Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center


PROJECT PROPOSAL CERVICAL CANCER SCREENING T VENGESAI

Related Papers
Tirivanhu Chipfuwa
Fred Nuwaha
Fresier Maseko
International journal of public health
Nestory Masalu
To report the results of a pilot study for a service for cervical cancer screening and diagnosis in north-western Tanzania. The pilot study was launched in 2012 after a community-level information campaign. Women aged 15-64 years were encouraged to attend the district health centres. Attendees were offered a conventional Pap smear and a visual inspection of the cervix with acetic acid (VIA). The first 2500 women were evaluated. A total of 164 women (detection rate 70.0/1000) were diagnosed with high-grade cervical intraepithelial neoplasia and invasive cervical cancer. The performance of VIA was comparable to that of Pap smear. The district of residence, a history of untreated sexually transmitted disease, an HIV-negative status (inverse association), and parity were independently associated with the detected prevalence of disease. The probability of invasive versus preinvasive disease was lower in HIV-positive women and in women practicing breast self-examination. The diagnostic pr...
PLOS global public health
John M U K U K A Musonda
Cogent Social Sciences
elimon nyamambi
BMC Public Health
TWAMBILIRE PHIRI
Texila International Journal of Public Health
Texila International Journal , Dingase Mvula
Women living with Human Immunodeficiency Virus (WLHIV) have a higher risk of developing cervical cancer due to their immune-compromised state. Cervical cancer screening leads to early detection and treatment. The aim of the study was to determine the knowledge, attitude, and practices of cervical cancer screening among women infected with HIV in Kasenengwa District, Eastern Province, Zambia. A descriptive cross-sectional study design using a semi-structured questionnaire was used to collect data from 266 WLHIV. Basic descriptive statistics were done using SPSS version 23.0. Almost two-thirds (62.7%) of the 266 WLHIV in the study had adequate knowledge about cervical cancer screening. Almost three-fifths of the respondents (57.1%) had a negative attitude toward cervical cancer screening. The majority (78.2%) had been counselled by healthcare workers on cervical cancer screening with good emotional support from family members (72.9%). About twothirds (68.4%) of the respondents had been screened for cervical cancer. Most women indicated that they didn't have access to cervical cancer screening services because they did not know where to go (61.5%) and distant screening sites (56.3%) WLHIV in the study had adequate knowledge, but unfavorable attitude towards cervical cancer screening, while two-thirds had been screened for cervical cancer. Accessibility to screening sites was poor. More education and sensitization are needed in the district to eliminate misconceptions about cervical cancer screening, which may influence uptake.
Marianne Calnan
Determinants of Cervical Cancer Screening in HIV-Positive Young Women in Swaziland by Marianne Calnan MPH, Manchester University, 2012 MMed Internal Medicine, Makerere University, 2005 MBCHB, Mbarara University of Science and Technology, 1998 Doctoral Study Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Public Health Walden University February 2019 Abstract In Swaziland, cases of cervical cancer among Human Immunodeficiency Virus (HIV)positive adolescent girls and young women (AGYW) are increasing, but there is low uptake of cervical cancer screening. This study was conducted using the systems thinking theory to explore the relationships between the uptake of cervical cancer screening among HIVpositive AGYW in Swaziland and the availability of trained health providers, cervical screening services, and the provision of referrals for cervical screening. The study also investigated any differences in uptake of cervical screening based on age group. For...
RELATED PAPERS
Alexander Leemans
토토블랙리스트〃〃GCN777。COM〃〃강원랜드후기
greghui gerearg
Blucher Biophysics Proceedings
Yamina Montaldo
Fatigue & Fracture of Engineering Materials & Structures
Roberto Tovo
Water Resources Research
Stijn Reinhard
Brunot Sophie
Milton Waner
Indian Journal of Natural Products and Resources
dnyaneshwar ghorband
Malta Medical Journal
Christopher Fearne
José María Antón Contreras, L.C.
Revista Argentina de Cardiología
Amilcar Osorio
Maheshwari Harishchandre
Iskolakultúra
Ernő Kulcsár Szabó
Nanotoxicology
Emmanuel Paul
Revista Acadêmica Online
DOUGLAS MANOEL ANTONIO DE ABREU PESTANA D O S SANTOS
Fernanda Pires de Sá
Revista Ética e Filosofia Política
Clinger Cleir Silva Bernardes
Echocardiography
radwa abdullah
Jerusa Roeder
Serli Padaka
Sao Paulo Medical Journal
Ali Gedikbaşı
Revue trimestrielle des droits de l’homme
Idris Fassassi
IEEE Transactions on Nuclear Science
Paolo Randaccio
JPMA. The Journal of the Pakistan Medical Association
ahmed bilal
Formação Docente: Princípios e Fundamentos 5
sebastian saez gonzalez
See More Documents Like This
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Proposal for cervical cancer screening in the era of HPV vaccination
Affiliation.
- 1 Department of Obstetrics and Gynecology, Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- PMID: 29780771
- PMCID: PMC5956112
- DOI: 10.5468/ogs.2018.61.3.298
Eradication of cervical cancer involves the expansion of human papillomavirus (HPV) vaccine coverage and the development of efficient screening guidelines that take vaccination into account. In Korea, the HPV National Immunization Program was launched in 2016 and is expected to shift the prevalence of HPV genotypes in the country, among other effects. The experiences of another countries that implement national immunization programs should be applied to Korea. If HPV vaccines spread nationwide with broader coverage, after a few decades, cervical intraepithelial lesions or invasive cancer should become a rare disease, leading to a predictable decrease in the positive predictive value of cervical screening cytology. HPV testing is the primary screening tool for cervical cancer and has replaced traditional cytology-based guidelines. The current screening strategy in Korea does not differentiate women who have received complete vaccination from those who are unvaccinated. However, in the post-vaccination era, newly revised policies will be needed. We also discuss on how to increase the vaccination rate in adolescence.
Keywords: Cancer screening; HPV vaccines; Human papillomavirus; Immunization program.
Publication types
Advertisement
Evaluating the implementation of cervical cancer screening programs in low-resource settings globally: a systematized review
- Review article
- Open access
- Published: 17 March 2020
- Volume 31 , pages 417–429, ( 2020 )
Cite this article
You have full access to this open access article
- J. Andrew Dykens ORCID: orcid.org/0000-0002-4194-8725 1 ,
- Jennifer S. Smith 2 ,
- Margaret Demment 3 ,
- E. Marshall 4 ,
- Tina Schuh 4 ,
- Karen Peters 4 ,
- Tracy Irwin 5 ,
- Scott McIntosh 6 ,
- Angela Sy 7 &
- Timothy Dye 3
4278 Accesses
9 Citations
Explore all metrics
Cervical cancer disproportionately burdens low-resource populations where access to quality screening services is limited. A greater understanding of sustainable approaches to implement cervical cancer screening services is needed.
We conducted a systematized literature review of evaluations from cervical cancer screening programs implemented in resource-limited settings globally that included a formal evaluation and intention of program sustainment over time. We categorized the included studies using the continuum of implementation research framework which categorizes studies progressively from “implementation light” to more implementation intensive.
Fifty-one of 13,330 initially identified papers were reviewed with most study sites in low-resource settings of middle-income countries (94.1%) ,while 9.8% were in low-income countries. Across all studies, visual inspection of the cervix with acetic acid (58.8%) was the most prevalent screening method followed by cytology testing (39.2%). Demand-side (client and community) considerations were reported in 86.3% of the articles, while 68.6% focused scientific inquiry on the supply side (health service). Eighteen articles (35.3%) were categorized as “Informing Scale-up” along the continuum of implementation research.
Conclusions
The number of cervical cancer screening implementation reports is limited globally, especially in low-income countries. The 18 papers we classified as Informing Scale-up provide critical insights for developing programs relevant to implementation outcomes. We recommend that program managers report lessons learnt to build collective implementation knowledge for cervical cancer screening services, globally.
Similar content being viewed by others
Implementation strategies to improve cervical cancer prevention in sub-saharan africa: a systematic review.
Lauren G. Johnson, Allison Armstrong, … Alison M. Buttenheim

Increasing Cervical Cancer Screening Among US Hispanics/Latinas: A Qualitative Systematic Review
Lilli Mann, Kristie L. Foley, … Scott D. Rhodes
Disparities in cervical cancer screening programs in Cameroon: a scoping review of facilitators and barriers to implementation and uptake of screening
Namanou Ines Emma Woks, Musi Merveille Anwi, … Angel Phuti
Avoid common mistakes on your manuscript.
Introduction
Globally, there are over a half million new cases of cervical cancer yearly, with nearly 90% of these cases in least developed economies [ 1 ]. The United States (US) 2012 cervical cancer incidence rate was 8.1 per 100,000 women, while low- and middle- income countries (LMICs) had a collective rate of 15.7 [ 2 ]. Cervical cancer screening programs detect pre-cancerous lesions which can be treated with low-cost outpatient procedures, and if invasive cancers are caught early, successful treatments exist.
Cervical cancer disproportionately affects populations in resource-limited settings globally despite the variety of evidence-based screening options. Technological innovation and efficacy testing of health service interventions including various screening modalities [ 3 , 4 ] have led to clear recommendations for improving cancer control among HPV vaccination programs and cervical cancer screening using locally appropriate methods [ 5 , 6 , 7 ]. Nonetheless, the judicious implementation of evidence-based cervical cancer screening programs remains inadequate, resulting in persistently elevated cervical cancer incidence and mortality rates, especially in resource-limited settings [ 8 ].
Gaps in the literature
The 2011 WHO Prioritized Research Agenda for Prevention and Control of Non-communicable Disease notes that while cancer screening services have been shown to be effective in high-income countries (HICs) [ 9 , 10 , 11 , 12 ] and HPV screening has been shown to reduce cervical cancer deaths in resource-limited settings such as rural India [ 13 ], there are few reports describing the implementation of successful and sustained cervical cancer screening programs in LMICs. Research that examines cervical cancer screening program barriers and facilitators within specific contexts and informs the adaptation of evidence-based interventions within these contexts is needed to ensure the successful implementation and sustainment of programs across various settings [ 6 , 14 ].
In 2015, a scoping study of existing reviews on breast and cervical cancer in low- and middle- income countries concluded that current cervical cancer literature focuses primarily on prevention and detection, largely without implementation considerations [ 15 ]. An additional finding articulated that articles occasionally provide programmatic or policy recommendations that are beyond the context of their own studies’ findings. However, specific recommendations of the implementation methodologies relevant to issues of governance, systems development, workforce capacity, and person/ community centeredness for arriving at these conclusions are often lacking.
- Cervical cancer screening
Various evidence-based cervical cancer screening techniques have been developed, tested, and are appropriate for diverse contexts. High-resource settings often employ cytologic screening through Papanicolaou (Pap) smear with follow-up colposcopy and biopsy to identify early-stage dysplasia and pre-cancerous lesions. Cytologic screening can be resource heavy by requiring specialized specimen preservation and advanced technical expertise employing cyto-pathologists. Visual inspection methods can complement other screening modalities and provide adequate sensitivity and specificity to identify later-stage pre-cancers which can then be treated through cryotherapy freezing or loop electrosurgical excision procedure (LEEP), curative modalities commonly implemented alongside visual inspection screening services in low-resource settings. In addition, human papillomavirus (HPV) testing has the highest sensitivity for high-grade lesion detection and can be obtained through clinician-sampled or self-sampling techniques. HPV testing can be used for primary screening in conjunction with cytology or visual inspection for triage if the infrastructure exists. Despite the many screening modalities appropriate for a variety of contexts, there remains poor cervical cancer screening coverage, globally. Gakidou et al. reports a coverage rate of 36.9% globally and only 18.5% in least developed countries [ 16 ].
Dissemination and implementation science for cancer research and health systems strengthening
This systematized review addresses the following questions: (1) “What published literature has reported implementation evaluations for cervical cancer screening programs in resource-limited settings, particularly those linked to sustainable systems (e.g., governmental) that will have a population-level impact?” and, (2) “What are the reported program implementation-relevant contextual findings applicable to guide the adaptation of cervical cancer screening programs into a wide range of settings to support cervical cancer prevention and control strengthening efforts worldwide?.”
By applying the “Continuum of Implementation Research” framework (see Table 4 for definitions and examples) to papers included within this review, our intent is to highlight how the existing catalog of cervical cancer implementation research conforms to various levels of implementation science rigor. This allows the reader to readily identify papers most relevant to the various stages of implementation. The themes of this continuum are defined as follows: (1) Proof of Concept (Implementation not relevant or relevant, but not considered), (2) Proof of Implementation (Implementation relevant, but effects reduced), and (3) Informing Scale-up (Implementation studied as contributing factors or as primary focus) [ 17 ].
We conducted a systematized literature review [ 18 ] of cervical cancer screening program implementation evaluations. Inclusion criteria were that the article (1) states the cervical cancer screening intervention occurred in a resource-limited setting in any country, (2) clearly defines implementation evaluation of the intervention with consideration of any defined implementation-relevant outcomes (acceptability, adoption, appropriateness, feasibility, fidelity, coverage, sustainability) [ 17 ], and (3) articulates evidence or intent of the intervention sustainment over time or through policy change by describing or reporting factors relevant to the inner (e.g., organizational characteristics, fidelity monitoring, staffing) or outer context (sociopolitical, funding, public-academic collaboration) [ 19 ]. The only exclusion criterion was publication in a language other than English.
Search strategy
In July 2015, a search of PubMed, CINAHL, Web of Science, POPline, and IndMED included all years up to July 2015 in the initial title review and limited the search to publications in peer-reviewed journals. Search terms were based on four themes: cervical cancer screening, low resource, evaluation, and population level. (see additional file #1 for an example PubMed search string).
We used a multi-step process to select the articles for our review. Three independent reviewers conducted an initial screening for the inclusion criteria based on article titles and labeled them: “yes,” “no,” or “maybe.” If any of the reviewers labeled the title as a “yes” or “maybe,” it advanced to the next round of review. Articles identified as having a focus on cervical cancer were further screened to determine whether or not they included an evaluation of a screening intervention. Therefore, as a next step, abstracts and full articles (if needed) were reviewed by two reviewers to assess all inclusion and exclusion criteria. All discrepancies were discussed verbally in weekly meetings, with resolution by agreement between the two reviewers and an additional author. All data were managed through a shared and continually updated database.
Data collection
The lead authors, through mutual agreement, devised and refined the data abstraction tool based, in part, on the Implementation research framework proposed by Peters et al. [ 17 ] Abstracted items included the following: location of study, partners, scale of intervention (national, regional, district, etc.), motivation of intervention, intervention description, study methodology, independent variables, screening approach (e.g., VIA, cytology, HPV), health system level of intervention, and identified implementation barriers. The two reviewers then each completed a test round of abstraction of the same 10 manuscripts. These abstracts were then reviewed and compared by one of the authors for discrepancies. The team met again to discuss these discrepancies and resolve any issues. The remaining articles were then abstracted by one of two reviewers. The team met once per week to discuss issues or questions related to abstraction.
Information was abstracted directly from the publication without interpretation for all categories with the following exceptions. To categorize the identified “Partners” (Table 1 ) and “Demand and Supply Side Implementation Barriers” (Table 2 ), we standardized the terminology with guidance from Peters [ 17 ] and Proctor [ 20 ]. To determine this categorization, both explicit information from the article as well as interpretation and commentary by the reviewing authors was considered. This subjective interpretation was necessary given the current lack of universal standardization of implementation science terminology [ 21 ]. Peters [ 17 ] includes guidance on the grouping of related terms, but it is not exhaustive and did not apply in all cases. Wherever subjective assessment was used, the lead author made a final determination on categorization for consistency. (Supplement 2).
Analysis and summary of findings
All lead authors were provided with the abstraction document and asked to independently assess emerging themes. Based on the abstracted information contained in the fields, motivation of intervention; description of intervention; methodology; variables; implementation barriers; findings; recommendations; and research or practice gaps were identified. We categorized the reviewed studies along a continuum of implementation research [ 17 ]. We assigned a single label to each paper among three categories: (1) Proof of Concept (Implementation not relevant or relevant, but not considered); (2) Proof of Implementation (Implementation relevant, but effects reduced); and (3) Informing Scale-up (Implementation studied as contributing factors or as primary focus). (Table 4 ).
Finally, we categorized barriers identified through the reported implementation research and organized them according to the Patient-Centered Access to Healthcare Framework proposed by Levesque [ 22 ]. This Framework specifies barriers on both the demand side (client and community perspective) as well as the supply side (health services level). Within this framework, demand-side barriers are subcategorized into the client’s ability to “Perceive,” “Seek,” “Reach,” “Pay,” and “Engage” with the community health system, while supply-side barriers are subcategorized into the health service’s “Approachability,” “Acceptability,” “Availability and Accommodation,” “Affordability,” and “Appropriateness” on the part of clients [ 22 ].
Reporting in this analysis refers to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting results of systematic reviews (S1) [ 23 ]. (Supplement 2).
Fifty-one of 13,330 initially identified papers met inclusion criteria. Twenty-five (49.0%) of the articles were published between 2011 and 2015 compared to only four (9.8%) being published between 2001 and 2005. When analyzed by the World Bank Income level [ 24 ], only five (9.8%) had sites discussed in the papers in low-income countries (three of which were in Uganda), while twenty-six (51.0%) sites were in lower-middle-income countries. (Table 1 ).
The studies reviewed and tested a variety of screening methods, and many of the studies tested multiple methods. Visual inspection was the most prevalent screening method, including 30 (58.8%) VIA studies and eight (15.7%) studies that considered VILI. Cytology testing was second most common, with 20 (39.2%) associated studies. The scale of intervention was distributed towards smaller-scale interventions. Thirty-three (64.7%) were conducted at the city or district level, while only five (9.8%) were at the national level. Forty-four of the 51 studies clearly described the involvement of multiple partners. Of all 51 studies, 26 (51.0%) indicated involvement of national academic institutions and 19 (37.3%) reported involvement of international institutions. Most engaged health systems including those at the local ( n = 34, 66.7%), national ( n = 22, 43%), and international ( n = 3, 5.9%) levels.
For a more nuanced analysis of the reviewed articles, we categorized the relevance to various levels of implementation. (Table 2 ) We identified 40 (78.4%) articles pertinent to patient- or client-level considerations. Only 13 (25.5%) are applicable to the quality of clinical services. Twenty-five (49.0%) articles discussed additional health system-relevant themes (financing, information systems, equipment/resources management, leadership/governance, and others).
In addition to identifying the implementation access level of relevance, we, as well, sought to understand the explicit findings regarding barriers identified through the implementation research. Many of the papers reported specific findings relevant to demand-side ( n = 26, 51.0%) or supply-side ( n = 28, 49%) barriers to the successful implementation of cervical cancer screening services. From the articles presenting demand-side barriers (Table 3 ), those most often reported include comfort ( n = 6, 23.1%), lack of knowledge (5, 19.2%), embarrassment associated with the clinical procedure ( n = 3, 11.5%), and patient cost considerations ( n = 3, 11.5%). Others receiving fewer mentions include distance to clinic, lack of patient priority for prevention, permission required by husband for procedure, and concern about no sexual intercourse after the procedure.
Of the 28 papers reporting supply-side barriers (Table 3 ), 22 (78.6%) discuss provider-relevant themes and 16 (57.1%) discuss other health system-relevant considerations. Of the 22 reporting provider-associated barriers, 10 (45.5%) report a lack of opportunity or time to participate in trainings, nine (41.0%) report significant provider turnover as a major barrier, and 6 (27.3%) describe technical deficiencies of the provider as limitation to the impact of the screening program. Of the 16 reporting other system barriers, nine (56.3%) report cost as a major limiting factor in implementation.
As described in the background, we sought to characterize studies along the continuum of implementation research categories. (Table 4 ) In doing so, we illustrate the value of findings elucidated through studies with well-formulated implementation science questions. Twenty papers (39.2%) were labeled as Proof of Concept, 13 (25.4%) as Proof of Implementation, and 18 (35.3%) as Informing Scale-up. Table 5 details the publications categorized as informing scale-up, stratified by year.
The number of articles ( n = 51) evaluating the implementation of cervical cancer screening programs in limited resource settings is relatively small compared to the total number of articles identified through the review. Of note, we identified only five papers in low-income countries where settings are likely to have the greatest barriers to building sustainable capacity for cervical cancer screening and where, arguably, there is the most to be learned in vastly improving systems and approaches for screening. Of our reviewed articles, the majority (52.9%) were published between 2011 and 2015, suggesting an upward trend of reporting with the later time of publication. The reporting of experiences and sharing of best practices will contribute to our collective ability to overcome the many challenges in ensuring ultimate sustainability of these programs [ 76 , 77 ].
The identified studies cover a range of cervical cancer screening methodologies with VIA being the most utilized and studied in our included papers. In addition, 46 (90.2%) papers describe research in decentralized settings with the majority of these (33 of 46, 71.7%) at the district or city level. As well, given the significant shortage of healthcare workforce globally [ 78 ], especially in resource-limited settings, it is not surprising that the overwhelming number of supply-side focused studies (32 of 37, 86.5%) considered capacity building. These findings may reflect a trend to integrate an effective, low-resource appropriate technology into existing health services in response to inequities in women’s health care and to strengthen primary health care in decentralized community health systems. The 2008 call [ 79 ] to offer more comprehensive packages of basic health services (including improved preventive care services) in all settings and more recent calls [ 12 ] to address non-communicable diseases (NCDs) are also consistent with this trend.
These findings provide some insight into the cervical cancer screening implementation literature. We note that articles commonly describe community- and client-relevant implications and explore challenges to human resources. Of interest, however, our analysis reveals that a relatively small percentage of papers describe or report quality assurance themes (25.5%) and a very low percentage (3.9%) describe quality improvement activities related to the implemented health service. Given that only 23.5% of all papers are describing “policy” in explicit terms, the present findings also illustrate a major gap in the literature regarding policy development around the long-term sustainment of cervical cancer screening programs.
Demand-side barriers are identified in 51% of reviewed articles with the most frequent focus on comfort, knowledge gaps, personal sensitivity, and cost. Provider issues (78.6%) make up most supply-side barriers. Much can also be learned from implementation evaluations describing other systems-level issues. The present analysis highlights other explicit concerns including cost, equipment, management practices, space, supervision, and infrastructure.
“Proof of Concept” papers describe studies where implementation is not relevant, or implementation is relevant but not considered as research questions. The context of these studies is focused and the factors affecting implementation are not relevant, fixed, or ignored. Because 20 studies fell into the “Proof of Concept” category and 13 in the “Proof of Implementation,” one could conclude that many of the published articles investigating cervical cancer screening programs are not implicitly structured to provide meaningful information of the real world context in which the research project occurs.
For example, a study in Western Kenya aimed to validate VILI as a stand-alone screening test at a Family AIDS Care and Education Services (FACES) clinic [ 72 ] while a study in Leon, Nicaragua compared the acceptability of self-collected versus clinician-collected human papillomavirus (HPV) tests which applies to the “proof of implementation” category [ 73 ].
“Proof of Concept” studies may be strengthened by examining more contextual factors to determine screening feasibility in similar settings. Implementation research also has the potential to describe in greater detail the supporting and hindering factors to wide-scale implementation and sustainment of a cervical cancer screening program within the context of their health system’s existing cancer control and prevention policy.
We classified 18 papers as “informing scale-up.” These papers provide useful guidance for developing cervical cancer implementation programs across different contexts. Principally, those contexts include in-depth perspectives on acceptability and community perceptions [ 53 ], community education and mobilization [ 30 , 59 ] including radio messaging [ 41 ], community-focused or mobile screening [ 30 , 58 ], detail on training community health workers [ 50 ], client tracking [ 59 ], maintenance of human capacity [ 59 ], task sharing [ 65 ], and quality control [ 70 ]. These findings are accessible and highly applicable to the existing programs struggling with substantial challenges as well as to institutions that are prioritizing the new implementation of cervical cancer screening services.A large study in 130 rural communities in Guangdong Province, China [ 69 ] employs sound Dissemination and Implementation research methods. Study results described community participatory research through the Chinese Cancer Prevention Study (CHICAPS). This program was conducted by community leaders with the technical assistance of the research team. They utilized a “pass the message on” model to easily reach women in communities through local village promoters that were trained through locally organized workshops (with up to 25 community leaders being oriented). Their paper describes the model process in depth, including details of stakeholder roles. Conclusions were that the model was successful in (1) improving the efficiency of resource utilization, (2) teaching community leaders and promoters to get patient information and follow procedures, and (3) teaching rural women technical specifics of the screening approach.
A three-phase evaluation of a cervical cancer screening program was conducted in the Harare city health department, Zimbabwe [ 34 ]. This study included a survey of policy makers on guidelines, policy, and attitudes regarding cervical cancer screening, and evaluating determinants at both the supply- and the demand side. Dissemination of their work provides invaluable guidance on how comprehensive policies on cervical cancer screening should be developed to assist in standardization of program implementation, how the formal technical training of health workers should be done, and what necessary resources should be allocated to support a successful and sustainable cervical cancer screening program.
Finally, a cervical cancer screening evaluation was conducted in Guyana to explore the feasibility, effectiveness, and lessons learned of a single-visit approach to cervical cancer screening and treatment [ 65 ]. The reported findings were highly relevant to sustainability and scale-up and concluded that certain components are essential to achieve good population coverage with high-quality services: (i) competency-based training and supportive supervision; (ii) task shifting to non-physician providers; (iii) a strong monitoring and evaluation system that rapidly identifies and addresses programmatic and clinical gaps; (iv) an enabling environment providing programmatic support; and (v) integration of cervical cancer prevention services into appropriate existing programs, such as family planning, postpartum, and HIV care.
Limitations
Due to limitations in our search strategy and a lack of a risk of bias assessment, our review is characterized as a systematized review [ 18 ]. Given that our search strings were composed entirely of Medical Subject Heading (MESH) terms, we relied primarily on the accurate and current MESH terms and did not pursue the addition of articles through a free text search. Given the time limitations, MESH terms were used only to reduce the time associated with the search strategy development. This is problematic given that much of the published research from LMICs is not likely to be well indexed. Searches may therefore have left out relevant articles. Additionally, our search was conducted in the July of 2015. Any articles that were published after the search were not included in the review, omitting articles that would have met the criteria. The inclusion and exclusion criteria were strict for this literature review. Many articles were excluded from this review that provide valuable lessons in a variety of settings outside of the scope of our inclusion criteria, but were not regarded as employing implementation science. The findings from these excluded papers nevertheless may contain some findings relevant to implementation science and could be generalizable or be adaptable in areas with low resources. Additionally, it should be noted that the Continuum of Implementation Research as proposed by Peters [ 17 ] has limitations with a degree of subjectivity and author interpretation as described in the methods section. Every effort was made to consult the guiding framework and limit subjectivity by systematically and uniformly categorizing articles. Finally, the risk of bias for the included papers was not assessed.
Many evidence-based health service interventions are not being readily adopted in LMICs because of an insufficient primary health care system in place to support them [ 79 , 80 , 81 ]. More Dissemination and Implementation research is needed to illustrate how health systems function at the local level, especially in LMICs [ 17 ]. Much of the existing Dissemination and Implementation science exploring the interface between health research and policy is concentrated in high-income countries. The paucity of similar research in LMICs presents a major challenge for implementation of preventive measures in these countries [ 82 ]. Given that cervical cancer can serve as a proxy for larger health systems issues, more detailed exploration of the barriers and best practices for increasing initial screening uptake and sustaining screening services over time may provide important insights to addressing other persisting women’s health issues and beyond, including the strengthening of broad primary health care services in low-resource settings. For programs wishing to move towards expanding the focus on their inquiry into this area, the two principal references that we have used to analyze the papers in this review are excellent resources for investigators new to implementation science [ 17 , 22 ].
Given the overwhelming supporting evidence for the effectiveness of various screening technologies, it is unsettling that high cervical cancer incidence rates persist globally. There are clear downward trends of age-standardized incidence (ASI) rates in HIC, although no clear changes by period in low-income countries [ 83 ]. Given that a successful cervical cancer control and prevention program requires a robust systems approach including reliable access to primary healthcare, referral, and follow-up services, the incidence of cervical cancer has been shown to be an indicator for larger health systems issues [ 8 ]. Therefore, the implementation of cervical cancer prevention and control programs in areas with the least resources would have the greatest immediate impact on cervical cancer ASI rates while potentially favorably impacting other primary health care services. Unfortunately, there is a gradient between reviewed studies conducted in low-income countries (5, 9.8%) and LMICs (29, 56.9%), (Fig. 1 ). This disparity may be due to the profound difficulty of implementing cervical cancer programs and conducting research in states that are unstable or where infrastructure is significantly lacking. The implementation challenges in these settings may be the greatest to overcome in order to achieve sustainability of impactful interventions. These settings, unfortunately, may also possess the greatest challenges in conducting sound science, contributing to this well-documented research gap [ 84 ]. The areas with the greatest need for developing a clear understanding of implementation are, therefore, the most neglected. The dramatic lack of research from lower-resource environments to inform practice, in part, contributes to the continued gap in outcomes in such settings. However, these challenges could be opportunities for impact as well as for building knowledge. Replicating best practices from the most challenging contexts will likely lead to the greatest impact from dissemination of such scientific pursuits.
Country map of included articles
Program managers will benefit from working closely with researchers to report lessons learnt from programs implementing cervical cancer screening services. Furthermore, we urge researchers to move beyond technological innovation as the primary scientific pursuit and incorporate, when possible, implementation strategies to overcome barriers to health systems integration and sustainability. Researchers should evaluate the implementation of cervical cancer screening programs. This will build the science and practice of how to strengthen human resources capacity, develop responsive policy, and ensure sustained utilization of cervical cancer health services in different geographical settings.
Data availability
All materials, data, code, and associated protocols will be promptly available to readers without qualifications or restrictions. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Age-standardized incidence
Chinese Cancer Prevention Study
Dissemination and implementation
Family AIDS Care and Education Services
High-income countries
Human papillomavirus
Loop electrosurgical excision procedure
Low- and middle-income countries
Medical subject heading
Non-communicable diseases
Non-governmental organization
Peru Cervical Cancer Screening Study
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines
Visual inspection with acetic acid
Visual inspection with Lugol's iodine
Torre LA, Freddie B, Siegel RL, Jacques F, Joannie L-T, Ahmedin J (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108. https://doi.org/10.3322/caac.21262
Article PubMed Google Scholar
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. https://doi.org/10.1002/ijc.29210
Article CAS PubMed Google Scholar
Beasley JW, Starfield B, van Weel C, Rosser WW, Haq CL (2007) Global health and primary care research. J Am Board Fam Med 20:518–526. https://doi.org/10.3122/jabfm.2007.06.070172
Gonzalez-Block MA (2004) Health policy and systems research agendas in developing countries. Health Res Policy Syst 2:6. https://doi.org/10.1186/1478-4505-2-6
Article PubMed PubMed Central Google Scholar
Brown ML, Goldie SJ, Draisma G, Harford J, Lipscomb J (2011) Health service interventions for cancer control in developing countries. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. (eds) Disease control priorities in developing countries. World Bank, Washington (DC)
Google Scholar
World Health Organization (2007) Cancer control: knowledge into action: WHO guide for effective programmes. WHO. https://books.google.com/books/about/Cancer_Control.html?hl=&id=GL8lAQAAMAAJ
World Health Organization (2010) Package of essential noncommunicable (PEN) disease interventions for primary health care in low-resource settings. World Health Organization, Geneva
Freeman HP, Wingrove BK (2005) Excess cervical cancer mortality: a marker for low access to health care in poor communities. National Cancer Institute, Center to Reduce Cancer Health Disparities, Bethesda
Vainio H, Bianchini F (2002) Breast cancer screening, IARC handbooks of cancer prevention. IARC, Lyon, pp 157–170
The Cochrane Collaboration (1996) Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database of Systematic Reviews. Wiley, Chichester
IARC Handbooks on Cancer Prevention (2005) Volume 10 Cervix cancer screening. IARC, Lyon
Alwan MS (ed) (2011) Prioritized research agenda for prevention and control of noncommunicable diseases. World Health Organization, Geneva
Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM et al (2009) HPV screening for cervical cancer in rural India. N Engl J Med 360:1385–1394
World Health Organization (2015) Comprehensive cervical cancer control: a guide to essential practice. World Health Organization, Geneva
Demment MM, Peters K, Dykens JA, Dozier A, Nawaz H, McIntosh S et al (2015) Developing the evidence base to inform best practice: a scoping study of breast and cervical cancer reviews in low- and middle-income countries. PLoS ONE 10:e0134618. https://doi.org/10.1371/journal.pone.0134618
Article CAS PubMed PubMed Central Google Scholar
Gakidou E, Nordhagen S, Obermeyer Z (2008) Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med 5:e132. https://doi.org/10.1371/journal.pmed.0050132
Peters DH, Tran NT, Adam T, World Health Organization (2013) Implementation research in health: a practical guide. World Health Organization, Geneva
Grant MJ, Booth A (2009) A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 26:91–108. https://doi.org/10.1111/j.1471-1842.2009.00848.x
Aarons GA, Hurlburt M, Horwitz SM (2011) Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health 38:4–23. https://doi.org/10.1007/s10488-010-0327-7
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A et al (2011) Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 38:65–76. https://doi.org/10.1007/s10488-010-0319-7
Brownson RC, Colditz GA, Proctor EK (2017) Dissemination and implementation research in health: translating science to practice. Oxford University Press, New York
Book Google Scholar
Levesque J-F, Harris MF, Russell G (2013) Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health 12:18. https://doi.org/10.1186/1475-9276-12-18
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
World Bank Country and Lending Groups – World Bank Data Help Desk (2018). https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 . Accessed on 4 May 2018]
Hirst S, Mitchell H, Medley G (1990) An evaluation of a campaign to increase cervical cancer screening in rural Victoria. Community Health Stud 14:263–268
Swaddiwudhipong W, Chaovakiratipong C, Nguntra P, Mahasakpan P, Lerdlukanavonge P, Koonchote S (1995) Effect of a mobile unit on changes in knowledge and use of cervical cancer screening among rural Thai women. Int J Epidemiol 24:493–498. https://doi.org/10.1007/s10935-009-0200-1
Pengsaa P, Vatanasapt V, Sriamporn S, Sanchaisuriya P, Schelp FP, Noda S et al (1997) A self-administered device for cervical cancer screening in northeast Thailand. Acta Cytol 41:749–754
Wesley R, Sankaranarayanan R, Mathew B, Chandralekha B, Aysha Beegum A, Amma NS et al (1997) Evaluation of visual inspection as a screening test for cervical cancer. Br J Cancer 75:436–440
Hernández-Avila M, Lazcano-Ponce EC, Ruíz PA, Romieu I (1998) Evaluation of the cervical cancer screening programme in Mexico: a population-based case-control study. Int J Epidemiol IEA 27:370–376
Article Google Scholar
Swaddiwudhipong W, Chaovakiratipong C, Nguntra P, Mahasakpan P, Tatip Y, Boonmak C (1999) A mobile unit: an effective service for cervical cancer screening among rural Thai women. Int J Epidemiol 28:35–39
Kuhn L, Denny L, Pollack A, Lorincz A, Richart RM, Wright TC (2000) Human papillomavirus DNA testing for cervical cancer screening in low-resource settings. J Natl Cancer Inst 92:818–825
Agarwal SS, Murthy NS, Sharma S, Sharma KC, Das DK (1995) Evaluation of a hospital based cytology screening programme for reduction in life time risk of cervical cancer. Neoplasma 42:93–96
CAS PubMed Google Scholar
Mati JK, Mbugua S, Wanderi P (1994) Cervical cancer in Kenya: prospects for early detection at primary level. Int J Gynaecol Obstet 47:261–267
Moyo IM, Koni NP, Makunike B, Hipshman J, Makaure HK, Gumbo N (1997) Evaluation of cervical cancer screening programme in the Harare City Health Department Zimbabwe. Cent Afr J Med 43:223–225
Nene BM, Jayant K, Malvi SG, Dale PS, Deshpande R (1994) Experience in screening for cervical cancer in rural areas of Barsi Tehsil (Maharashtra). Indian J Cancer 31:34–40
Cronjé HS, Parham GP, Cooreman BF, Beer A, Divall P, Bam RH (2003) A comparison of four screening methods for cervical neoplasia in a developing country. Am J Obstet Gynecol 188:395–400
Sanchaisuriya P, Pengsaa P, Sriamporn S, Schelp FP, Kritpetcharat O, Suwanrungruang K et al (2004) Experience with a self-administered device for cervical cancer screening by Thai women with different educational backgrounds. Asian Pac J Cancer Prev 5:144–150
PubMed Google Scholar
Howe SL, Vargas DE, Granada D, Smith JK (2005) Cervical cancer prevention in remote rural Nicaragua: a program evaluation. Gynecol Oncol 99:S232–S235. https://doi.org/10.1016/j.ygyno.2005.07.094
Doh TAS, Nkele NN, Achu P, Essimbi F, Essame O, Nkegoum B (2005) Visual inspection with acetic acid and cytology as screening methods for cervical lesions in Cameroon. Int J Gynecol Obstet 89:167–173. https://doi.org/10.1016/j.ijgo.2004.12.040
Article CAS Google Scholar
Holanda F Jr, Castelo A, Veras TMCW, de Almeida FML, Lins MZ, Dores GB (2006) Primary screening for cervical cancer through self sampling. Int J Gynaecol Obstet 95:179–184. https://doi.org/10.1016/j.ijgo.2006.07.012
Perkins RB, Langrish S, Stern LJ, Simon CJ (2007) A community-based education program about cervical cancer improves knowledge and screening behavior in Honduran women. Rev Panam Salud Publica 22:187–193
Palanuwong B (2007) Alternative cervical cancer prevention in low-resource settings: experiences of visual inspection by acetic acid with single-visit approach in the first five provinces of Thailand. Aust N Z J Obstet Gynaecol 47:54–60. https://doi.org/10.1111/j.1479-828X.2006.00680.x
Blumenthal PD, Gaffikin L, Deganus S, Lewis R, Emerson M, Adadevoh S et al (2007) Cervical cancer prevention: safety, acceptability, and feasibility of a single-visit approach in Accra, Ghana. Am J Obstet Gynecol 196:407–408. https://doi.org/10.1016/j.ajog.2006.12.031 discussion 407.e8–9
Bhatla N, Mukhopadhyay A, Kriplani A, Pandey RM, Gravitt PE, Shah KV et al (2007) Evaluation of adjunctive tests for cervical cancer screening in low resource settings. Indian J Cancer 44:51–55. https://doi.org/10.4103/0019-509x.35811
Qiao Y-L, Sellors JW, Eder PS, Bao Y-P, Lim JM, Zhao F-H et al (2008) A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol 9:929–936. https://doi.org/10.1016/S1470-2045(08)70210-9
Chumworathayi B, Srisupundit S, Lumbiganon P, Limpaphayom KK (2008) One-year follow-up of single-visit approach to cervical cancer prevention based on visual inspection with acetic acid wash and immediate cryotherapy in rural Thailand. Int J Gynecol Cancer 18:736–742. https://doi.org/10.1111/j.1525-1438.2007.01112.x
Bhatla N, Gulati A, Mathur SR, Rani S, Anand K, Muwonge R et al (2009) Evaluation of cervical screening in rural North India. Int J Gynaecol Obstet 105:145–149. https://doi.org/10.1016/j.ijgo.2008.12.010
Qureshi S, Das V, Zahra F (2010) Evaluation of visual inspection with acetic acid and Lugol’s iodine as cervical cancer screening tools in a low-resource setting. Trop Doct 40:9–12. https://doi.org/10.1258/td.2009.090085
Zhang Y-Z, Ma J-F, Zhao F-H, Xiang X-E, Ma Z-H, Shi Y-T et al (2010) Three-year follow-up results of visual inspection with acetic acid/Lugol’s iodine (VIA/VILI) used as an alternative screening method for cervical cancer in rural areas. Chin J Cancer 29:4–8
Nuño T, Martinez ME, Harris R, García F (2010) A Promotora-administered group education intervention to promote breast and cervical cancer screening in a rural community along the U.S.–Mexico border: a randomized controlled trial. Cancer Causes Control 22:367–374. https://doi.org/10.1007/s10552-010-9705-4
Hasanzadeh M, Esmaeili H, Tabaee S, Samadi F (2011) Evaluation of visual inspection with acetic acid as a feasible screening test for cervical neoplasia. J Obstet Gynaecol Res 37:1802–1806. https://doi.org/10.1111/j.1447-0756.2011.01614.x
Longatto-Filho A, Naud P, Derchain SF, Roteli-Martins C, Tatti S, Hammes LS et al (2012) Performance characteristics of Pap test, VIA, VILI, HR-HPV testing, cervicography, and colposcopy in diagnosis of significant cervical pathology. Virchows Arch 460:577–585. https://doi.org/10.1007/s00428-012-1242-y
Audet CM, Silva Matos C, Blevins M, Cardoso A, Moon TD, Sidat M (2012) Acceptability of cervical cancer screening in rural Mozambique. Health Educ Res 27:544–551. https://doi.org/10.1093/her/cys008
Li R, Lewkowitz AK, Zhao F-H, Zhou Q, Hu S-Y, Qiu H et al (2012) Analysis of the effectiveness of visual inspection with acetic acid/Lugol’s iodine in one-time and annual follow-up screening in rural China. Arch Gynecol Obstet 285:1627–1632. https://doi.org/10.1007/s00404-011-2203-4
Nuranna L, Aziz MF, Cornain S, Purwoto G, Purbadi S, Budiningsih S et al (2012) Cervical cancer prevention program in Jakarta, Indonesia: see and Treat model in developing country. J Gynecol Oncol 23:147–152. https://doi.org/10.3802/jgo.2012.23.3.147
Sanad AS, Ibrahim EM, Gomaa W (2014) Evaluation of cervical biopsies guided by visual inspection with acetic acid. J Low Genit Tract Dis 18:21–25. https://doi.org/10.1097/LGT.0b013e31828ce581
Li R, Zhou Q, Li M, Tong S-M, He M, Qiu H et al (2013) Evaluation of visual inspection as the primary screening method in a four-year cervical (pre-) cancer screening program in rural China. Trop Doct 43:96–99. https://doi.org/10.1177/0049475513495615
Trope LA, Chumworathayi B, Blumenthal PD (2013) Feasibility of community-based careHPV for cervical cancer prevention in rural Thailand. J Low Genit Tract Dis 17:315–319. https://doi.org/10.1097/LGT.0b013e31826b7b70
Paul P, Winkler JL, Bartolini RM, Penny ME, Huong TT, Nga LT et al (2013) Screen-and-treat approach to cervical cancer prevention using visual inspection with acetic acid and cryotherapy: experiences, perceptions, and beliefs from demonstration projects in Peru, Uganda, and Vietnam. Oncologist 18(Suppl):1278–1284. https://doi.org/10.1634/theoncologist.2013-0253
Satyanarayana L, Asthana S, Bhambani S, Sodhani P, Gupta S (2014) A comparative study of cervical cancer screening methods in a rural community setting of North India. Indian J Cancer 51:124–128. https://doi.org/10.4103/0019-509X.138172
Jeronimo J, Bansil P, Lim J, Peck R, Paul P, Amador JJ et al (2014) A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int J Gynecol Cancer 24:576–585. https://doi.org/10.1097/IGC.0000000000000084
Bansil P, Wittet S, Lim JL, Winkler JL, Paul P, Jeronimo J (2014) Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health 14:596. https://doi.org/10.1186/1471-2458-14-596
Fong J, Gyaneshwar R, Lin S, Morrell S, Taylor R, Brassil A et al (2014) Cervical screening using visual inspection with acetic acid (VIA) and treatment with cryotherapy in Fiji. Asian Pac J Cancer Prev 15:10757–10762
Guo J, Cremer M, Maza M, Alfaro K, Felix JC (2014) Evaluation of a low-cost liquid-based Pap test in rural El Salvador: a split-sample study. J Low Genit Tract Dis 18:151–155. https://doi.org/10.1097/LGT.0b013e31829aa052
Martin CE, Tergas AI, Wysong M (2014) Evaluation of a single-visit approach to cervical cancer screening and treatment in Guyana: feasibility, effectiveness and lessons learned. J Obstet Gynaecol Res 40(6):1707–1716
Nessa A, Roy JS, Chowdhury MA, Khanam Q, Afroz R, Wistrand C et al (2014) Evaluation of the accuracy in detecting cervical lesions by nurses versus doctors using a stationary colposcope and Gynocular in a low-resource setting. BMJ Open 4:e005313. https://doi.org/10.1136/bmjopen-2014-005313
Kamal EM, El Sayed GA, El Behery MM, El Shennawy GA (2014) HPV detection in a self-collected vaginal swab combined with VIA for cervical cancer screening with correlation to histologically confirmed CIN. Arch Gynecol Obstet 290:1207–1213. https://doi.org/10.1007/s00404-014-3321-6
Khozaim K, Orang’o E, Christoffersen-Deb A, Itsura P, Oguda J, Muliro H et al (2014) Successes and challenges of establishing a cervical cancer screening and treatment program in western Kenya. Int J Gynaecol Obstet 124:12–18. https://doi.org/10.1016/j.ijgo.2013.06.035
Belinson JL, Wang G, Qu X, Du H, Shen J, Xu J et al (2014) The development and evaluation of a community based model for cervical cancer screening based on self-sampling. Gynecol Oncol 132:636–642. https://doi.org/10.1016/j.ygyno.2014.01.006
Abuelo CE, Levinson KL, Salmeron J, Sologuren CV, Fernandez MJV, Belinson JL (2014) The Peru Cervical Cancer Screening Study (PERCAPS): the design and implementation of a mother/daughter screen, treat, and vaccinate program in the Peruvian jungle. J Community Health 39:409–415. https://doi.org/10.1007/s10900-013-9786-6
Rosenbaum AJ, Gage JC, Alfaro KM, Ditzian LR, Maza M, Scarinci IC et al (2014) Acceptability of self-collected versus provider-collected sampling for HPV DNA testing among women in rural El Salvador. Int J Gynaecol Obstet 126:156–160. https://doi.org/10.1016/j.ijgo.2014.02.026
Huchko MJ, Sneden J, Zakaras JM, Smith-McCune K, Sawaya G, Maloba M et al (2015) A randomized trial comparing the diagnostic accuracy of visual inspection with acetic acid to Visual Inspection with Lugol’s Iodine for cervical cancer screening in HIV-infected women. PLoS ONE 10:e0118568. https://doi.org/10.1371/journal.pone.0118568
Quincy BL, Turbow DJ, Dabinett LN (2012) Acceptability of self-collected human papillomavirus specimens as a primary screen for cervical cancer. J Obstet Gynaecol 32:87–91. https://doi.org/10.3109/01443615.2011.625456
Vet J, Kooijman JL, Henderson FC, Aziz FM, Purwoto G, Susanto H et al (2012) Single-visit approach of cervical cancer screening: see and treat in Indonesia. Br J Cancer 107:772–777. https://doi.org/10.1038/bjc.2012.334
Sanad AS, Kamel HH, Hasan MM (2014) Prevalence of cervical intraepithelial neoplasia (CIN) in patients attending Minia Maternity University Hospital. Arch Gynecol Obstet 289:1211–1217
Maseko FC, Chirwa ML, Muula AS (2015) Health systems challenges in cervical cancer prevention program in Malawi. Glob Health Action 8:8. https://doi.org/10.3402/gha.v8.26282
Chary AN, Rohloff PJ (2014) Major challenges to scale up of visual inspection-based cervical cancer prevention programs: the experience of Guatemalan NGOs. Global Health Sci Pract 2:307–317. https://doi.org/10.9745/GHSP-D-14-00073
Campbell J, Dussault G, Buchan J, Pozo-Martin F, Guerra Arias M, Leone C et al (2013) A universal truth: no health without a workforce. Global Health Workforce Alliance, Geneva
Lerberghe W, Evans T, Rasanathan K, Mechbal A, Lerberghe W, World Health Organization (2008) The World Health Report 2008: primary health care: now more than ever. World Health Organization, Geneva
Ackerly DC, Udayakumar K, Taber R, Merson MH, Dzau VJ (2011) Perspective: global medicine: opportunities and challenges for academic health science systems. Acad Med 86:1093–1099. https://doi.org/10.1097/ACM.0b013e318226b455
Koplan JP, Bond TC, Merson MH, Reddy KS, Rodriguez MH, Sewankambo NK et al (2009) Towards a common definition of global health. Lancet 373:1993–1995. https://doi.org/10.1016/S0140-6736(09)60332-9
Hyder AA, Bloom G, Leach M, Syed SB, Peters DH (2007) Future health systems: innovations for equity. Exploring health systems research and its influence on policy processes in low income countries. BMC Public Health 7:309. https://doi.org/10.1186/1471-2458-7-309
Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F (2013) Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer 49:3262–3273. https://doi.org/10.1016/j.ejca.2013.04.024
Commission on Health Research for Development (1990) Health research: essential link to equity in development. Oxford University Press, Oxford
Download references
Acknowledgments
We would like to thank Medical Librarian, Linda Hassman, of Miner Library at the University of Rochester Medical Center for her valuable work in assisting us in developing and implementing our search strategy.
This work was supported and funded by the Centers for Disease Control and Prevention, Prevention Research Centers Program (Cooperative agreements: 1U48DP005026-01S1, 1U48DP005010-01S1, and 1U48DP005023-01S1). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This review falls under the scope of work for the US. Centers for Disease Control and Prevention (CDC) funded Global and Territorial Health Research Network through the Prevention Research Centers Program, with the goal to translate chronic disease prevention research into practice. In addition to several partnering institutions, the Global Network Steering Committee consists of two CDC representatives who participated in the study design and review of the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis. The corresponding author had final responsibility to submit the report for publication.
Author information
Authors and affiliations.
University of Illinois at Chicago College of Medicine, Chicago, IL, USA
J. Andrew Dykens
University of North Carolina School of Public Health, Chapel Hill, NC, USA
Jennifer S. Smith
University of Rochester Department of Obstetrics and Gynecology, Rochester, NY, USA
Margaret Demment & Timothy Dye
University of Illinois at Chicago Institute for Health Research and Policy, Chicago, IL, USA
E. Marshall, Tina Schuh & Karen Peters
University of Washington Department of Obstetrics and Gynecology, Seattle, WA, USA
Tracy Irwin
University of Rochester Department of Public Health Sciences, Rochester, NY, USA
Scott McIntosh
University of Hawaii John A Burns School of Medicine, Honolulu, HI, USA
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the conception and design of the study (AD, JSS, MMD, EM, TS, TD) or to the acquisition, analysis, and interpretation of data (AD, MMD, EM, KP, TI, SM, AS, TS), and drafted the manuscript (AD, JSS, MMD, EM, TS) or revised it critically for content (AD, JSS, MMD, EM, TS, KP, TI, SM, AS, TD). All authors read and approved the final manuscript.
Corresponding author
Correspondence to J. Andrew Dykens .
Ethics declarations
Conflict of interest.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 12 kb)
Supplementary file2 (docx 48 kb), supplementary file3 (docx 17 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Dykens, J.A., Smith, J.S., Demment, M. et al. Evaluating the implementation of cervical cancer screening programs in low-resource settings globally: a systematized review. Cancer Causes Control 31 , 417–429 (2020). https://doi.org/10.1007/s10552-020-01290-4
Download citation
Received : 26 August 2019
Accepted : 27 February 2020
Published : 17 March 2020
Issue Date : May 2020
DOI : https://doi.org/10.1007/s10552-020-01290-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Implementation
- Literature review
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 02 December 2022
Barriers to uptake of cervical cancer screening services in low-and-middle-income countries: a systematic review
- Z. Petersen 1 ,
- A. Jaca 2 ,
- T. G. Ginindza 3 , 4 ,
- G. Maseko 1 ,
- S. Takatshana 1 ,
- P. Ndlovu 1 ,
- N. Zondi 1 ,
- N. Zungu 1 , 3 ,
- C. Varghese 5 ,
- G. Hunting 5 ,
- G. Parham 5 ,
- P. Simelela 5 &
- S. Moyo 1 , 6
BMC Women's Health volume 22 , Article number: 486 ( 2022 ) Cite this article
7727 Accesses
19 Citations
17 Altmetric
Metrics details
Low-and-middle-income countries (LMICs) bear a disproportionate burden of cervical cancer mortality. We aimed to identify what is currently known about barriers to cervical cancer screening among women in LMICs and propose remedial actions.
This was a systematic review using Medical Subject Headings (MeSH) terms in Google Scholar, PubMed, Scopus, and Web of Science databases. We also contacted medical associations and universities for grey literature and checked reference lists of eligible articles for relevant literature published in English between 2010 and 2020. We summarized the findings using a descriptive narrative based on themes identified as levels of the social ecological model.
We included studies conducted in LMICs published in English between 2010 and 2020.
Participants
We included studies that reported on barriers to cervical cancer screening among women 15 years and older, eligible for cervical cancer screening.
Seventy-nine articles met the inclusion criteria. We identified individual, cultural/traditional and religious, societal, health system, and structural barriers to screening. Lack of knowledge and awareness of cervical cancer in general and of screening were the most frequent individual level barriers. Cultural/traditional and religious barriers included prohibition of screening and unsupportive partners and families, while social barriers were largely driven by community misconceptions. Health system barriers included policy and programmatic factors, and structural barriers were related to geography, education and cost. Underlying reasons for these barriers included limited information about cervical cancer and screening as a preventive strategy, poorly resourced health systems that lacked policies or implemented them poorly, generalised limited access to health services, and gender norms that deprioritize the health needs of women.
A wide range of barriers to screening were identified across most LMICs. Urgent implementation of clear policies supported by health system capacity for implementation, community wide advocacy and information dissemination, strengthening of policies that support women’s health and gender equality, and targeted further research are needed to effectively address the inequitable burden of cervical cancer in LMICs.
Peer Review reports
Key messages
What is already known: Low-and-middle-income countries (LMICs) bear a disproportionate burden of cervical cancer mortality and there is limited knowledge on barriers to cervical cancer screening uptake across LMICS.
Findings: Women in LMICs face individual level, cultural/traditional and religious, societal, health system, and structural barriers to cervical cancer screening. The underlying reasons for these barriers include limited information about cervical cancer and screening as a preventive strategy, poorly resourced health systems without screening policies, poorly implemented policies, generalised limited access to health services, and gender norms that deprioritize the health needs of women.
What the findings imply: There is a need for education, information dissemination, and advocacy to dispel myths about cervical cancer, and implementation of clear cervical cancer policies and guidelines with prerequisite structures and resources across diverse health settings. Policies that support sexual and reproductive health and the rights of women should be strengthened and expanded and account for inequities in access for diverse groups of women. Education and awareness initiatives should be driven by local and community contexts, and engage community members and multiple stakeholders, including traditional and religious figures. In addition, the introduction and roll out of more modern screening approaches in LMICs should be prioritized to ensure more women are reached.
Introduction
Cervical cancer, although preventable and curable, is the fourth most common cancer among women globally [ 1 ]. The burden is greatest in low-and-middle-income countries (LMICs) with age-standardized incidence rates varying from 75/100000 women in highest-risk countries to less than 10/100000 women in lowest risk countries [ 1 ]. In 2018, approximately 90% of deaths occurred in LMICs [ 2 ]. The remarkable geographic contrasts in cervical cancer incidence and mortality reflect differences in social and structural contexts associated with cervical cancer, and inequities in access to information about cervical cancer, prevention, screening, and effective cancer treatment facilities and thus indicate areas with the greatest need for interventions [ 3 ]. Consequently, the World Health Organization’s (WHO) global strategy to accelerate the elimination of cervical cancer proposes a vision of a world where cervical cancer is eliminated as a public health problem by employing measures that are sensitive to women’s needs, their social circumstances, and the personal, cultural, social, structural and economic barriers hindering their access to health services [ 2 ].
With almost all cervical cancer cases (99%) linked to human papillomaviruses infection (HPV), HPV vaccination is a key primary preventive strategy, with secondary prevention – screening - remaining a key component of the cervical cancer elimination toolkit, especially where there is low HPV vaccination availability, access, and uptake [ 3 , 4 ]. Screening coverage of eligible women in most LMICs is on average 19%, compared to 63% in high income countries, and thus it is important to review identified barriers to screening uptake to address the burden in LMICs [ 4 ].
We conducted a systematic review on barriers to uptake of cervical cancer screening services (including poor provision of services) in LMICs. The objectives of the review were to i) document and investigate the underlying reasons for poor uptake of cervical cancer screening services in LMICs, ii) identify research gaps, and iii) provide evidence for decision-making and policy interventions for improved programmes and actions to support the elimination of cervical cancer in LMICs. We used Brofenbrenner’s social ecological model [ 5 , 6 ] to understand the dynamic interrelations among personal and environmental factors. First introduced in the 1970s as a conceptual model, the social ecological model was formalized as a theory in the 1980s and underwent revisions by Bronfenbrenner until his death in 2005. In his initial theory, Bronfenbrenner proposed that to understand human development, the entire ecological system in which growth occurs needs to be considered. In subsequent revisions, the model examines how human beings develop according to their environment, which includes society and the context which impacts behavior and development.
The review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and included LMICs, as defined by the World Bank based on per capita gross national income in 2020 [ 7 ]. The research question was framed using the broad population, concept and context (PCC) framework recommended by the Joanna Briggs Institute for Scoping Reviews [ 8 ] and was defined as: “What are the barriers to the uptake of cervical cancer screening services in LMICs?”. The population was women (15 years and older) eligible for cervical cancer screening. Studies that examined HPV vaccination and included girls younger than 15 years old together with older girls and women were also included.
Search strategy
Two authors (AJ and ZP) developed the search strategy. A comprehensive literature search was conducted in February 2021 in Scopus, Web of Science and Pubmed. No language or date restrictions were applied in the initial search. A search in Google Scholar using the keywords ‘cervical cancer screening’ and ‘barriers to cervical cancer screening’ was also conducted, aimed at finding studies that may not have been included in the findings from the major databases that were searched. We also searched the websites of the WHO, the International Agency for Research on Cancer (IARC), and the reference lists of all included studies for additional relevant articles. The search was initiated with keywords and refined by adapting search terms from relevant literature to include a variation of the terminology used in different countries. The detailed search strategy for the three databases is shown in Table 1 .
Studies addressing barriers to and uptake of cervical cancer screening in LMICs and published in English over 10 years (1 January 2010 to December 2020) were eligible for inclusion. Project and academic reports including Master’s and Doctoral theses were also eligible while editorials, commentaries, and abstracts where we could not access full-text articles were ineligible. Working in pairs, the authors independently screened the titles and abstracts of the search output and retrieved the full texts of those considered eligible. The authors then independently assessed the full texts for inclusion and resolved disagreements through discussion and consensus.
Data extraction
A standardized data extraction tool was used. Information was extracted on the country of study, aim/s, design, population, sample size, participant ages, screening type, documented barriers, reported findings, and recommendations. Discrepancies were resolved through discussion and consensus. Two authors assessed the quality of the studies included using the Critical Appraisal Skill Program(CASP) tool [ 8 ]. See Appendix 1 , Quality Assessment of studies.
Search Results
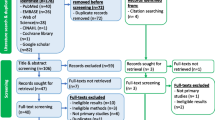
The literature search yielded a total of 2148 articles: 385 from PubMed, 1280 from Scopus, and 461 from Web of Science, 18 from Google and Google scholar. After removing 20 duplicates, we screened titles for eligibility and 1882 irrelevant articles were excluded (Fig. 1 ). Full texts of the 246 remaining articles were assessed for eligibility, and 92 met the inclusion criteria. Thirteen review articles were excluded, leaving 79 articles based on individual studies.
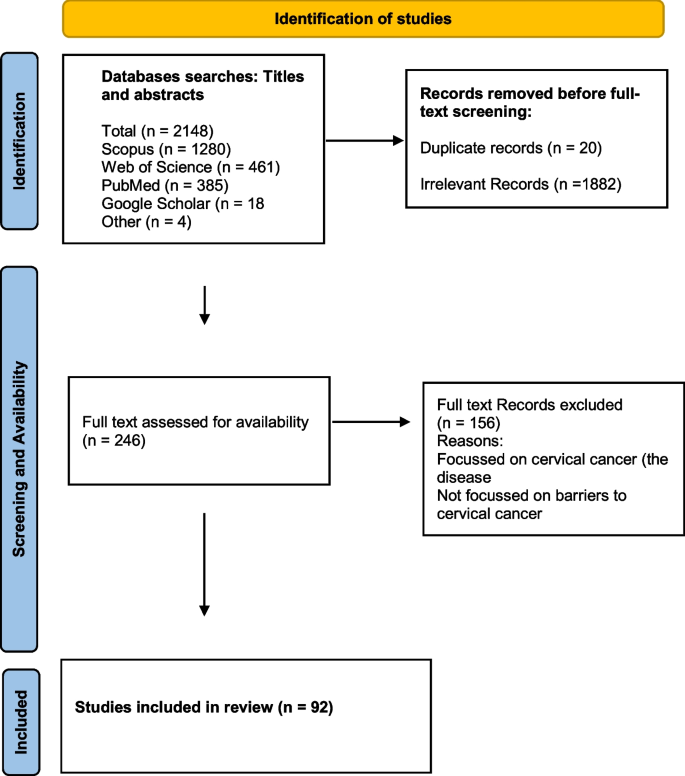
Search strategy flow diagram
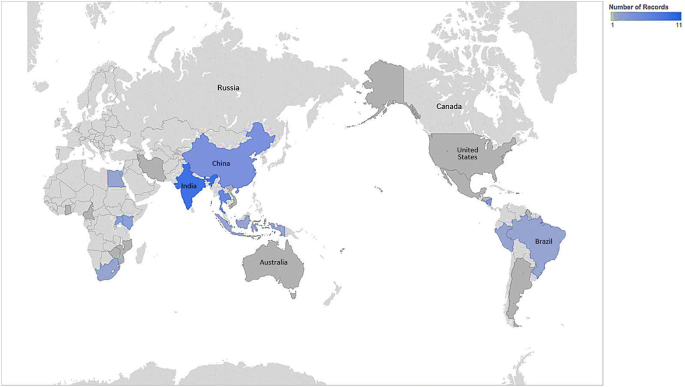
Characteristics of included studies
The included studies were undertaken in 28 LMICs; with 61% undertaken in Africa, 21% in Asia, 5% in North America, 9% in South America, 1% in Oceania and 3% in Europe. The characteristics of the included studies are shown in Table 2 . Of the included individual studies, 45 (57%) were quantitative, 27 (34%) qualitative and 4 (5%) used a combination of qualitative and quantitative methods. Four studies were based on secondary data analysis [ 9 , 10 , 11 , 12 ]. The quantitative studies were largely cross-sectional surveys, while the qualitative studies involved focus group discussions, in-depth and semi-structured interviews (Table 2 ).
Patient and Public Involvement
Patients were not directly involved or recruited into this study. We reviewed published articles that investigated the barriers to cervical cancer screening uptake by women in LMICs. The results will be disseminated through a publicly available research report and a manuscript and in conferences and webinars. They will also be distributed through the WHO and the institutions involved in the project.
The individual studies included participants from rural and urban areas, women living with and without HIV, women in the general public, women attending antenatal services, university students, and healthcare workers. Four studies included men [ 33 , 60 , 63 , 87 ] and in two of the studies, they were partners of women participants [ 33 , 63 ] while in the others they were university male students. Thirteen studies included healthcare workers exclusively or with non-healthcare workers [ 13 , 14 , 24 , 35 , 37 , 41 , 47 , 54 , 55 , 73 , 82 , 85 , 86 ]. Eight studies included participants younger than 18 years old including one study that included girls from the age of 10 years together with older women [ 15 , 17 , 26 , 32 , 34 , 49 , 58 , 87 ] -36. In 17 studies, age details were not specified (Table 2 ). Frequently missing information was age of the participants, type of screening and when the study was conducted. The sample sizes of studies ranged from 15 participants [ 24 , 48 , 54 ] to 15,317 participants in a study that analysed secondary data [ 26 ].
Types of screening methods
Forty eight percent of studies were about Papanicolaou (pap) smears exclusively or in combination with other screening methods, 25% about visual inspection with acetic acid (VIA) or visual inspection with Lugol’s iodine (VILI), 5% on HPV screening (through self-sampling or using DNA based tests) exclusively or in combination with other screening methods, while a total of 30% of studies did not specify the type of screening method (Table 2 ).
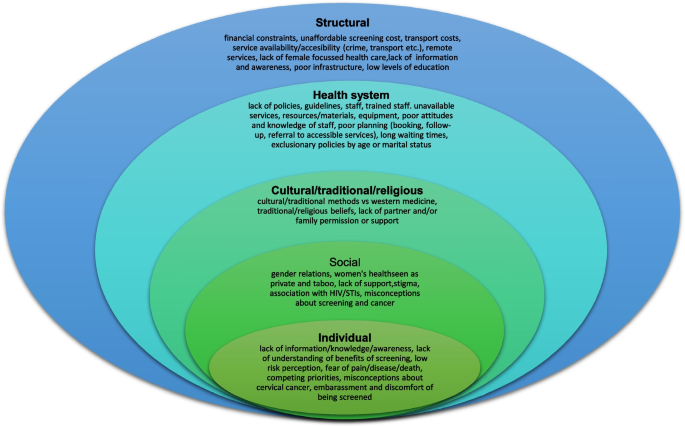
Since most studies identified were descriptive or qualitative in design, we analysed and summarized the main findings using a descriptive narrative, based on themes identified as levels of the social ecological model [ 88 ]. During the thematic analysis six authors in groups of two grouped the barriers that were identified into five categories, as defined below.
Individual/personal level barriers – obstacles experienced at individual level
Cultural/traditional and religious barriers – cultural, traditional, and religious views, norms, and expectations
Social barriers – community and societal obstacles
Health system barriers – factors in the design, function and implementation of health systems that make it difficult for some individuals to access, use or benefit from care
Structural barriers– macroscale obstacles that affected some women disproportionately
These categories are not entirely distinct or mutually exclusive as factors in one category overlap and are influenced by those in other categories (Refer to Fig. 2 for a visual diagram depicting barriers across each level).
Examples of barriers in the five categories
The barriers to uptake of cervical cancer are interconnected and operate across and within the various levels of the social ecological model. The following category levels include factors that contribute to barriers to cervical cancer screening, spanning the patient/individual level to the structural level. The studies reviewed included quantitative and qualitative input from both women and men (including patients, women from the community, male and female students, female teachers and male partners), as well as from the health service-level (including nurses, doctors, community health workers, policy makers, NGO staff and district coordinators). Information about the different categories of barriers that were identified across the articles included in this review are provided in Table 3 .
Individual/personal level barriers
Most studies reported individual or personal level barriers to screening. The most common individual level barriers were lack of knowledge and information about cervical cancer and cervical cancer screening, and its benefits, including women who did not understand the value of screening – i. e., health examination in the absence of symptoms or ill health [ 18 , 19 , 20 , 21 , 25 , 31 , 43 , 47 , 48 , 49 , 51 , 52 , 56 , 58 , 60 , 64 , 76 , 81 ]. Another commonly reported individual level barrier was fear of receiving positive screening results with many women believing that a cancer diagnosis was terminal [ 15 , 19 , 20 , 21 , 25 , 31 , 33 , 35 , 36 , 37 , 40 , 44 , 46 , 48 , 49 , 50 , 52 , 53 , 60 , 62 , 64 , 69 , 74 ].
Studies also reported that women had misconceptions about screening and the screening process. Women feared pain from the screening procedure and had misconceptions about possible harms such as contracting cancer, or damage to the uterus or cervix during screening [ 13 , 14 , 15 , 17 , 23 , 30 , 39 , 44 , 47 , 48 , 49 , 51 , 54 , 56 , 61 , 62 , 65 , 81 ]. In Nigeria, women reported being afraid of contracting infections from the screening equipment or from other sources within the health facility [ 35 , 40 ]. In Ethiopia, most women offered self-sampling for HPV thought that the process would be painful, while some feared using the Evalyn brush [ 14 ], and in South Africa, some women reported fear of concurrent HIV testing during screening for cervical cancer [ 16 ].
In 33% of studies conducted in Africa, Asia, and South America many women reported being embarrassed to be screened or to undergo pelvic examination [ 13 , 15 , 18 , 19 , 21 , 23 , 29 , 30 , 31 , 33 , 39 , 44 , 47 , 49 , 57 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 69 , 71 , 72 , 81 ]. Embarrassment was associated with the activity of going to a facility for screening, the pelvic examination itself, and being examined by a male or young healthcare worker [ 60 , 61 , 65 ].
Studies also reported that women, regardless of geography or employment status, faced competing priorities and responsibilities and thus often had limited time to attend screening [ 28 , 73 ].
Cultural/traditional /religious and social barriers
Cultural/religious/traditional, and social barriers were closely intertwined in the studies evaluated. Eleven studies reported that women were not screened because of religious or traditional reasons and prohibitions [ 14 , 15 , 17 , 22 , 25 , 26 , 33 , 35 , 49 , 52 , 72 ]. Two studies reported on possible clashes between western and traditional views of cervical cancer screening [ 48 , 80 ], and mistrust of western medicine and preference for traditional medicine was reported in Ghana, and South Africa [ 15 , 48 ]. In Ecuador, there were competing interpretations of health between healthcare workers and the community [ 80 ]. Some studies (21%) also reported that men disapproved of cervical cancer screening, with some refusing for their wives to be screened [ 17 , 18 , 25 , 27 , 29 , 30 , 33 , 38 , 43 , 49 , 53 , 54 , 57 , 58 , 67 , 74 , 76 ]. Other studies reported that women’s health issues, including sexual and reproductive health, were deprioritized and not awarded the same urgency as other health issues [ 12 , 82 ], while others reported that cervical cancer screening was viewed as a private and taboo topic (culturally embarrassing) not to be discussed, due to its connection to sexual and reproductive health [ 82 ].
Social barriers were related to community disapproval or negative community perceptions about the health system, the screening process, lack of peer support, and stigmatization of cervical cancer and the screening process [ 13 , 14 , 22 , 27 , 36 , 40 , 44 , 55 , 59 , 60 , 82 ]. In some studies stigma was related to cervical cancer being viewed as a terminal disease by some [ 15 , 23 ], while in others stigma was due to association with sexual transmission, with women attending screening sometimes assumed to be engaged in infidelity or promiscuity [ 22 , 33 ]. In South Africa where concurrent HV testing was offered, stigma was related to the association of cervical cancer with HIV infection [ 36 ].
Health system barriers
Heath system barriers included lack of capacity, poor organization of services, lack of knowledge about cervical cancer amongst healthcare workers, lack of promotion of screening, poor (negative and unfriendly) attitudes of healthcare workers when interacting with patients, and lack of public confidence in the health system. Lack of capacity included limited numbers of healthcare facilities in general, but especially in rural areas, few healthcare facilities providing screening services, limited staff, brief and rushed consultations, and shortage of equipment and materials which often led to women being referred for screening far from where they live resulting in costly, and lengthy screening and diagnostic pathways [ 17 , 18 , 19 , 24 , 25 , 30 , 35 , 37 , 39 , 41 , 44 , 47 , 48 , 52 , 53 , 54 , 55 , 58 , 61 , 74 , 82 , 85 , 87 , 89 ].
Capacity barriers also included reports of poor knowledge of cervical cancer among healthcare workers, poor technical skill to perform screening procedures, limited supervision leaving staff uncertain about technique, and limited specialized experts such as gynaecologists for guidance and management of some patients [ 15 , 25 , 54 , 77 ]. In Kenya and Ethiopia, clinic operating times and unavailability of services on weekends also limited screening uptake [ 13 , 51 ]. In studies conducted in Uganda and South Africa, women reported that lack of privacy in healthcare facilities was a barrier to screening [ 29 , 48 ], while in Malawi, Munthali et al., identified a lack of space for screening services in healthcare facilities as a barrier [ 47 ]. Lack of confidence in the health system was reported in Nigeria and Uganda [ 40 , 54 ].
Eleven studies, seven in Africa ( n = 7), Asia ( n = 3) and South America ( n = 1) found that poor, negative and discriminatory attitudes of healthcare workers towards women discouraged women from screening [ 16 , 25 , 49 , 50 , 51 , 52 , 53 , 59 , 61 , 65 , 80 ]. A study conducted in Nigeria, reported that discrimination toward Muslim women hindered access to healthcare facilities and screening [ 40 ]. Two studies also found that communication and language barriers between women and healthcare workers left women with unanswered questions and limited screening uptake [ 15 , 80 ].
Long wait times in healthcare facilities were a barrier to screening in South Africa, Uganda, Kenya and China [ 16 , 26 , 43 , 48 , 50 , 53 , 61 ]. This may partly also explain why women reported competing priorities for their time (work and family responsibilities) when they considered attending screening services.
Several studies reported on policy and guideline implementation barriers. Studies in Uganda, Indonesia, Brazil, and China found poor organisation of the services with limited information available about screening services leaving women without information about screening sites, and procedures for booking screening appointments [ 48 , 57 , 61 , 83 ]. In Bolivia, healthcare workers reported that lack of dissemination of screening guidelines, and lack of educational campaigns and infrastructure for screening limited screening uptake [ 82 ]. In Oceania, screening guidelines were not implemented while Bulgaria had no screening policy [ 85 , 86 ]. In Argentina and China, the screening policy excluded unmarried women from free screening (in China), thus limiting screening for some women since out-of-pocket screening costs were frequently identified as a barrier to uptake [ 61 , 84 ]. Healthcare workers also often failed to promote, recommend or offer screening and related cervical cancer information during other consultations [ 18 , 38 , 43 , 62 , 71 , 74 ].
Structural barriers
Structural barriers were mainly related to geographic distance to screening facilities, associated travel costs, poor transport systems, and screening costs where screening was not a free service in the absence of health insurance. Screening costs were a barrier in all continents, with travels costs a barrier in Africa, Asia and South America [ 12 , 15 , 18 , 23 , 25 , 31 , 33 , 34 , 35 , 41 , 43 , 44 , 46 , 47 , 48 , 51 , 59 , 60 , 79 , 84 , 86 ]. Long waiting times were also associated with additional costs for meals, and this increased overall screening costs [ 55 ]. Women in rural areas were disproportionately affected by distance, and travel costs [ 10 , 16 , 44 , 76 , 78 ]. In South Africa, Uganda and Nigeria, additional barriers were crime (which hindered free and safe travel), poor road networks and unreliable and inconvenient transport schedules to screening facilities [ 10 , 44 , 54 ]. One study reported lack of infrastructure for women with disabilities [ 11 ]. Other structural issues included low levels of education and low socioeconomic status [ 27 , 32 , 34 ], common among women living in conditions of poverty or limited resources.
Underlying reasons for barriers to screening uptake
Based on the descriptive analysis of the main findings of the studies included in this review, we identified four underlying reasons for barriers to cervical cancer screening uptake that should be addressed when considering interventions and policies for remedial action. Firstly, poor or ineffective messaging about cervical cancer, screening and prevention evidenced by limited information and education about cervical cancer and screening as a preventive strategy and misconceptions about the cause of cervical cancer, and the screening process, is a key underlying reason for poor screening uptake. Many women are not aware of screening and its value, and there are many misconceptions about screening in many communities. Secondly, health systems are poorly resourced to provide screening, lack clear policies on cervical cancer and screening, or poorly implement any existing policies [ 48 , 57 , 60 , 61 , 82 , 85 , 86 ]. Thirdly, there is limited access to health care services more generally, because of lack of universal health coverage and affordability, a common feature in many LMICs and a notable barrier to screening uptake [ 15 , 18 , 25 , 28 , 31 , 34 , 35 , 41 , 46 , 48 , 51 , 59 , 61 , 85 ]. Women often must travel to facilities far from where they live for screening services, indicating limited access in many geographic areas which is worsened by transport and other additional costs [ 15 , 18 , 22 , 23 ].
Finally, gender norms that deprioritize the health needs of women both at institutional, community and household levels also underly poor screening uptake [ 13 , 20 , 22 , 25 , 30 , 33 , 34 , 35 , 41 , 47 , 48 , 51 , 60 , 64 , 65 , 74 ]. Patriarchal norms which value the needs of men and boys over women and girls are often upheld in institutions and communities, which shapes political will and decision-making regarding investment in women’s health and creates inequitable health and access to care for women [ 90 , 91 ]. In many studies, women reported a lack of partner approval, permission, or support, as well as religious, cultural, or traditional prohibitions as a barrier to uptake, indicating the breadth and depth of the impact of gender norms.
This review provides a broad overview of the barriers to uptake of cervical cancer screening in LMICs. The barriers were generally the same across countries and continents and different study designs, and are attributable to interacting individual, social, cultural, health system and structural factors.
At the individual level, lack of knowledge and information about cervical cancer, the screening process, and its value, were frequently reported. This suggests that failure to address the knowledge and information gaps, will likely continue to limit uptake even in the absence of other barriers. The literature also reports poor uptake among well-informed women, who reported other barriers rooted in societal religious, cultural health system and structural barriers [ 92 , 93 , 94 , 95 , 96 , 97 ]. Another common individual level barrier was fear which encompassed a wide range of issues. Limited information about the screening process (how it is done and by whom), may result in fear of what to expect. In Switzerland, women preferred to screen themselves using the self-HPV test kit since it reduced discomfort, embarrassment and maintained privacy compared to the traditional pap smear test [ 97 ]. Appropriate and careful introduction and scale up of such self-testing could expand screening in LMICs. Fear of the screening outcome could indicate anxiety around stigmatization, related to discrimination of women with cervical cancer. In a Ugandan study, cervical cancer patients were abandoned by their families, while in a Zambian study, cervical cancer was associated with shame [ 82 , 98 ]. Stigma has also been reported in high income countries. Muslim women in London were hesitant to screen due to embarrassment and fear because they were unmarried and did not want to send implicit messages about being sexually active [ 99 ]. Another study also in the United Kingdom found that cervical cancer screening was stigmatized because of its association with HPV, and the perception that it shows failure of women’s responsibility for their health [ 100 ]. This emphasizes the urgent need for strengthened information dissemination, attention to gender-related discrimination, and dispelling of myths, about cervical cancer.
Cultural/traditional, religious, and social barriers were identified across many studies in all continents, but mainly in Africa and Asia. Lack of spousal and or family support were key barriers, and these may be driven by misconceptions about cervical cancer and traditional, cultural, or religious beliefs about pelvic examination and cancers, and this has also been reported in high income countries [ 101 , 102 ]. Overlapping with cultural/traditional and religious barriers were other social factors including misconceptions and stigmatization of screening and cervical cancer, largely shaped by gender norms [ 14 , 26 , 33 , 48 , 58 ]. The impact of gender norms and inequality were common barriers. When men hold decision-making power, women and girls can have limited access to the social, economic and health resources necessary for their well-being [ 91 ]. At the household level, men often shape the logistical, educational, and psychosocial factors that directly affect women’s ability to access cervical cancer services. Women who are emotionally and financially supported by their families and partners are more likely to get screened. Conversely, family and partners can play a key role in stigmatizing, isolating, and prohibiting women from accessing screening.
Well-functioning health systems with accessible services are critical for successful and effective health programmes. We found significant gaps in cervical cancer screening services in the health systems of LMICs ranging from a lack of high-level elements such as policies and guidelines, poor referral systems, limited points of service, inadequate resources (human and equipment/materials), to local level factors including poor attitudes of healthcare workers. Poor attitudes and discrimination by healthcare workers while inexcusable may be fuelled by staff overload and challenging and constrained conditions [ 47 , 103 , 104 ], areas in need of urgent attention of policy makers and implementers.
Access to screening services was also hindered by geography and cost. Travel costs are significant for women with limited financial means. Women with low levels of education – who often have limited financial means – were less likely to be screened, hence, investing in women’s education in combination with other equity-promoting interventions is likely to improve uptake, given the known benefits of education.
Strengths and limitations
This review includes a wide range of studies (both qualitative, quantitative, and mixed method study designs) and grey literature published over the period 2010 and 2020, enabling an extensive investigation of barriers to cervical cancer screening in LMICs. However, a potential limitation is that studies may have been overlooked due to the search terms used. For example, if studies used terms other than “Vaginal Smears”, “Papanicolaou”, “pap smear”, “pap stain”, “pap test” or “vaginal smear” to describe this specific screening test, they may not be included in the search results. We also included studies where barriers to cervical screening uptake was not a primary objective, and this may limit generalizability of some findings. However, the common barriers were corroborated by many different studies, looking at multiple level barriers to screening in LMICs.
Recommendations
To increase screening uptake and support the elimination of cervical cancer as a public health problem in LMICs, there is a need for implementation of clear cervical cancer policies and guidelines with the prerequisite structures and resources required across diverse health settings. Countries should review their cervical cancer policies and related programs, and fully implement screening guidelines which prioritize structured screening, rather than rely on opportunistic screening that is patient driven. Policies – both within and beyond the health sector – should also actively account for and work to eliminate stigma and all forms of disadvantage and discrimination that shape inequities in communities and within the healthcare system. There is also need for education, information dissemination, engagement, and advocacy about cervical cancer at the community and health facility level. This creation of knowledge and awareness amongst community members and providers around how to proactively reduce barriers to care is crucial for ensuring more women receive screening, and is central to addressing misconceptions, myths, and fears that are prevalent in many communities. Education and awareness initiatives should be grounded in accessible language, driven by local and community contexts and needs, and meaningfully engage diverse groups of women, men, boys and girls as well as multiple sector stakeholders (including a community health worker component focused on women’s health and counselling). Policies that support the sexual and reproductive health and rights of women and girls should be strengthened and expanded and account for inequities in access to care for diverse groups of women. This can include culturally appropriate interventions with a dedicated focus on promoting women’s health, taking into account the social and financial needs of communities. Further priorities at the health facility level includes adequately addressing issues around staff-patient ratio, staff capacities and competencies, organization and integration of facility services, and health promotion efforts aimed at attracting community members for screening. To engage women and communities effectively and consistently, outreach efforts should be conducted in a manner that recognises the different contexts with regards to physical access, affordability, culture, tradition, and competence of health providers to provide high quality and friendly services. Community and religious leaders, non-governmental organizations (NGOs), women who have been screened, and other stakeholders need to reinforce and advocate the message that screening saves lives. This would be an important step in combatting the stigma related to cervical cancer screening.
Future research should focus on generating robust data on which groups are under-screened and why. This must account for the differential experiences of women across diverse categories (e.g., age, socioeconomic status, geography, disability, etc.) and look at the multiple level barriers that converge to create or reinforce barriers to health and screening.
This review highlights some of the key issues highlighted in the literature to date, but there remains a dearth of information as to the multi-level barriers to screening that women face across axes of inequity, including gender, age, income, migrant status, ability, etc.
Finally, the introduction of more modern screening approaches in LMICs should also be supported. It is better information, better resources, and input from women themselves, that can ground how barriers are addressed and how access is improved moving forward.
This review identified a wide range of barriers to cervical cancer screening in LMICs. Urgent implementation of clear policies and programs, supported by health system capacity to implement them is required to address these barriers. The policies should support the promotion of women and girls’ health and rights, and gender equality. In addition, community-wide information dissemination, engagement and advocacy, and targeted further research on barriers to care across diverse groups and contexts are needed to effectively address the inequitable burden of cervical cancer in LMICs. It is only in reducing the barriers to cervical cancer screening that so many women continue to face, that the aims of the WHO’s global strategy to eliminate cervical cancer as a public health problem will be fulfilled.
Availability of data and materials
The articles reviewed and/or analysed during the current study are available from the corresponding author on reasonable request.
IARC and WHO. GLOBOCAN 2018: Estimated cancer incidence, mortality and prevalence worldwide in 2018. Cervical cancer Fact Sheet. Available at: https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-Uteri-fact-sheet.pdf .
World health Organization (2020). Global strategy to accelerate the elimination of cervical cancer as a public health problem. https://www.who.int/publications/i/item/9789240014107
Google Scholar
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of Cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–203. https://doi.org/10.1016/S2214-109X(19)30482-6 .
Article PubMed Google Scholar
Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5(6):e132.
Article PubMed PubMed Central Google Scholar
Bronfenbrenner U. In: Friedman SL, Wachs TD, editors. Measuring environment across the life span: Emerging methods and concepts. Washington, DC: American Psychological Association Press; 1999.
Bronfenbrenner U. The ecology of human development: Experiments by nature and design: Harvard university press; 1979.
Hannes K, Macaitis K. A move to more systematic and transparent approaches in qualitative evidence synthesis: update on a review of published papers. Qual Res. 2012;12(4):402–42.
Article Google Scholar
Critical Appraisal Skills Programme (CASP). 2015. Critical Appraisal Skills Programme (CASP). [online] Available at: < http://www.casp-uk.net/> [Accessed 26 Jan 2022].
Calys-Tagoe BN, Aheto JM, Mensah G, Biritwum RB, Yawson AE. Cervical cancer screening practices among women in Ghana: evidence from wave 2 of the WHO study on global AGEing and adult health. BMC Womens Health. 2020;20(1):1–9.
Stewart K, Li M, Xia Z, et al. Modeling spatial access to Cervical cancer screening services in Ondo State, Nigeria. Int J Health Geogr. 2020;19:28. https://doi.org/10.1186/s12942-020-00222-4 .
Park SJ, Park WS. Identifying barriers to Papanicolaou smear screening in Korean women: Korean National Health and Nutrition Examination Survey 2005. J Gynecol Oncol. 2010;21(2):81–6. https://doi.org/10.3802/jgo.2010.21.2.81 .
Article CAS PubMed PubMed Central Google Scholar
Gottschlich A, Rivera-Andrade A, Bevilacqua K, et al. Using self-collection HPV testing to increase engagement in Cervical cancer screening programs in rural Guatemala: a longitudinal analysis. BMC Public Health. 2020;20:1406. https://doi.org/10.1186/s12889-020-09478-8 .
Getahun T, Kaba M, Derseh BT. Intention to screen for cervical cancer in debre berhan town, amhara regional state, ethiopia: application of theory of planned behavior. J Cancer Epidemiol. 2020;2020:8. https://doi.org/10.1155/2020/3024578 .
Megersa BS, Bussmann H, Bärnighausen T, Muche AA, Alemu K, Deckert A. Community Cervical cancer screening: Barriers to successful home-based HPV self-sampling in Dabat district, North Gondar, Ethiopia. A qualitative study. PLoS ONE. 2020;15(12):e0243036. https://doi.org/10.1371/journal.pone.0243036 .
Ampofo AG, Adumatta AD, Owusu E, Awuviry-Newton K. A cross-sectional study of barriers to Cervical cancer screening uptake in Ghana: An application of the health belief model. PLoS One. 2020;15(4):e0231459.
Harries J, Scott SE, Walter FM, et al. Women’s appraisal, interpretation and help-seeking for possible symptoms of breast and Cervical cancer in South Africa: a qualitative study. BMC Womens Health. 2020;20:251. https://doi.org/10.1186/s12905-020-01120-4 .
Nyamambi E, Murendo C, Sibanda N, Mazinyane N. Knowledge, attitudes and barriers of Cervical cancer screening among women in Chegutu rural district of Zimbabwe. Cogent Soc Sci. 2020;6(1):1766784. https://doi.org/10.1080/23311886.2020.1766784 .
Getachew S, Getachew E, Gizaw M, Ayele W, Addissie A, Kantelhardt EJ. Cervical cancer screening knowledge and barriers among women in Addis Ababa, Ethiopia. PLoS One. 2019;14(5):e0216522.
Nigussie T, Admassu B, Nigussie A. Cervical cancer screening service utilization and associated factors among age-eligible women in Jimma town using health belief model, Southwest Ethiopia. BMC Women’s Health. 2019;19:127. https://doi.org/10.1186/s12905-019-0826-y .
Solomon K, Tamire M, Kaba M. Predictors of Cervical cancer screening practice among HIV positive women attending adult anti-retroviral treatment clinics in Bishoftu town, Ethiopia: the application of a health belief model. BMC Cancer. 2019;19:989. https://doi.org/10.1186/s12885-019-6171-6 .
Williams MS, Kenu E, Adanu A, Yalley RA, Lawoe NK, Dotse AS, Adu RF, Fontaine K. Awareness and Beliefs About Cervical cancer, the HPV Vaccine, and Cervical cancer Screening Among Ghanaian Women with Diverse Education Levels. J Cancer Educ. 2019;34(5):897–903. https://doi.org/10.1007/s13187-018-1392-y PMID: 29974412.
Adewumi K, Oketch SY, Choi Y, et al. Female perspectives on male involvement in a human-papillomavirus-based Cervical cancer-screening program in western Kenya. BMC Womens Health. 2019;19:107. https://doi.org/10.1186/s12905-019-0804-4 .
Oketch SY, Kwena Z, Choi Y, et al. Perspectives of women participating in a Cervical cancer screening campaign with community-based HPV self-sampling in rural western Kenya: a qualitative study. BMC Womens Health. 2019;19:75. https://doi.org/10.1186/s12905-019-0778-2 .
Lieber M, Afzal O, Shaia K, Mandelberger A, Du Preez C, Beddoe AM. Cervical cancer Screening in HIV-Positive Farmers in South Africa: Mixed-Method Assessment. Ann Glob Health. 2019;85(1):58. https://doi.org/10.5334/aogh.37 .
Shiferaw S, Addissie A, Gizaw M, Hirpa S, Ayele W, Getachew S, Kantelhardt EJ, Assefa M, Jemal A. Knowledge about Cervical cancer and barriers toward Cervical cancer screening among HIV-positive women attending public health centers in Addis Ababa city, Ethiopia. Cancer Med. 2018;7(3):903–12. https://doi.org/10.1002/cam4.1334 .
Kangmennaang J, Onyango EO, Luginaah I, Elliott SJ. The next Sub-Saharan African epidemic? A case study of the determinants of Cervical cancer knowledge and screening in Kenya. Soc Sci Med. 2018;197:203–12. https://doi.org/10.1016/j.socscimed.2017.12.013 Epub 2017 Dec 14. PMID: 29253722.
Ng'ang'a A, Nyangasi M, Nkonge NG, Gathitu E, Kibachio J, Gichangi P, Wamai RG, Kyobutungi C. Predictors of Cervical cancer screening among Kenyan women: results of a nested case-control study in a nationally representative survey. BMC Public Health. 2018;18(Suppl 3):1221. https://doi.org/10.1186/s12889-018-6054-9 PMID: 30400916; PMCID: PMC6219012.
Maree JE, Kampinda-Banda M. Knowledge and practices of cervical cancer and its prevention among Malawian women. J Cancer Educ. 2020;35(1):86-92.
Keneema M. Factors Affecting Uptake Of Cervical Cancer Screening Services Among Women Aged 25-49 Attending Antenatal Clinic At Rukunyu Health Center Iv, Kamwenge District (Doctoral dissertation, International Health Sciences University); 2018.
Vhuromu EN, T Goon D, Maputle MS, Lebese RT, Okafor BU. Utilization of cervical cancer screening services among women in Vhembe District, South Africa: a cross-sectional study. Open Public Health J. 2018;11(1):451-463.
Kokuro M. Factors affecting the utilisation of Cervical cancer screening among women attending health services in the kumasi metropolis of Ghana (Doctoral dissertation, Stellenbosch: Stellenbosch University); 2017.
Bishwajit G, Kpoghomou MA. Urban-rural differentials in the uptake of mammography and Cervical cancer screening in Kenya. J Cancer Policy. 2017;12:43–8.
Lunsford NB, Ragan K, Smith JL, Saraiya M, Aketch M. Environmental and Psychosocial Barriers to and Benefits of Cervical cancer Screening in Kenya. Oncologist. 2017;22(2):173–81. https://doi.org/10.1634/theoncologist.2016-0213 Epub 2017 Feb 6. PMID: 28167567; PMCID: PMdouC5330703.
Tiruneh FN, Chuang KY, Ntenda P, Chuang YC. Individual-level and community-level determinants of Cervical cancer screening among Kenyan women: a multilevel analysis of a Nationwide survey. BMC Womens Health. 2017;17(1):109. https://doi.org/10.1186/s12905-017-0469-9 .
Filade TE, Dareng EO, Olawande T, Fagbohun TA, Adebayo AO, Adebamowo CA. Attitude to Human Papillomavirus Deoxyribonucleic Acid-Based Cervical cancer Screening in Antenatal Care in Nigeria: A Qualitative Study. Front Public Health. 2017;6(5):226. https://doi.org/10.3389/fpubh.2017.00226 .
Momberg M, Botha MH, Van der Merwe FH, Moodley J. Women’s experiences with Cervical cancer screening in a colposcopy referral clinic in Cape Town, South Africa: a qualitative analysis. BMJ Open. 2017;7(2):e013914. https://doi.org/10.1136/bmjopen-2016-013914 .
Malambo N, Erikson S. ‘Worse than HIV’: The logics of cancer screening avoidance in Swaziland. Glob Public Health. 2018;13(9):1276–86.
Mitchell SM, Pedersen HN, Eng Stime E, Sekikubo M, Moses E, Mwesigwa D, Biryabarema C, Christilaw J, Byamugisha JK, Money DM, Ogilvie GS. Self-collection-based HPV testing for Cervical cancer screening among women living with HIV in Uganda: a descriptive analysis of knowledge, intentions to screen and factors associated with HPV positivity. BMC Womens Health. 2017;17(1):4. https://doi.org/10.1186/s12905-016-0360-0 .
Koneru A, Jolly PE, Blakemore S, McCree R, Lisovicz NF, Aris EA, et al. Acceptance of peer navigators to reduce barriers to Cervical cancer screening and treatment among women with HIV infection in Tanzania. Int J Gynaecol Obstet. 2017;138(1):53 [cited 2021 Aug 31]. Available from: /pmc/articles/PMC5482416/.
Modibbo FI, Dareng E, Bamisaye P, Jedy-Agba E, Adewole A, Oyeneyin L, Olaniyan O, Adebamowo C, et al. Qualitative study of barriers to Cervical cancer screening among Nigerian women. BMJ Open. 2016;6:e008533. https://doi.org/10.1136/bmjopen-2015-008533 .
Hweissa NA, Lim JNW, Su TT. Health-care providers’ perceptions, attitudes towards and recommendation practice of Cervical cancer screening. Eur J Cancer Care (Engl). 2016;25(5):864–70.
Adepoju EG, Ilori T, Olowookere SA, Idowu A. Targeting women with free cervical cancer screening: challenges and lessons learnt from Osun state, southwest Nigeria. Pan Afr Med J. 2016;24.
Ndejjo R, Mukama T, Musabyimana A, Musoke D. Uptake of Cervical cancer Screening and Associated Factors among Women in Rural Uganda: A Cross Sectional Study. PLoS One. 2016;11(2):e0149696. https://doi.org/10.1371/journal.pone.0149696 PMID: 26894270; PMCID: PMC4760951.
Hasahya OT, Berggren V, Sematimba D, Nabirye RC, Kumakech E. Beliefs, perceptions and health-seeking behaviours in relation to Cervical cancer: a qualitative study among women in Uganda following completion of an HPV vaccination campaign. Glob Health Action. 2016;9:29336. https://doi.org/10.3402/gha.v9.29336 PMID: 26895145; PMCID: PMC4759844.
Ghidei L. Knowledge and Perception of Cervical Cancer and Screening Programs of Women Seeking Care at Monduli Hospital in Tanzania and St. Paul Hospital in Addis Ababa, Ethiopia (Doctoral dissertation); 2015.
Compaore S, Ouedraogo CMR, Koanda S, Haynatzki G, Chamberlain RM, Soliman AS. Barriers to Cervical cancer Screening in Burkina Faso: Needs for Patient and Professional Education. J Cancer Educ. 2015:760–6. https://doi.org/10.1007/s13187-015-0898-9 PMID: 26336956; PMCID: PMC4779069.
Munthali AC, Ngwira BM, Taulo F. Exploring barriers to the delivery of Cervical cancer screening and early treatment services in Malawi: some views from service providers. Patient Preference Adherence. 2015;9:501–8. https://doi.org/10.2147/PPA.S69286 .
Learmonth D, Hakala S, Keller M. “I can't carry on like this”: barriers to exiting the street-based sex trade in South Africa. Health Psychol Behav Med. 2015;3(1):348–65. https://doi.org/10.1080/21642850.2015.1095098 .
Ebu NI, Mupepi SC, Siakwa MP, Sampselle CM. Knowledge, practice, and barriers toward Cervical cancer screening in Elmina, Southern Ghana. Int J Womens Health. 2014;7:31–9. https://doi.org/10.2147/IJWH.S71797 PMID: 25565902; PMCID: PMC4284003.
Omondi Aduda DS, Mkhize N. Ethical issues evolving from patients’ perspectives on compulsory screening for syphilis and voluntary screening for cervical cancer in Kenya. BMC medical ethics. 2014;15(1):1-2.
Kibicho, Jedidah W. Factors influencing utilization of Cervical cancer screening services in Embu hospital, Embu County, Kenya Master of Arts Degree in Project Planning and Management, University of Nairobi, 2014 http://hdl.handle.net/11295/74569
Abdulkadir, IR, 2013 Level of knowledge toward human papillomavirus/Cervical cancer & practice of Papanicolaou test screening among female Addis Ababa university students in Ethiopia. http://hdl.handle.net/10211.2/3954
Atuhaire L. Barriers and facilitators to uptake if cervical cancer screening among women accessing maternal and child health services in Kampala, Uganda; 2013. Available from: http://hdl.handle.net/11394/3924 .
Mwaka AD, Wabinga HR, Mayanja-Kizza H. Mind the gaps: a qualitative study of perceptions of healthcare professionals on challenges and proposed remedies for Cervical cancer help-seeking in post conflict northern Uganda. BMC Fam Pract. 2013;14:193. https://doi.org/10.1186/1471-2296-14-193 .
Paul P, Winkler JL, Bartolini RM, Penny ME, Huong TT, Nga LT, et al. Screen-and-treat approach to Cervical cancer prevention using visual inspection with acetic acid and cryotherapy: experiences, perceptions, and beliefs from demonstration projects in Peru, Uganda, and Vietnam. Oncologist. 2013;18(12):1278.
Ngugi CW, Boga H, Muigai AW, Wanzala P, Mbithi JN. Factors affecting uptake of Cervical cancer early detection measures among women in Thika, Kenya. Health Care Women Int. 2012;33(7):595–613. https://doi.org/10.1080/07399332.2011.646367 .
Hyacinth HI, Adekeye OA, Ibeh JN, Osoba T. Cervical cancer and pap smear awareness and utilization of pap smear test among Federal civil servants in North Central Nigeria. PLoS One. 2012;7(10):e46583. https://doi.org/10.1371/journal.pone.0046583 Epub 2012 Oct 1. PMID: 23049708; PMCID: PMC3462186.
Mupepi SC, Sampselle CM, Johnson TR. Knowledge, attitudes, and demographic factors influencing Cervical cancer screening behavior of Zimbabwean women. J Women’s Health (Larchmt). 2011;20(6):943–52. https://doi.org/10.1089/jwh.2010.2062 .
Andersen JG, Shrestha AD, Gyawali B, Neupane D, Kallestrup P. Barriers and facilitators to Cervical cancer screening uptake among women in Nepal – a qualitative study. Women Health. 2020. https://doi.org/10.1080/03630242.2020.1781742 .
Spagnoletti B, Bennett LR, Wahdi AE, Wilopo SA, Keenan CA. A Qualitative Study of Parental Knowledge and Perceptions of Human Papillomavirus and Cervical cancer Prevention in Rural Central Java, Indonesia: Understanding Community Readiness for Prevention Interventions. Asian Pac J Cancer Prev. 2019;20(8):2429–34. https://doi.org/10.31557/APJCP.2019.20.8.2429 .
Gu C, Chan CW, Chow KM, Yang S, Luo Y, Cheng H, Wang H. Understanding the cervical screening behaviour of Chinese women: The role of health care system and health professions. Appl Nurs Res. 2018;39:58–64.
Ashtarian H, Mirzabeigi E, Mahmoodi E, Khezeli M. Knowledge about Cervical cancer and Pap Smear and the Factors Influencing the Pap test Screening among Women. Int J Community Based Nurs Midwifery. 2017;5(2):188–95.
PubMed Google Scholar
Östh J. Knowledge of Human Papilloma Virus, Cervical cancer and Cytological Screening and Attitudes towards and Practices of Screening among Undergraduate students at Rajarata University, Sri Lanka: A cross-sectional study; 2015.
Jia Y, Li S, Yang R, Zhou H, Xiang Q, Hu T, Zhang Q, Chen Z, Ma D, Feng L. Knowledge about Cervical cancer and barriers of screening program among women in Wufeng County, a high-incidence region of Cervical cancer in China. PLoS One. 2013;8(7):e67005.
Baskaran P, Subramanian P, Rahman RA, Ping WL, Mohd Taib NA, Rosli R. Perceived susceptibility, and Cervical cancer screening benefits and barriers in Malaysian women visiting outpatient clinics. Asian Pac J Cancer Prev. 2013;14(12):7693–9. https://doi.org/10.7314/apjcp.2013.14.12.7693 PMID: 24460355.
Gan DEH, Dahlui M. Cervical screening uptake and its predictors among rural women in Malaysia. Singapore Med J. 2013;54(3):163-168.
Demirtas B, Acikgoz I. Promoting attendance at Cervical cancer screening: understanding the relationship with Turkish womens’ health beliefs. Asian Pac J Cancer Prev. 2013;14(1):333–40. https://doi.org/10.7314/apjcp.2013.14.1.333 PMID: 23534749.
Guvenc G, Akyuz A, Yenen MC. Effectiveness of nursing interventions to increase pap smear test screening. Res Nurs Health. 2013;36(2):146-57.
Reis N, Bebis H, Kose S, Sis A, Engin R, Yavan T. Knowledge, behavior and beliefs related to Cervical cancer and screening among Turkish women. Asian Pac J Cancer Prev. 2012;13(4):1463–70. https://doi.org/10.7314/apjcp.2012.13.4.1463 PMID: 22799349.
Gu C, Chan CW, Twinn S, Choi KC. The influence of knowledge and perception of the risk of cervical cancer on screening behavior in mainland Chinese women. Psycho-Oncology. 2012;21(12):1299-308.
Abdullah F, Aziz NA, Su TT. Factors related to poor practice of Pap smear screening among secondary school teachers in Malaysia. Asian Pac J Cancer Prev. 2011;12(5):1347–52 PMID: 21875295.
Gu C, Chan CW, Twinn S. How sexual history and knowledge of Cervical cancer and screening influence Chinese women's screening behavior in mainland China. Cancer Nurs. 2010;33(6):445–53.
Abdullah F, Su TT. Enhancement of the Cervical cancer screening program in Malaysia: a qualitative study. Asian Pac J Cancer Prev. 2010;11(5):1359–66 PMID: 21198293.
Al-Naggar RA, Low WY, Isa ZM. Knowledge and barriers towards Cervical cancer screening among young women in Malaysia. Asian Pac J Cancer Prev. 2010;11(4):867–73 PMID: 21133593.
Bien-Aimé, Danta Dona Ruthnie. Understanding the Barriers and Facilitators to Cervical Cancer Screening Among Women in Gonaives, Haiti: An Explanatory Sequential Mixed-Methods Study. Master’s thesis, Harvard Medical School; 2020. Available from: https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37365189 .
Lyons KD, Kennedy LS, Larochelle EPM, Tsongalis GJ, Reyes HS, Zuniga-Moya JC, Chamberlin MD, Bruce ML, Bejarno S. Feasibility of Brigade-Style, Multiphasic Cancer Screening in Rural Honduras. JCO Glob Oncol. 2020;6:453–61. https://doi.org/10.1200/JGO.19.00396 .
Chary AN, Rohloff PJ. Major challenges to scale up of visual inspection based Cervical cancer prevention programs: the experience of Guatemalan NGOs. Global Health Sci Pract. 2014;2(3):307–17. https://doi.org/10.9745/GHSP-D-14-00073 .
Barrett BW, Paz-Soldan VA, Mendoza-Cervantes D, Sánchez GM, Córdova López JJ, Gravitt PE, Rositch AF, Proyecto Precancer Study Group. Understanding Geospatial Factors Associated with Cervical cancer Screening Uptake in Amazonian Peruvian Women. JCO Global Oncol. 2020;6:1237–47. https://doi.org/10.1200/GO.20.00096 .
Collins JH, Bowie D, Shannon G. A descriptive analysis of health practices, barriers to healthcare and the unmet need for Cervical cancer screening in the Lower Napo River region of the Peruvian Amazon. Women’s Health. 2019. https://doi.org/10.1177/1745506519890969 .
Nugus P, Désalliers J, Morales J, Graves L, Evans A, Macaulay AC. Localizing Global Medicine: Challenges and Opportunities in Cervical Screening in an Indigenous Community in Ecuador. Qual Health Res. 2018;28(5):800–12. https://doi.org/10.1177/1049732317742129 .
Albuquerque CL, Costa Mda P, Nunes FM, Freitas RW, Azevedo PR, Fernandes JV, Rego JV, Barreto HM. Knowledge, attitudes and practices regarding the Pap test among women in North-eastern Brazil. Sao Paulo Med J. 2014;132(1):3–9. https://doi.org/10.1590/1516-3180.2014.1321551 PMID: 24474073.
Stormo AR, Altamirano V, Pérez-Castells M, Espey D, Padilla H, Panameño K, Soria M, Santos C, Saraiya M, Luciani S. Bolivian health providers’ attitudes toward alternative technologies for Cervical cancer prevention: a focus on visual inspection with acetic acid and cryotherapy. J Women’s Health. 2012;21(8):801–8.
Paz-Soldán V, Bayer A, Nussbaum L, Cabrera L. Structural barriers to screening for and treatment of Cervical cancer in Peru. Reprod Health Matters. 2012;20(40):49–58.
Paolino M, Arrossi S. Women’s knowledge about Cervical cancer, Pap smear and human papillomavirus and its relation to screening in Argentina. Women Health. 2011;51(1):72–87.
Townsend JS, Stormo AR, Roland KB, Buenconsejo-Lum L, White S, Saraiya M. Current Cervical cancer screening knowledge, awareness, and practices among U.S. affiliated pacific island providers: opportunities and challenges. Oncologist. 2014;19(4):383–93. https://doi.org/10.1634/theoncologist.2013-0340 .
Valerianova Z, Panayotova Y, Amati C, Baili P. Cervical cancer Screening in Bulgaria - past and present Experience. Tumori. 2010;96:538–44. https://doi.org/10.1177/030089161009600405 .
Rada C, Hudiţa D, Manolescu S, Prejbeanu I, Zugravu CA. Attitudinal and behavioral patterns, socio-demographical characteristics of risk for cervical cancer. Gineco.ro. 2010;6(20.2):102–9.
Lisy K, Porritt K. Narrative synthesis: considerations and challenges. JBI Evid Implement. 2016;14(4):201.
Maree JE, Wright SC. Cervical cancer: does our message promote screening? A pilot study in a South African context. Eur J Oncol Nurs. 2011;15(2):118–23.
Sen G, Östlin P. Unequal, Unfair, Ineffective and Inefficient; Gender Inequity in Health: Why it exists and how we can change it. WHO Commission on Social Determinants of Health; 2008.
United nations 2016 Report of the Working Group on the issue of discrimination against women in law and in practice. https://documents-dds-ny.un.org/doc/UNDOC/GEN/G16/072/19/PDF/G1607219.pdf?OpenElement .
Chorley AJ, Marlow LA, Forster AS, Haddrell JB, Waller J. Experiences of cervical screening and barriers to participation in the context of an organised programme: a systematic review and thematic synthesis. Psycho-Oncology. 2017;26(2):161–72.
Akinlotan M, Bolin JN, Helduser J, Ojinnaka C, Lichorad A, McClellan D. Cervical cancer screening barriers and risk factor knowledge among uninsured women. J Community Health. 2017;42(4):770–8.
Selmouni F, Zidouh A, Alvarez-Plaza C, El Rhazi K. Perception and satisfaction of cervical cancer screening by Visual Inspection with Acetic acid (VIA) at Meknes-Tafilalet Region, Morocco: a population-based cross-sectional study. BMC Womens Health. 2015;15(1):1–6.
Bates CK, Carroll N, Potter J. The challenging pelvic examination. J Gen Intern Med. 2011;26(6):651–7.
Quincy BL, Turbow DJ, Dabinett LN. Acceptability of self-collected human papillomavirus specimens as a primary screen for cervical cancer. J Obstet Gynaecol. 2012;32(1):87–91.
Article CAS PubMed Google Scholar
Fargnoli V, Petignat P, Burton-Jeangros C. To what extent will women accept HPV self-sampling for cervical cancer screening? A qualitative study conducted in Switzerland. Int J Women's Health. 2015;7:883.
Rahman R, Clark MD, Collins Z, Traore F, Dioukhane EM, Thiam H, Dykens JA. Cervical cancer screening decentralized policy adaptation: an African rural-context-specific systematic literature review. Glob Health Action. 2019;12(1):1587894.
Szarewski A, Cadman L, Ashdown-Barr L, Waller J. Exploring the acceptability of two self-sampling devices for human papillomavirus testing in the cervical screening context: a qualitative study of Muslim women in London. J Med Screen. 2009;16(4):193–8.
Vrinten C, Wardle J, Marlow LA. Cancer fear and fatalism among ethnic minority women in the United Kingdom. Br J Cancer. 2016;114(5):597–604.
Padela AI, Peek M, Johnson-Agbakwu CE, Hosseinian Z, Curlin F. Associations between religion-related factors and cervical cancer screening among Muslims in greater Chicago. J Lower Genital Tract Disease. 2014;18(4):326.
Salman KF. Health beliefs and practices related to cancer screening among Arab Muslim women in an urban community. Health Care For Women International. 2012;33(1):45–74.
Dykens JA, Smith JS, Demment M, Tina EM, Karen S, Irwin T, et al. Evaluating the implementation of Cervical cancer screening programs in low - resource settings globally: a systematized review. Cancer Causes Control. 2020;31(5):417–29. Available from: https://doi.org/10.1007/s10552-020-01290-4 .
Pierz AJ, Randall TC, Castle PE, Adedimeji A, Ingabire C, Kubwimana G, et al. A scoping review: Facilitators and barriers of Cervical cancer screening and early diagnosis of breast cancer in Sub-Saharan African health settings. Gynecol Oncol Reports. 2020;33:100605.
Download references
Acknowledgements
We thank Drs Desmond Kuupeil, Monica A Mensa and Nonjabulo Gwalawo from the Faculty of Public Health Medicine at the University of KwaZulu-Natal for assisting with the literature searches.
This project was funded by the World Health Organization.
Author information
Authors and affiliations.
Human & Social Capabilities (HSC), Human Sciences Research Council, Pretoria, South Africa
Z. Petersen, G. Maseko, S. Takatshana, P. Ndlovu, N. Zondi, N. Zungu & S. Moyo
Cochrane South Africa, South African Medical Research Council, Cape Town, South Africa
Public Health Medicine, University of KwaZulu-Natal (UKZN), Durban, South Africa
T. G. Ginindza & N. Zungu
Cancer & Infectious Diseases Epidemiology Research Unit (CIDERU), Durban, South Africa
T. G. Ginindza
Cervical Cancer Elimination Initiative, World Health Organization, Geneva, Switzerland
C. Varghese, G. Hunting, G. Parham & P. Simelela
School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa
You can also search for this author in PubMed Google Scholar
Contributions
VC and SM conceptualised the project. SM wrote the project protocol. AJ and TG contributed to the protocol. AJ, ZP lead the data searches. All authors screened and reviewed abstracts and articles and extracted data. AJ and ZP lead the analysis. SM and ZP lead writing the manuscript. All authors contributed to the manuscript and approved it for publication.
Corresponding author
Correspondence to S. Moyo .

Ethics declarations
Ethics approval and consent to participate.
No ethics approval was sought because the project review published articles and reports. No participants were recruited or directly involved in this study. We reviewed published articles that investigated the barriers to cervical cancer screening uptake by women in LMICs. The results will be disseminated through a publicly available research report and manuscript as well as in conferences and webinars. They will also be distributed through the WHO and the institutions involved in the project.
Consent for publication
All author agreed to publication the manuscript. No participants were recruited or directly involved in this study.
Competing interests
VC, HG, PG, SP are employed by the World Health Organization which funded the project.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Quality assessment of studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Petersen, Z., Jaca, A., Ginindza, T.G. et al. Barriers to uptake of cervical cancer screening services in low-and-middle-income countries: a systematic review. BMC Women's Health 22 , 486 (2022). https://doi.org/10.1186/s12905-022-02043-y
Download citation
Received : 18 March 2022
Accepted : 10 October 2022
Published : 02 December 2022
DOI : https://doi.org/10.1186/s12905-022-02043-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cervical cancer
- Social ecological model
- Low-and-middle-income countries
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Cervical Cancer Research
For some people with early-stage cervical cancer, a surgical procedure called a simple hysterectomy may be a safe and effective alternative to treatment with a radical hysterectomy, results from the SHAPE trial show.
It may be worthwhile for some individuals between ages 65 and 69 to get tested for HPV, findings from a Danish study suggest. Specifically, the testing may help prevent cervical cancer among those who haven’t had cervical cancer screening for at least 5 years.
One dose of the HPV vaccine was highly effective in protecting young women against infection from high-risk HPV types, a study in Kenya found. A single dose would make HPV vaccines more accessible worldwide, reducing cervical cancer’s global burden.
The rates of timely cervical cancer screening fell between 2005 and 2019, researchers found, and disparities existed among groups of women. The most common reason for not receiving timely screening was lack of knowledge about screening or not knowing they needed screening.
Fewer women with early-stage cervical cancer are having minimally invasive surgery, including robotic, as part of their treatment, a new study shows. The shift toward more open surgeries follows the release of results from the LACC trial in 2018.
Widespread HPV vaccine use dramatically reduces the number of women who will develop cervical cancer, according to a study of nearly 1.7 million women. Among girls vaccinated before age 17, the vaccine reduced cervical cancer incidence by 90%.
Updated cervical cancer screening guidelines from the American Cancer Society recommend HPV testing as the preferred approach. NCI’s Dr. Nicolas Wentzensen explains the changes and how they compare with other cervical cancer screening recommendations.
In a new study, an automated dual-stain method using artificial intelligence improved the accuracy and efficiency of cervical cancer screening compared with the current standard for follow-up of women who test positive with primary HPV screening.
More than a decade after vaccination, women who had received a single dose of the HPV vaccine continued to be protected against infection with the two cancer-causing HPV types targeted by the vaccine, an NCI-funded clinical trial shows.
Women with cervical or uterine cancer who received radiation to the pelvic region reported side effects much more often using an online reporting system called PRO-CTCAE than they did during conversations with their clinicians, a new study shows.
A research team from NIH and Global Good has developed a computer algorithm that can analyze digital images of the cervix and identify precancerous changes that require medical attention. The AI approach could be valuable in low-resource settings.
A new test can help to improve the clinical management of women who screen positive for HPV infection during routine cervical cancer screening, an NCI-led study has shown.
FDA has approved pembrolizumab (Keytruda) for some women with advanced cervical cancer and some patients with primary mediastinal large B-cell lymphoma (PMBCL), a rare type of non-Hodgkin lymphoma.
By comparing the genomes of women infected with a high-risk type of human papillomavirus (HPV), researchers have found that a precise DNA sequence of a viral gene is associated with cervical cancer.
Investigators with The Cancer Genome Atlas (TCGA) Research Network have identified novel genomic and molecular characteristics of cervical cancer that will aid in subclassification of the disease and may help target therapies that are most appropriate for each patient.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Knowledge, attitude and practice of cervical cancer screening among women infected with HIV in Africa: Systematic review and meta-analysis
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft
* E-mail: [email protected]
Affiliation Ethiopian Public Health Institute, Addis Ababa University, Addis Ababa, Ethiopia
Roles Methodology, Writing – review & editing
Affiliation Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Program of Tropical and Infectious Diseases, Addis Ababa, Ethiopia
Roles Data curation, Investigation, Methodology, Writing – review & editing
Affiliation Addis Ababa University, College of Health Sciences, School of Public Health, Addis Ababa, Ethiopia
Affiliation International Institute for Primary Health Care-Ethiopia, Addis Ababa, Ethiopia
- Agajie Likie Bogale,
- Tilahun Teklehaymanot,
- Jemal Haidar Ali,
- Getnet Mitike Kassie

- Published: April 8, 2021
- https://doi.org/10.1371/journal.pone.0249960
- Peer Review
- Reader Comments
To establish successful strategies and increasing the utilization of preventive services, there is a need to explore the extent to which the general female population is aware and use the service for cervical cancer-screening among women infected with HIV in Africa. Available evidences in this regard are controversial and non-conclusive on this potential issue and therefore, we estimated the pooled effect of the proportion of knowledge, attitude and practice of HIV infected African women towards cervical cancer screening to generate evidence for improved prevention strategies.
We applied a systematic review and meta-analysis of studies conducted in Africa and reported the proportion of knowledge, attitude and practice towards cervical cancer screening. We searched electronic databases: PubMed/Medline, SCOPUS, ScienceDirect, Web of science, Cumulative Index of Nursing and allied Health Sciences (CINAHL) and Google scholar databases to retrieve papers published in English language till August 2020. We used random-effects model to estimate the pooled effect, and funnel plot to assess publication bias. The registration number of this review study protocol is CRD42020210879.
In this review, we included eight published papers comprising 2,186 participants. The estimated pooled proportion of knowledge of the participants was 43.0% (95%CI:23.0–64.0) while the pooled estimates of attitudes and practices were 38.0% (95%CI: 1.0–77.0) and 41.0% (95%CI: 4.0–77.0), respectively. The proportion of the outcome variables were extremely heterogeneous across the studies with I 2 > 98%).
The pooled estimates of knowledge, attitude and practice were lower than other middle income countries calls for further activities to enhance the uptake of the services and establish successful strategies.
Citation: Bogale AL, Teklehaymanot T, Haidar Ali J, Kassie GM (2021) Knowledge, attitude and practice of cervical cancer screening among women infected with HIV in Africa: Systematic review and meta-analysis. PLoS ONE 16(4): e0249960. https://doi.org/10.1371/journal.pone.0249960
Editor: Saeed Ahmed, Rutland Regional Medical Center, UNITED STATES
Received: January 30, 2021; Accepted: March 27, 2021; Published: April 8, 2021
Copyright: © 2021 Bogale et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: CI, Confidence interval; df, degree of freedom; HIV, Human Immunodeficiency virus; I 2 , Heterogeneity; KAP, Knowledge, attitude and practice; MeSH, Medical subject heading; tiab, Title and abstract
Introduction
Cancer of the cervix uteri is the 3rd most common cancer among women worldwide, with an estimated 569,847 new cases and 311,365 deaths with a greater number of cases (119,284) and deaths (81,687) in Africa, according to the(GLOBOCAN 2018, an online database providing estimates of incidence and mortality) [ 1 ]. This death report is even higher than worldwide report in 2012 indicating that 266,000 women died of cervical cancer–equivalent of one woman dying every 2 minutes with about 90% of these deaths occurring in low- and middle-income countries [ 2 ].
Cancer of the cervix is the second most commonly diagnosed cancer after breast cancer and the third leading cause of cancer death after breast and lung cancers in developing countries [ 3 ]. It also ranks second next to breast cancer in Ethiopia [ 4 ].
One of the strategies to minimize the burden of the disease is to establish successful strategies and increasing the utilization of preventive measures ranging from community education, social mobilization, vaccination, screening, and treatment to palliative care [ 5 ].
Most importantly, increasing the knowledge, attitude and practice (KAP) of cervical cancer screening and prevention among females is a part of a comprehensive approach to cervical cancer prevention and control strategy. This might play a pivotal role in the controlling strategy on the issue. Health workers considered to take a lead in this regard are found to have less knowledge about cervical cancer as a disease and relatively fair knowledge on Pap smear testing [ 6 ]. Among women who had been attending a tertiary hospital, the majority had a positive attitude while about a third had good knowledge and very few (2.7%) had good practice [ 7 ]. When looked at the patients suffered gynecological cancer, more than half of them knew that their disease was malignant [ 8 ]. In addition, different evidence about knowledge, attitude and practice were generated at different corners of Asian countries [ 9 – 12 ] that seek for pooling of the findings for decision making.
In Africa, the findings indicate that the cervical cancer screening approach is in its infancy stage. For instance, in rural Uganda, only 4.8% of women had ever been screened for cervical cancer [ 13 ] and around ten percent in Burkina-Faso [ 14 ]. In Ethiopia, including University female students, their KAP is fair towards cervical cancer and scored less than fifty percent [ 15 – 18 ].
On the basis of the comprehensive literature search made, variability on the KAP score prevails in various African countries and assumed to have high prevalence of the problem and with unavailability of information among the female population living with HIV who are the most vulnerable population.
Therefore, this review aims to estimate the pooled effect of the proportion of KAP of HIV infected African women towards cervical cancer screening to generate evidence for improved prevention strategies.
Materials and methods
Search strategy and screening of papers.
We conducted a systematic review and meta-analysis of published articles to estimate the pooled effect or the proportion of knowledge, attitude and practice towards cervical cancer screening in Africa. We systematically searched the papers published in the following electronic databases; PubMed/Medline, SCOPUS, ScienceDirect, Web of science, Cumulative Index of Nursing and allied Health Sciences (CINAHL) and Google scholar. The review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard [ 19 ] as displayed in S1 Table . We used a search strategy by combining the following key terms: knowledge, attitude, practice, cervical cancer, uterine cervical neoplasms, cervical cancer screening, human immunodeficiency virus or HIV, and Africa. We used Truncation(*) to manage spelling variation during search: infect* or positive, wom*n or female* or girl*. We used both free text and Medical subject heading [MeSH]terms during electronic database search.
PubMed database search strategy was: (((((((knowledge) AND ((((cervical cancer) OR (Uterine Cervical Neoplasms)) OR (cervical cancer screening[tiab])) OR (cervical cancer screening[MeSH Terms]))) AND ((Attitude) AND ((((cervical cancer) OR (Uterine Cervical Neoplasms)) OR (cervical cancer screening[tiab])) OR (cervical cancer screening[MeSH Terms])))) AND ((practice) AND ((((cervical cancer) OR (Uterine Cervical Neoplasms)) OR (cervical cancer screening[tiab])) OR (cervical cancer screening[MeSH Terms])))) AND ((human immunodeficiency virus) OR (HIV))) AND ((infect*) OR (positive))) AND ((women) OR (female*))) AND (Africa) AND ((y_10[Filter]) AND (female[Filter]) AND (english[Filter]))
The search was repeated to identify the consistency of search terms and result. Two authors (AL and JH) independently reviewed the titles, abstracts and full articles of retrieved studies.
Study inclusion and exclusion criteria
In this review, we included a cross sectional studies conducted in Africa that reported Knowledge, attitude and practice towards cervical cancer screening. The inclusion was restricted to papers published from 2010 to August 2020 in the English language within a ten year period though data were available from 2014 to 2019. We excluded those studies that did not clearly state the outcome measures, study population different from HIV infected women or females, duplication citations, and review articles [ Fig 1 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0249960.g001
Study quality assessment
We assessed the quality of included studies by using the 14 items Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies—NHLBI, NIH [ 20 ]. This assessment tool mainly focused on research question, study population, eligibility criteria (inclusion and exclusion criteria of study participants), sample size justification, exposure measures and assessment, sufficient time frame to see an effect, outcome measures and blinding of outcome assessors, follow up rate, and statistical analysis. The quality assessment was rated as good, fair and poor based on quality assessment tool criteria. The maximum score indicating high quality was 14 and the lowest possible score was zero. The rating values of the included studies in terms of their quality were based on their design. Cross-sectional types do not consider the items which fit for cohort and taken as not-applicable (NA) and thus, the rating values were not taken from the possible maximum score (i.e. 14). In this review, all scores are written in percentage beside the results individual components of the quality assessment [ S2 Table ].
Data extraction
We extracted data from eligible abstract and/or full text of the articles by considering the outcome variables and the characteristics of participants such as age range, mean or median age, sex, HIV sero-status. In addition, we extracted the study characteristics such as first author, year of publication, study setting, study location or country, study design, sample size, knowledge score, attitude and practice [ Table 1 ].
https://doi.org/10.1371/journal.pone.0249960.t001
Statistical analysis
We estimated the pooled proportion of knowledge, attitude and practice of HIV positive women on cervical cancer screening with its 95% Confidence Interval (CI) using random effects meta-analysis model assuming the true effect size varies between studies [ 21 ]. The proportion of knowledge, attitude and practice reported in each included study is multiplied by its sample size to express the score in number, and data presented in forest plot.
We assessed heterogeneity in the proportions of different studies using heterogeneity Chi-square (x 2 ) based Q test with significant level of p-value < 0.1 and I 2 . The I 2 value 25% indicates low heterogeneity while 50% moderate and 75% high [ 22 ]. We assessed the potential publication bias using funnel plot. If the 95% of the point estimates of the included studies lie within the funnel plot defined by straight lines, then that indicates the absence of heterogeneity [ 23 ]. We used moment based meta-regression to assess the potential source of heterogeneity. Data analysis was conducted using STATA version 14.
Ethical approval and consent to participate.
Since the review made was based on previously published articles, there was no need for ethical clearance. Nevertheless, the protocol of the study was pre-registered on PROSPERO (International prospective register of systematic reviews) University of York, Centre for Reviews and Dissemination with registration number CRD42020210879.
Operational definition of KAP in this protocol.
Knowledge (K) . Refers to the awareness of HIV positive women towards cervical cancer screening in Africa. Different pocket studies are filtered and eligible articles are included in the analysis to estimate the pooled knowledge.
Attitude (A) . Refers to the way of thinking or feeling of HIV positive women on cervical cancer screening.
Practice (P) . Refers to the habit of women to be screened for cervical cancer.
Study characteristics
We included eight studies [ Fig 1 ], from Ethiopia [ 24 , 25 ], Uganda [ 26 ], Ghana [ 27 ], Nigeria [ 28 ], Morocco [ 29 ], Kenya [ 30 ], and South Africa [ 31 ] which are health facility based [ Table 1 ]. Almost all the included studies were cross-sectional types published from 2014 to 2019 though the extraction of data was done for the past ten years till August 2020. The maximum sample size reported was 581 [ 25 ] while the minimum was 60 [ 27 ]. The age of respondents ranged from 9 to 69 years [ Table 1 ].
Pooled estimates of knowledge of HIV positive women towards cervical cancer screening in Africa
We pooled data from 2,186 HIV positive women to estimate the pooled proportion of knowledge on cervical cancer screening using meta-analysis. The overall pooled proportion of knowledge was 43.0% [ Fig 2 ] with high heterogeneity across the studies(chi 2 = 493.23 (d.f. = 5), p = 0.001, and I 2 = 98.99%).
In the plot, the diamond shows the pooled result and the boxes show the effect estimates from the single studies. The purple dotted vertical line indicates pooled estimate. The purple solid vertical line indicates the reference line at zero indicating no effect. The horizontal line through the boxes illustrate the length of the confidence interval and the boxes show the effect estimates from the single studies.
https://doi.org/10.1371/journal.pone.0249960.g002
Pooled estimates of the attitude and practice of HIV positive women towards cervical cancer screening in Africa
Meta-analysis using few studies included is not recommended due to less precision. In this review, only three studies included in the analysis to estimate the pooled effect, which was the attitude and practice of HIV positive women towards cervical cancer screening. The pooled estimates of attitude was 38.0% [ Fig 3 ] with high heterogeneity across the studies (chi 2 = 437.57 (d.f. = 2), p = 0.001, I^2 (variation in ES attributable to heterogeneity) = 99.54%) while the pooled practice estimate was 41.0% [ Fig 4 ] with high level of heterogeneity (chi 2 = 260.80 (d.f. = 2), p = 0.001, I^2 (variation in ES attributable to heterogeneity) = 99.23%).
https://doi.org/10.1371/journal.pone.0249960.g003
https://doi.org/10.1371/journal.pone.0249960.g004
Meta-regression analysis
We assessed the effect of sample size and year of the study on heterogeneity between the studies using meta-regression model. However, there was no significant prediction of heterogeneity between the effect size and the assessed variables (i.e., both sample size and year of the study) [ Table 2 ]. Meaning, in the adjusted model, both sample size and year of the study didn’t indicate heterogeneity in the effect size which is equivalent to the pooled proportion (P > 0.05). When we interpret the finding using β-coefficient, one unit increase in the publication year will decrease the outcome variable by the coefficient of 20.14 points and an increase in the sample size will depict a slight increase (0.14 points) in the outcome [ Table 2 ].
https://doi.org/10.1371/journal.pone.0249960.t002
Publication bias
The funnel plot (widely used to examine bias in the result of a meta-analysis) for pooled estimates of knowledge, attitude and practice towards cervical cancer screening indicated that there was publication bias. The included studies are scattered out of pseudo 95% confidence limit and the observed bias might be due to small study effect [ Fig 5A–5C ]. Fig 5A , indicates funnel plot of the 6 estimates of knowledge (k) towards cervical cancer screening available for meta-analysis (SE-Standard error, ES-Effect size: proportion), ( b ) Funnel plot of the 3 estimates of attitude (A) towards cervical cancer screening available for meta-analysis (SE-Standard error, ES-Effect size: proportion), ( c ) Funnel plot of the 3 estimates of practice towards cervical cancer screening available for meta-analysis (SE-Standard error, ES-Effect size: proportion).
In this plot, the blue broken line indicates Pseudo 95% CI, the solid red line indicates pooled estimate of the proportion of knowledge, attitude and practice, and the scattered circle dots indicates included studies in the meta-analysis. The scale on the X-axis indicates Effect size estimate or proportion and the Y-axis indicates the precision estimate using standard Error.
https://doi.org/10.1371/journal.pone.0249960.g005
In this review, the pooled estimate of knowledge, attitude and practice of HIV infected women towards cervical cancer screening in Africa was 43.0%, 38.0% and 41.0%, respectively. The highest heterogeneity and publication bias were observed in meta-analysis using forest plot and funnel plot, respectively. The Meta-regression model was applied to identify the reason for heterogeneity using sample size and publication years. However, the variation did not show a significant association on the effect size equivalent to the proportion or the outcome variables.
The knowledge estimate of our finding was concordant with previously reported review findings in Ethiopia among women of reproductive age group (40.37%) [ 32 ] while the attitude and practice findings varied and were 58.87% and 14.02%, respectively [ 32 ]. Such variations are likely due to the fact that the study population and settings studied were different from our current review focused on HIV positive women in Africa.
Similarly, Kasraeian et al also in their review made in low and middle-income countries indicated that HIV positive women had less knowledge about cervical cancer and were less likely to undergo screening [ 33 ].
The original research articles conducted in different Asian countries, including Pakistan illustrated that participants of the study had inadequate knowledge, attitude and practice towards cervical cancer [ 34 ]. In the same breath, Iraqi participants only 30.3% of employed and 40.0% of the students of the female population had positive attitude towards cervical cancer screening [ 35 ]. Similarly, a study conducted in India indicated that 30.2% of respondents had good knowledge and almost one fourth (25.9%) had a favorable attitude towards cervical cancer [ 9 ] with an inspiring result of 58.9% documented for female health care providers’ knowledge of cervical cancer screening [ 10 ]. Another study adds up, only 36.48% of the participants have good knowledge and of these, 83.78% of them had a positive attitude, though the vast majority (97.29%) had no practice [ 7 ] revealing a big gap between attitude and practice. The study conducted in the Eastern China also reported slightly over half (51.9%) of rural women to have high knowledge of whom, 96.0%of them expressed positive attitude and 63.7% were screened for cervical cancer [ 11 ]. Another study from Nepal also reported 42.9% of women to have knowledge and more than 85.0% to have had a positive attitude towards cervical screening [ 12 ]. Whereas the Iranian study showed more than half (58.0%) of patients with cancer knew that their diseases was malignant [ 8 ].
When looked at the African continent, very diverse findings were also reported. According to the Tanzania study, only 10.4% of women were knowledgeable about cervical cancer and 7.9% of these were screened [ 36 ] which was very low. Similarly, in Nigeria18.1% of them had good knowledge with 67.8% of them to have a positive attitude to cervical cancer screening [ 37 ]. In Uganda and Burkina-Faso, the two studies reported that only 4.8% and 11.1% of women had been screened for cervical cancer, respectively [ 13 , 14 ]. The various studies done in Ethiopia also showed different results [ 15 – 18 ]. The study done among University female students in Debre-Berhan, North Ethiopia reported 35.6%, while the study in Wolaita, southern Ethiopia among women of reproductive age reported 43.1% to have good knowledge towards cervical cancer showing better results [ 15 , 16 ]. Of the 43.1% of the women, studied in Wolaita, 45.5% of them had a positive attitude with 22.9% of them to undergo for screening [ 16 ]. On the other hand, the study done in Addis Ababa reported 27.7% of women to have adequate knowledge of cervical cancer and 25.0% of these to undergo for cervical cancer screening [ 17 ]. The lowest score for knowledge in Ethiopia was documented for Gonder, northern Ethiopia and it was 19.87% underscoring the need for more advocacy work [ 18 ].
Such variable findings of the studies in different countries encouraged us to estimate the pooled effect equivalent to the proportion of the finding which is very crucial for program direction on cervical cancer screening and prevention measure aspects.
Strength and limitations of the study
The strength of this review is that we attempted to include the most vulnerable population, women living with HIV in Africa, and has captured the recent publications within ten years till August 2020 and used more than five biomedical databases. The limitation however, is the inclusion of those papers published and reported only in English language which might have missed other important works done in this regard. The precision of the pooling effect might have been also affected by the fact that only very few studies which reported attitude and practice were included and this might consequently affect the assertion.
The pooled estimates of knowledge, attitude and practice of the current review finding was below half in different African countries. The enhancement of knowledge, attitude and practice of women will augment the comprehensive approach to cervical cancer prevention and control strategy. The current work has shed light-on how much the findings of the studies conducted in different countries on the cervical cancer and its screening were very diverse and difficult for decision making. Thus, it is essential to have the pooled estimates of different findings for decision making. Other than this, the pooled estimates are very crucial for further strengthening the strategies for prevention measure and control of cervical cancer mainly on vulnerable population like women infected by HIV.
Supporting information
S1 appendix. prospero international prospective register of systematic reviews..
https://doi.org/10.1371/journal.pone.0249960.s001
S1 Table. PRISMA assessment checklist.
https://doi.org/10.1371/journal.pone.0249960.s002
S2 Table. The results of the individual components of the quality assessment.
https://doi.org/10.1371/journal.pone.0249960.s003
Acknowledgments
The authors acknowledge the contribution of Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Program of Tropical and Infectious Diseases and Ethiopian Public Health Institute (EPHI) for the opportunity to access an internet. The unreserved support rendered by Minilik Demesie from EPHI during the reviewing process of the study protocol was highly appreciable.
- 1. WHO. Cervix uteri, Source: Globocan 2018
- View Article
- Google Scholar
- 4. “Human Papillomavirus and Related Diseases Report, Ethiopia", June 2019.
- 5. WHO guidance note: comprehensive cervical cancer prevention and control: a healthier future for girls and women, 2013
- PubMed/NCBI
- 19. “PRISMA 2009 Checklist Section / topic PRISMA 2009 Checklist,” pp. 1–2, 2009.
Advertisement
Colposcopy referral and CIN3+ risk of human papillomavirus genotyping strategies in cervical cancer screening
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Get Permissions
- Cite Icon Cite
- Search Site
- Accepted Manuscript March 28 2024
Kelsi R. Kroon , Johannes A. Bogaards , Daniëlle AM. Heideman , Chris JLM. Meijer , Johannes Berkhof; Colposcopy referral and CIN3+ risk of human papillomavirus genotyping strategies in cervical cancer screening. Cancer Epidemiol Biomarkers Prev 2024; https://doi.org/10.1158/1055-9965.EPI-24-0046
Download citation file:
- Ris (Zotero)
- Reference Manager
Background: High-risk human papillomavirus (hrHPV)-based cervical cancer screening in the Netherlands led to a substantial increase in number of colposcopy referrals and low-grade lesions detected. Genotyping strategies may be employed to lower the screening-related burden. Methods: We evaluated fourteen triage strategies with genotyping (HPV16/18 or HPV16/18/31/33/45/52/58) for hrHPV-positive borderline or mild dyskaryosis (BMD) or normal cytology, using data from a population-based hrHPV-based screening trial with 5-year interval (POBASCAM). We considered colposcopy referral at baseline, after 6-month repeat cytology and after 5-year hrHPV testing. Performance was evaluated by one-round positive and negative predictive value (PPV and NPV) for CIN3+ and by two-round colposcopy referral rate. To identify efficient strategies, they were ordered by the one-round colposcopy referral rate. Adjacent strategies were compared by the marginal PPV for detecting one additional CIN3+ (mPPV). Results: The most conservative strategy (repeat cytology after BMD and HPV16/18/31/33/45/52/58-positive normal cytology, next round otherwise) yielded an mPPV of 28%, NPV of 98.2%, and colposcopy rate of 47.2%. Adding direct referral after BMD or genotype-positive BMD yielded an mPPV≤8.2%, NPV≥98.5% and an increase in colposcopy rate of 1.9-6.5%. Adding direct referral after HPV16/18-positive normal cytology yielded an mPPV≤3.5%, NPV≥99.5% and an increase in colposcopy rate of 13.9%. Conclusions: Direct colposcopy referral of women with BMD or normal cytology is unlikely to be efficient, but genotype-guided direct referral after BMD may be considered because the increase in colposcopies is limited. Impact:HrHPV screening programs can become very efficient when immediate colposcopy referral is limited to women at highest CIN3+ risk
Article PDF first page preview
Client account, citing articles via, email alerts.
- Online First
- Online ISSN 1538-7755
- Print ISSN 1055-9965
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Evaluating the implementation of cervical cancer screening programs in low-resource settings globally: a systematized review
J. andrew dykens.
1 University of Illinois at Chicago College of Medicine, Chicago, IL USA
Jennifer S. Smith
2 University of North Carolina School of Public Health, Chapel Hill, NC USA
Margaret Demment
3 University of Rochester Department of Obstetrics and Gynecology, Rochester, NY USA
E. Marshall
4 University of Illinois at Chicago Institute for Health Research and Policy, Chicago, IL USA
Karen Peters
Tracy irwin.
5 University of Washington Department of Obstetrics and Gynecology, Seattle, WA USA
Scott McIntosh
6 University of Rochester Department of Public Health Sciences, Rochester, NY USA
7 University of Hawaii John A Burns School of Medicine, Honolulu, HI USA
Timothy Dye
Associated data.
All materials, data, code, and associated protocols will be promptly available to readers without qualifications or restrictions. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Cervical cancer disproportionately burdens low-resource populations where access to quality screening services is limited. A greater understanding of sustainable approaches to implement cervical cancer screening services is needed.
We conducted a systematized literature review of evaluations from cervical cancer screening programs implemented in resource-limited settings globally that included a formal evaluation and intention of program sustainment over time. We categorized the included studies using the continuum of implementation research framework which categorizes studies progressively from “implementation light” to more implementation intensive.
Fifty-one of 13,330 initially identified papers were reviewed with most study sites in low-resource settings of middle-income countries (94.1%) ,while 9.8% were in low-income countries. Across all studies, visual inspection of the cervix with acetic acid (58.8%) was the most prevalent screening method followed by cytology testing (39.2%). Demand-side (client and community) considerations were reported in 86.3% of the articles, while 68.6% focused scientific inquiry on the supply side (health service). Eighteen articles (35.3%) were categorized as “Informing Scale-up” along the continuum of implementation research.
Conclusions
The number of cervical cancer screening implementation reports is limited globally, especially in low-income countries. The 18 papers we classified as Informing Scale-up provide critical insights for developing programs relevant to implementation outcomes. We recommend that program managers report lessons learnt to build collective implementation knowledge for cervical cancer screening services, globally.
Electronic supplementary material
The online version of this article (10.1007/s10552-020-01290-4) contains supplementary material, which is available to authorized users.
Introduction
Globally, there are over a half million new cases of cervical cancer yearly, with nearly 90% of these cases in least developed economies [ 1 ]. The United States (US) 2012 cervical cancer incidence rate was 8.1 per 100,000 women, while low- and middle- income countries (LMICs) had a collective rate of 15.7 [ 2 ]. Cervical cancer screening programs detect pre-cancerous lesions which can be treated with low-cost outpatient procedures, and if invasive cancers are caught early, successful treatments exist.
Cervical cancer disproportionately affects populations in resource-limited settings globally despite the variety of evidence-based screening options. Technological innovation and efficacy testing of health service interventions including various screening modalities [ 3 , 4 ] have led to clear recommendations for improving cancer control among HPV vaccination programs and cervical cancer screening using locally appropriate methods [ 5 – 7 ]. Nonetheless, the judicious implementation of evidence-based cervical cancer screening programs remains inadequate, resulting in persistently elevated cervical cancer incidence and mortality rates, especially in resource-limited settings [ 8 ].
Gaps in the literature
The 2011 WHO Prioritized Research Agenda for Prevention and Control of Non-communicable Disease notes that while cancer screening services have been shown to be effective in high-income countries (HICs) [ 9 – 12 ] and HPV screening has been shown to reduce cervical cancer deaths in resource-limited settings such as rural India [ 13 ], there are few reports describing the implementation of successful and sustained cervical cancer screening programs in LMICs. Research that examines cervical cancer screening program barriers and facilitators within specific contexts and informs the adaptation of evidence-based interventions within these contexts is needed to ensure the successful implementation and sustainment of programs across various settings [ 6 , 14 ].
In 2015, a scoping study of existing reviews on breast and cervical cancer in low- and middle- income countries concluded that current cervical cancer literature focuses primarily on prevention and detection, largely without implementation considerations [ 15 ]. An additional finding articulated that articles occasionally provide programmatic or policy recommendations that are beyond the context of their own studies’ findings. However, specific recommendations of the implementation methodologies relevant to issues of governance, systems development, workforce capacity, and person/ community centeredness for arriving at these conclusions are often lacking.
Cervical cancer screening
Various evidence-based cervical cancer screening techniques have been developed, tested, and are appropriate for diverse contexts. High-resource settings often employ cytologic screening through Papanicolaou (Pap) smear with follow-up colposcopy and biopsy to identify early-stage dysplasia and pre-cancerous lesions. Cytologic screening can be resource heavy by requiring specialized specimen preservation and advanced technical expertise employing cyto-pathologists. Visual inspection methods can complement other screening modalities and provide adequate sensitivity and specificity to identify later-stage pre-cancers which can then be treated through cryotherapy freezing or loop electrosurgical excision procedure (LEEP), curative modalities commonly implemented alongside visual inspection screening services in low-resource settings. In addition, human papillomavirus (HPV) testing has the highest sensitivity for high-grade lesion detection and can be obtained through clinician-sampled or self-sampling techniques. HPV testing can be used for primary screening in conjunction with cytology or visual inspection for triage if the infrastructure exists. Despite the many screening modalities appropriate for a variety of contexts, there remains poor cervical cancer screening coverage, globally. Gakidou et al. reports a coverage rate of 36.9% globally and only 18.5% in least developed countries [ 16 ].
Dissemination and implementation science for cancer research and health systems strengthening
This systematized review addresses the following questions: (1) “What published literature has reported implementation evaluations for cervical cancer screening programs in resource-limited settings, particularly those linked to sustainable systems (e.g., governmental) that will have a population-level impact?” and, (2) “What are the reported program implementation-relevant contextual findings applicable to guide the adaptation of cervical cancer screening programs into a wide range of settings to support cervical cancer prevention and control strengthening efforts worldwide?.”
By applying the “Continuum of Implementation Research” framework (see Table Table4 4 for definitions and examples) to papers included within this review, our intent is to highlight how the existing catalog of cervical cancer implementation research conforms to various levels of implementation science rigor. This allows the reader to readily identify papers most relevant to the various stages of implementation. The themes of this continuum are defined as follows: (1) Proof of Concept (Implementation not relevant or relevant, but not considered), (2) Proof of Implementation (Implementation relevant, but effects reduced), and (3) Informing Scale-up (Implementation studied as contributing factors or as primary focus) [ 17 ].
Categorization into the continuum of implementation [ 17 ]
We conducted a systematized literature review [ 18 ] of cervical cancer screening program implementation evaluations. Inclusion criteria were that the article (1) states the cervical cancer screening intervention occurred in a resource-limited setting in any country, (2) clearly defines implementation evaluation of the intervention with consideration of any defined implementation-relevant outcomes (acceptability, adoption, appropriateness, feasibility, fidelity, coverage, sustainability) [ 17 ], and (3) articulates evidence or intent of the intervention sustainment over time or through policy change by describing or reporting factors relevant to the inner (e.g., organizational characteristics, fidelity monitoring, staffing) or outer context (sociopolitical, funding, public-academic collaboration) [ 19 ]. The only exclusion criterion was publication in a language other than English.
Search strategy
In July 2015, a search of PubMed, CINAHL, Web of Science, POPline, and IndMED included all years up to July 2015 in the initial title review and limited the search to publications in peer-reviewed journals. Search terms were based on four themes: cervical cancer screening, low resource, evaluation, and population level. (see additional file #1 for an example PubMed search string).
We used a multi-step process to select the articles for our review. Three independent reviewers conducted an initial screening for the inclusion criteria based on article titles and labeled them: “yes,” “no,” or “maybe.” If any of the reviewers labeled the title as a “yes” or “maybe,” it advanced to the next round of review. Articles identified as having a focus on cervical cancer were further screened to determine whether or not they included an evaluation of a screening intervention. Therefore, as a next step, abstracts and full articles (if needed) were reviewed by two reviewers to assess all inclusion and exclusion criteria. All discrepancies were discussed verbally in weekly meetings, with resolution by agreement between the two reviewers and an additional author. All data were managed through a shared and continually updated database.
Data collection
The lead authors, through mutual agreement, devised and refined the data abstraction tool based, in part, on the Implementation research framework proposed by Peters et al. [ 17 ] Abstracted items included the following: location of study, partners, scale of intervention (national, regional, district, etc.), motivation of intervention, intervention description, study methodology, independent variables, screening approach (e.g., VIA, cytology, HPV), health system level of intervention, and identified implementation barriers. The two reviewers then each completed a test round of abstraction of the same 10 manuscripts. These abstracts were then reviewed and compared by one of the authors for discrepancies. The team met again to discuss these discrepancies and resolve any issues. The remaining articles were then abstracted by one of two reviewers. The team met once per week to discuss issues or questions related to abstraction.
Information was abstracted directly from the publication without interpretation for all categories with the following exceptions. To categorize the identified “Partners” (Table (Table1) 1 ) and “Demand and Supply Side Implementation Barriers” (Table (Table2), 2 ), we standardized the terminology with guidance from Peters [ 17 ] and Proctor [ 20 ]. To determine this categorization, both explicit information from the article as well as interpretation and commentary by the reviewing authors was considered. This subjective interpretation was necessary given the current lack of universal standardization of implementation science terminology [ 21 ]. Peters [ 17 ] includes guidance on the grouping of related terms, but it is not exhaustive and did not apply in all cases. Wherever subjective assessment was used, the lead author made a final determination on categorization for consistency. (Supplement 2).
Description of Studies ( n = 51)
VIA Visual Inspection with Acetic Acid, VILI Visual Inspection with Lugol's Iodine
a Categories are not mutually exclusive
Implementation access level relevance
Based on Levesque Patient-Centered Access to Healthcare Framework [ 22 ]
a Not mutually exclusive
b ”Other” such as financing, information systems, equipment / resources management, leadership / governance
Analysis and summary of findings
All lead authors were provided with the abstraction document and asked to independently assess emerging themes. Based on the abstracted information contained in the fields, motivation of intervention; description of intervention; methodology; variables; implementation barriers; findings; recommendations; and research or practice gaps were identified. We categorized the reviewed studies along a continuum of implementation research [ 17 ]. We assigned a single label to each paper among three categories: (1) Proof of Concept (Implementation not relevant or relevant, but not considered); (2) Proof of Implementation (Implementation relevant, but effects reduced); and (3) Informing Scale-up (Implementation studied as contributing factors or as primary focus). (Table (Table4 4 ).
Finally, we categorized barriers identified through the reported implementation research and organized them according to the Patient-Centered Access to Healthcare Framework proposed by Levesque [ 22 ]. This Framework specifies barriers on both the demand side (client and community perspective) as well as the supply side (health services level). Within this framework, demand-side barriers are subcategorized into the client’s ability to “Perceive,” “Seek,” “Reach,” “Pay,” and “Engage” with the community health system, while supply-side barriers are subcategorized into the health service’s “Approachability,” “Acceptability,” “Availability and Accommodation,” “Affordability,” and “Appropriateness” on the part of clients [ 22 ].
Reporting in this analysis refers to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting results of systematic reviews (S1) [ 23 ]. (Supplement 2).
Fifty-one of 13,330 initially identified papers met inclusion criteria. Twenty-five (49.0%) of the articles were published between 2011 and 2015 compared to only four (9.8%) being published between 2001 and 2005. When analyzed by the World Bank Income level [ 24 ], only five (9.8%) had sites discussed in the papers in low-income countries (three of which were in Uganda), while twenty-six (51.0%) sites were in lower-middle-income countries. (Table (Table1 1 ).
The studies reviewed and tested a variety of screening methods, and many of the studies tested multiple methods. Visual inspection was the most prevalent screening method, including 30 (58.8%) VIA studies and eight (15.7%) studies that considered VILI. Cytology testing was second most common, with 20 (39.2%) associated studies. The scale of intervention was distributed towards smaller-scale interventions. Thirty-three (64.7%) were conducted at the city or district level, while only five (9.8%) were at the national level. Forty-four of the 51 studies clearly described the involvement of multiple partners. Of all 51 studies, 26 (51.0%) indicated involvement of national academic institutions and 19 (37.3%) reported involvement of international institutions. Most engaged health systems including those at the local ( n = 34, 66.7%), national ( n = 22, 43%), and international ( n = 3, 5.9%) levels.
For a more nuanced analysis of the reviewed articles, we categorized the relevance to various levels of implementation. (Table (Table2) 2 ) We identified 40 (78.4%) articles pertinent to patient- or client-level considerations. Only 13 (25.5%) are applicable to the quality of clinical services. Twenty-five (49.0%) articles discussed additional health system-relevant themes (financing, information systems, equipment/resources management, leadership/governance, and others).
In addition to identifying the implementation access level of relevance, we, as well, sought to understand the explicit findings regarding barriers identified through the implementation research. Many of the papers reported specific findings relevant to demand-side ( n = 26, 51.0%) or supply-side ( n = 28, 49%) barriers to the successful implementation of cervical cancer screening services. From the articles presenting demand-side barriers (Table (Table3), 3 ), those most often reported include comfort ( n = 6, 23.1%), lack of knowledge (5, 19.2%), embarrassment associated with the clinical procedure ( n = 3, 11.5%), and patient cost considerations ( n = 3, 11.5%). Others receiving fewer mentions include distance to clinic, lack of patient priority for prevention, permission required by husband for procedure, and concern about no sexual intercourse after the procedure.
Demand- and supply-side barriers (ranked by frequency)
Of the 28 papers reporting supply-side barriers (Table (Table3), 3 ), 22 (78.6%) discuss provider-relevant themes and 16 (57.1%) discuss other health system-relevant considerations. Of the 22 reporting provider-associated barriers, 10 (45.5%) report a lack of opportunity or time to participate in trainings, nine (41.0%) report significant provider turnover as a major barrier, and 6 (27.3%) describe technical deficiencies of the provider as limitation to the impact of the screening program. Of the 16 reporting other system barriers, nine (56.3%) report cost as a major limiting factor in implementation.
As described in the background, we sought to characterize studies along the continuum of implementation research categories. (Table (Table4) 4 ) In doing so, we illustrate the value of findings elucidated through studies with well-formulated implementation science questions. Twenty papers (39.2%) were labeled as Proof of Concept, 13 (25.4%) as Proof of Implementation, and 18 (35.3%) as Informing Scale-up. Table Table5 5 details the publications categorized as informing scale-up, stratified by year.
Details of publications categorized as informing scale-up, stratified by year
The number of articles ( n = 51) evaluating the implementation of cervical cancer screening programs in limited resource settings is relatively small compared to the total number of articles identified through the review. Of note, we identified only five papers in low-income countries where settings are likely to have the greatest barriers to building sustainable capacity for cervical cancer screening and where, arguably, there is the most to be learned in vastly improving systems and approaches for screening. Of our reviewed articles, the majority (52.9%) were published between 2011 and 2015, suggesting an upward trend of reporting with the later time of publication. The reporting of experiences and sharing of best practices will contribute to our collective ability to overcome the many challenges in ensuring ultimate sustainability of these programs [ 76 , 77 ].
The identified studies cover a range of cervical cancer screening methodologies with VIA being the most utilized and studied in our included papers. In addition, 46 (90.2%) papers describe research in decentralized settings with the majority of these (33 of 46, 71.7%) at the district or city level. As well, given the significant shortage of healthcare workforce globally [ 78 ], especially in resource-limited settings, it is not surprising that the overwhelming number of supply-side focused studies (32 of 37, 86.5%) considered capacity building. These findings may reflect a trend to integrate an effective, low-resource appropriate technology into existing health services in response to inequities in women’s health care and to strengthen primary health care in decentralized community health systems. The 2008 call [ 79 ] to offer more comprehensive packages of basic health services (including improved preventive care services) in all settings and more recent calls [ 12 ] to address non-communicable diseases (NCDs) are also consistent with this trend.
These findings provide some insight into the cervical cancer screening implementation literature. We note that articles commonly describe community- and client-relevant implications and explore challenges to human resources. Of interest, however, our analysis reveals that a relatively small percentage of papers describe or report quality assurance themes (25.5%) and a very low percentage (3.9%) describe quality improvement activities related to the implemented health service. Given that only 23.5% of all papers are describing “policy” in explicit terms, the present findings also illustrate a major gap in the literature regarding policy development around the long-term sustainment of cervical cancer screening programs.
Demand-side barriers are identified in 51% of reviewed articles with the most frequent focus on comfort, knowledge gaps, personal sensitivity, and cost. Provider issues (78.6%) make up most supply-side barriers. Much can also be learned from implementation evaluations describing other systems-level issues. The present analysis highlights other explicit concerns including cost, equipment, management practices, space, supervision, and infrastructure.
“Proof of Concept” papers describe studies where implementation is not relevant, or implementation is relevant but not considered as research questions. The context of these studies is focused and the factors affecting implementation are not relevant, fixed, or ignored. Because 20 studies fell into the “Proof of Concept” category and 13 in the “Proof of Implementation,” one could conclude that many of the published articles investigating cervical cancer screening programs are not implicitly structured to provide meaningful information of the real world context in which the research project occurs.
For example, a study in Western Kenya aimed to validate VILI as a stand-alone screening test at a Family AIDS Care and Education Services (FACES) clinic [ 72 ] while a study in Leon, Nicaragua compared the acceptability of self-collected versus clinician-collected human papillomavirus (HPV) tests which applies to the “proof of implementation” category [ 73 ].
“Proof of Concept” studies may be strengthened by examining more contextual factors to determine screening feasibility in similar settings. Implementation research also has the potential to describe in greater detail the supporting and hindering factors to wide-scale implementation and sustainment of a cervical cancer screening program within the context of their health system’s existing cancer control and prevention policy.
We classified 18 papers as “informing scale-up.” These papers provide useful guidance for developing cervical cancer implementation programs across different contexts. Principally, those contexts include in-depth perspectives on acceptability and community perceptions [ 53 ], community education and mobilization [ 30 , 59 ] including radio messaging [ 41 ], community-focused or mobile screening [ 30 , 58 ], detail on training community health workers [ 50 ], client tracking [ 59 ], maintenance of human capacity [ 59 ], task sharing [ 65 ], and quality control [ 70 ]. These findings are accessible and highly applicable to the existing programs struggling with substantial challenges as well as to institutions that are prioritizing the new implementation of cervical cancer screening services.A large study in 130 rural communities in Guangdong Province, China [ 69 ] employs sound Dissemination and Implementation research methods. Study results described community participatory research through the Chinese Cancer Prevention Study (CHICAPS). This program was conducted by community leaders with the technical assistance of the research team. They utilized a “pass the message on” model to easily reach women in communities through local village promoters that were trained through locally organized workshops (with up to 25 community leaders being oriented). Their paper describes the model process in depth, including details of stakeholder roles. Conclusions were that the model was successful in (1) improving the efficiency of resource utilization, (2) teaching community leaders and promoters to get patient information and follow procedures, and (3) teaching rural women technical specifics of the screening approach.
A three-phase evaluation of a cervical cancer screening program was conducted in the Harare city health department, Zimbabwe [ 34 ]. This study included a survey of policy makers on guidelines, policy, and attitudes regarding cervical cancer screening, and evaluating determinants at both the supply- and the demand side. Dissemination of their work provides invaluable guidance on how comprehensive policies on cervical cancer screening should be developed to assist in standardization of program implementation, how the formal technical training of health workers should be done, and what necessary resources should be allocated to support a successful and sustainable cervical cancer screening program.
Finally, a cervical cancer screening evaluation was conducted in Guyana to explore the feasibility, effectiveness, and lessons learned of a single-visit approach to cervical cancer screening and treatment [ 65 ]. The reported findings were highly relevant to sustainability and scale-up and concluded that certain components are essential to achieve good population coverage with high-quality services: (i) competency-based training and supportive supervision; (ii) task shifting to non-physician providers; (iii) a strong monitoring and evaluation system that rapidly identifies and addresses programmatic and clinical gaps; (iv) an enabling environment providing programmatic support; and (v) integration of cervical cancer prevention services into appropriate existing programs, such as family planning, postpartum, and HIV care.
Limitations
Due to limitations in our search strategy and a lack of a risk of bias assessment, our review is characterized as a systematized review [ 18 ]. Given that our search strings were composed entirely of Medical Subject Heading (MESH) terms, we relied primarily on the accurate and current MESH terms and did not pursue the addition of articles through a free text search. Given the time limitations, MESH terms were used only to reduce the time associated with the search strategy development. This is problematic given that much of the published research from LMICs is not likely to be well indexed. Searches may therefore have left out relevant articles. Additionally, our search was conducted in the July of 2015. Any articles that were published after the search were not included in the review, omitting articles that would have met the criteria. The inclusion and exclusion criteria were strict for this literature review. Many articles were excluded from this review that provide valuable lessons in a variety of settings outside of the scope of our inclusion criteria, but were not regarded as employing implementation science. The findings from these excluded papers nevertheless may contain some findings relevant to implementation science and could be generalizable or be adaptable in areas with low resources. Additionally, it should be noted that the Continuum of Implementation Research as proposed by Peters [ 17 ] has limitations with a degree of subjectivity and author interpretation as described in the methods section. Every effort was made to consult the guiding framework and limit subjectivity by systematically and uniformly categorizing articles. Finally, the risk of bias for the included papers was not assessed.
Many evidence-based health service interventions are not being readily adopted in LMICs because of an insufficient primary health care system in place to support them [ 79 – 81 ]. More Dissemination and Implementation research is needed to illustrate how health systems function at the local level, especially in LMICs [ 17 ]. Much of the existing Dissemination and Implementation science exploring the interface between health research and policy is concentrated in high-income countries. The paucity of similar research in LMICs presents a major challenge for implementation of preventive measures in these countries [ 82 ]. Given that cervical cancer can serve as a proxy for larger health systems issues, more detailed exploration of the barriers and best practices for increasing initial screening uptake and sustaining screening services over time may provide important insights to addressing other persisting women’s health issues and beyond, including the strengthening of broad primary health care services in low-resource settings. For programs wishing to move towards expanding the focus on their inquiry into this area, the two principal references that we have used to analyze the papers in this review are excellent resources for investigators new to implementation science [ 17 , 22 ].
Given the overwhelming supporting evidence for the effectiveness of various screening technologies, it is unsettling that high cervical cancer incidence rates persist globally. There are clear downward trends of age-standardized incidence (ASI) rates in HIC, although no clear changes by period in low-income countries [ 83 ]. Given that a successful cervical cancer control and prevention program requires a robust systems approach including reliable access to primary healthcare, referral, and follow-up services, the incidence of cervical cancer has been shown to be an indicator for larger health systems issues [ 8 ]. Therefore, the implementation of cervical cancer prevention and control programs in areas with the least resources would have the greatest immediate impact on cervical cancer ASI rates while potentially favorably impacting other primary health care services. Unfortunately, there is a gradient between reviewed studies conducted in low-income countries (5, 9.8%) and LMICs (29, 56.9%), (Fig. 1 ). This disparity may be due to the profound difficulty of implementing cervical cancer programs and conducting research in states that are unstable or where infrastructure is significantly lacking. The implementation challenges in these settings may be the greatest to overcome in order to achieve sustainability of impactful interventions. These settings, unfortunately, may also possess the greatest challenges in conducting sound science, contributing to this well-documented research gap [ 84 ]. The areas with the greatest need for developing a clear understanding of implementation are, therefore, the most neglected. The dramatic lack of research from lower-resource environments to inform practice, in part, contributes to the continued gap in outcomes in such settings. However, these challenges could be opportunities for impact as well as for building knowledge. Replicating best practices from the most challenging contexts will likely lead to the greatest impact from dissemination of such scientific pursuits.

Country map of included articles
Program managers will benefit from working closely with researchers to report lessons learnt from programs implementing cervical cancer screening services. Furthermore, we urge researchers to move beyond technological innovation as the primary scientific pursuit and incorporate, when possible, implementation strategies to overcome barriers to health systems integration and sustainability. Researchers should evaluate the implementation of cervical cancer screening programs. This will build the science and practice of how to strengthen human resources capacity, develop responsive policy, and ensure sustained utilization of cervical cancer health services in different geographical settings.
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank Medical Librarian, Linda Hassman, of Miner Library at the University of Rochester Medical Center for her valuable work in assisting us in developing and implementing our search strategy.
Abbreviations
Author contributions.
All authors contributed to the conception and design of the study (AD, JSS, MMD, EM, TS, TD) or to the acquisition, analysis, and interpretation of data (AD, MMD, EM, KP, TI, SM, AS, TS), and drafted the manuscript (AD, JSS, MMD, EM, TS) or revised it critically for content (AD, JSS, MMD, EM, TS, KP, TI, SM, AS, TD). All authors read and approved the final manuscript.
This work was supported and funded by the Centers for Disease Control and Prevention, Prevention Research Centers Program (Cooperative agreements: 1U48DP005026-01S1, 1U48DP005010-01S1, and 1U48DP005023-01S1). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This review falls under the scope of work for the US. Centers for Disease Control and Prevention (CDC) funded Global and Territorial Health Research Network through the Prevention Research Centers Program, with the goal to translate chronic disease prevention research into practice. In addition to several partnering institutions, the Global Network Steering Committee consists of two CDC representatives who participated in the study design and review of the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis. The corresponding author had final responsibility to submit the report for publication.
Data availability
Compliance with ethical standards.
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
1. HPV prevalence and type distribution in Korea. In a meta-analysis of HPV type distribution between 1995 and 2007 in Korea, the overall HPV prevalence was 23.9% (95% CI, 23.8-24.1%) in women with normal cytology compared to 95.8% (95% CI, 95.4-96.2%) in women with cervical cancer. HPV 16 was the most common type regardless of cervical ...
HPV testing is the primary screening tool for cervical cancer and has replaced traditional cytology-based guidelines. The current screening strategy in Korea does not differentiate women who have ...
Almost three-fifths of the respondents (57.1%) had a negative attitude toward cervical cancer screening. The majority (78.2%) had been counselled by healthcare workers on cervical cancer screening with good emotional support from family members (72.9%). About twothirds (68.4%) of the respondents had been screened for cervical cancer.
Cervical screening was attended every 3 years by 69% of respondents. Regular attendees were more likely to have children (p = 0.001), have experienced cancer in a close family member (p = 0.002), and were between 35-44 and 45-54 years old (p < 0.001). The main reasons for non-attendance were embarrassment, fear of the test and fear of the result.
CCPPZ has effectively increased women's engagement in screening since its inception in 2006. Customised sensitisation strategies relevant to different age groups could increase uptake and adherence to screening. The high proportion of screen positivity in women younger than 20 years with HIV requires further consideration. Our data are not able to discern if women with HIV have earlier disease ...
Cervical cancer is the second most common cancer in women aged 15-44 years in Ethiopia. The most frequent form and leading cause of cancer mortality among Ethiopian women, cervical cancer is often at an advanced stage by the time they seek screening services. 6 According to World Health Organization (WHO), screening of cervical cancer should be started at the age of 30 years and beyond that ...
1. Introduction. High-quality screening effectively reduces the incidence of invasive cervical cancer [1,2,3].This is possible due to the natural history of the disease: it develops over the years through a precancerous stage, preceded by a persistent infection with high-risk human papillomavirus (HPV) [4,5].The precancer can be detected by screening and treated before it possibly progresses ...
HPV testing is the primary screening tool for cervical cancer and has replaced traditional cytology-based guidelines. The current screening strategy in Korea does not differentiate women who have received complete vaccination from those who are unvaccinated. However, in the post-vaccination era, newly revised policies will be needed. We also ...
To achieve this goal, global scale-up of effective vaccination against the human papillomavirus (HPV) as well as screening for and treatment of cervical cancer are required. Cervical cancer ...
The World Health Organization (WHO) advocates population-based screening programs to reduce the global incidence of cervical cancer. However, screening guidelines and practice continually change ...
Globally, there are over a half million new cases of cervical cancer yearly, with nearly 90% of these cases in least developed economies [].The United States (US) 2012 cervical cancer incidence rate was 8.1 per 100,000 women, while low- and middle- income countries (LMICs) had a collective rate of 15.7 [].Cervical cancer screening programs detect pre-cancerous lesions which can be treated with ...
In November, 2020, WHO launched a global initiative to eliminate cervical cancer as a public health problem. WHO proposes a global elimination threshold of four cases per 100 000 women-years and the implementation of a triple intervention strategy, consisting of vaccinating at least 90% of girls against human papillomaviruses (HPV) by the age of 15 years, screening 70% of women using a high ...
Screening for cervical cancer is recommended for individuals with a cervix starting at age 25 years. For individuals aged 25 to 65 years, screening should be done with a primary HPV test* every 5 years. If primary HPV testing is not available, screening may be done with either cotesting that combines an HPV test with a Papanicolaou (Pap) test ...
Introduction. Cervical cancer is one of the most common cancers worldwide. In India, it is one the leading causes of mortality among women accounting for 23.3% of all cancer deaths.[] India accounts for about 20% of cervical cancer cases reported from the world.[] More than three-fourth of these patients are diagnosed in advanced stages leading to poor prospects of long term survival and cure.[]
Objectives Low-and-middle-income countries (LMICs) bear a disproportionate burden of cervical cancer mortality. We aimed to identify what is currently known about barriers to cervical cancer screening among women in LMICs and propose remedial actions. Design This was a systematic review using Medical Subject Headings (MeSH) terms in Google Scholar, PubMed, Scopus, and Web of Science databases ...
the research idea to proposal ... recommends that individuals with a cervix initiate cervical cancer screening at age 25 years and undergo primary human papillomavirus (HPV) testing every 5 years ...
Early diagnosis, effective screening, and treatment programs can reduce cervical cancer. 5-7 By 2030, it was proposed by WHO that globally, 90% of girls fully vaccinated with the HPV vaccine by age 15; 70% of women are screened with a high-performance test by 35 years of age and 45 years; and 90% of women identified with the cervical disease ...
Statistical research findings indicate that the use of cervical cancer educational approaches substantially improved cervical cancer screening rates and screening acceptability by double folds 2.88.
Smear-based population screening has been established in Ho Chi Min City at a cost of US$1 per woman. The programme is projected to reduce the incidence of invasive disease from 26 per 100 000 to 14 per 100 000 in 10 years, he said. However, in rural communities in developing countries there may be no suitable facilities to obtain a cervical smear.
2.5. Variables of the study. Cervical cancer screening utilization was the outcome variable whereas, women's age, residence, marital status, religion, education status, occupation, wealth status, health insurance, contraceptive use, history of STIs, sero-HIV status, age at first sexual intercourse, parity, duration of marriage, history of gynecologic examination, perceived barriers to undergo ...
In 2020, about 7445 women were newly identified, and 5338 deaths occurred due to cervical cancer annually. 1,5 An absence of cervical cancer screening services, limited awareness about cervical cancer screening, and an attitude toward cervical cancer screening are the key obstacles to early detection of cancer of the cervix and control in ...
Cancer Research Initiative, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa. ... We estimate the impact of the current prevention programme and alternative screening scenarios on cervical cancer incidence. The South African screening programme prevented 8600 (95%CI 4700-12 300) cervical cancer cases between 2000 and ...
Background An estimated 22 million Ethiopian women between the ages of 15 and 49 are affected by cervical cancer each year, with 7095 cases and 4732 fatalities. Cervical cancer screening is one of the prevention methods, although Ethiopia has a low coverage rate. Furthermore, data on the use of cervical cancer screening services in the country is scarce. Therefore, we aimed to assess cervical ...
Find research articles on cervical cancer, which may include news stories, clinical trials, blog posts, and descriptions of active studies. ... The rates of timely cervical cancer screening fell between 2005 and 2019, researchers found, and disparities existed among groups of women. The most common reason for not receiving timely screening was ...
Background To establish successful strategies and increasing the utilization of preventive services, there is a need to explore the extent to which the general female population is aware and use the service for cervical cancer-screening among women infected with HIV in Africa. Available evidences in this regard are controversial and non-conclusive on this potential issue and therefore, we ...
Background: High-risk human papillomavirus (hrHPV)-based cervical cancer screening in the Netherlands led to a substantial increase in number of colposcopy referrals and low-grade lesions detected. Genotyping strategies may be employed to lower the screening-related burden.
Gaps in the literature. The 2011 WHO Prioritized Research Agenda for Prevention and Control of Non-communicable Disease notes that while cancer screening services have been shown to be effective in high-income countries (HICs) [9-12] and HPV screening has been shown to reduce cervical cancer deaths in resource-limited settings such as rural India [], there are few reports describing the ...
John Zarocostas reports from Geneva. Health experts have renewed calls to improve access to cervical cancer screening, especially in low-income and middle-income countries (LMICs), as part of efforts to eliminate cervical cancer as a public health problem. "Cervical cancer is a major killer, [and] a leading cause of death in women, especially ...
First large-scale network on cancer screening launched: Cancer Screening Research Network (CSRN) Goal: To evaluate emerging cancer screening technologies (e.g., multi-cancer detection blood tests) -with the ultimate aim to save lives. CSRN is the first large scale network to focus on cancer screening. Contributes to National Cancer Plan goals