 2 according to the following equation: \(\ce{2CuCl2 + 4KI \rightarrow 2CuI + 4KCl + I2}\) .
- How many molecules of I 2 are produced?
- What mass of I 2 is produced?
1.28 × 10 23 molecules I 2
PROBLEM \(\PageIndex{3}\)
Silver is often extracted from ores as K[Ag(CN) 2 ] and then recovered by the reaction
\(\ce{2K[Ag(CN)2]}(aq)+\ce{Zn}(s)\rightarrow \ce{2Ag}(s)+\ce{Zn(CN)2}(aq)+\ce{2KCN}(aq)\)
- How many molecules of Zn(CN) 2 are produced by the reaction of 35.27 g of K[Ag(CN) 2 ]?
- What mass of Zn(CN) 2 is produced?
5.337 × 10 22 molecules
10.41 g Zn(CN) 2
PROBLEM \(\PageIndex{4}\)
What mass of silver oxide, Ag 2 O, is required to produce 25.0 g of silver sulfadiazine, AgC 10 H 9 N 4 SO 2 , from the reaction of silver oxide and sulfadiazine?
\(\ce{2C10H10N4SO2 + Ag2O \rightarrow 2AgC10H9N4SO2 + H2O}\)
8.12 g Ag 2 O
PROBLEM \(\PageIndex{5}\)
Carborundum is silicon carbide, SiC, a very hard material used as an abrasive on sandpaper and in other applications. It is prepared by the reaction of pure sand, SiO 2 , with carbon at high temperature. Carbon monoxide, CO, is the other product of this reaction. Write the balanced equation for the reaction, and calculate how much SiO 2 is required to produce 3.00 kg of SiC.
\(\ce{SiO2 + 3C \rightarrow SiC + 2CO}\)
4.50 kg SiO 2
PROBLEM \(\PageIndex{6}\)
Automotive air bags inflate when a sample of sodium azide, NaN 3 , is very rapidly decomposed.
\(\ce{2NaN3}(s) \rightarrow \ce{2Na}(s) + \ce{3N2}(g)\)
What mass of sodium azide is required to produce 2.6 ft 3 (73.6 L) of nitrogen gas with a density of 1.25 g/L?
PROBLEM \(\PageIndex{7}\)
Urea, CO(NH 2 ) 2 , is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. What is the maximum mass of urea that can be manufactured from the CO 2 produced by combustion of 1.00×10 3 kg of carbon followed by the reaction?
\[\ce{CO2}(g)+\ce{2NH3}(g)\rightarrow \ce{CO(NH2)2}(s)+\ce{H2O}(l)\]
5.00 kg Urea
PROBLEM \(\PageIndex{8}\)
In an accident, a solution containing 2.5 kg of nitric acid was spilled. Two kilograms of Na 2 CO 3 was quickly spread on the area and CO 2 was released by the reaction. Was sufficient Na 2 CO 3 used to neutralize all of the acid? (in this reaction, water and sodium nitrate are the other two products)
No, you will need 2.1 kg of sodium carbonate to neutralize 2.5 kg of nitric acid.
PROBLEM \(\PageIndex{9}\)
A compact car gets 37.5 miles per gallon on the highway. If gasoline contains 84.2% carbon by mass and has a density of 0.8205 g/mL, determine the mass of carbon dioxide produced during a 500-mile trip (3.785 liters per gallon).
1.28 × 10 5 g CO 2
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/[email protected] ).
- Adelaide Clark, Oregon Institute of Technology

IMAGES
VIDEO
COMMENTS
Steps to Solve Stoichiometry Problems. In this section, we will outline a step-by-step approach to solving stoichiometry problems. By following these steps, students can simplify complex problems and arrive at accurate solutions. Step 1: Balance the chemical equation - Begin by ensuring that the chemical equation is balanced, with an equal ...
Stoichiometry Practice Problems. This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts. The links to the corresponding topics are given below. The Mole and Molar Mass.
PROBLEM 5.2.1.1 5.2.1. 1. Write the balanced equation and determine the information requested. Don't worry about state symbols in these reactions. The number of moles and the mass (in grams) of chlorine, Cl 2, required to react with 10.0 g of sodium metal, Na, to produce sodium chloride, NaCl. The number of moles and the mass (in milligrams) of ...