- Resources Home 🏠
- Try SciSpace Copilot
- Search research papers
- Add Copilot Extension
- Try AI Detector
- Try Paraphraser
- Try Citation Generator
- April Papers
- June Papers
- July Papers


How To Write A Research Summary

It’s a common perception that writing a research summary is a quick and easy task. After all, how hard can jotting down 300 words be? But when you consider the weight those 300 words carry, writing a research summary as a part of your dissertation, essay or compelling draft for your paper instantly becomes daunting task.
A research summary requires you to synthesize a complex research paper into an informative, self-explanatory snapshot. It needs to portray what your article contains. Thus, writing it often comes at the end of the task list.
Regardless of when you’re planning to write, it is no less of a challenge, particularly if you’re doing it for the first time. This blog will take you through everything you need to know about research summary so that you have an easier time with it.
What is a Research Summary?
A research summary is the part of your research paper that describes its findings to the audience in a brief yet concise manner. A well-curated research summary represents you and your knowledge about the information written in the research paper.
While writing a quality research summary, you need to discover and identify the significant points in the research and condense it in a more straightforward form. A research summary is like a doorway that provides access to the structure of a research paper's sections.
Since the purpose of a summary is to give an overview of the topic, methodology, and conclusions employed in a paper, it requires an objective approach. No analysis or criticism.
Research summary or Abstract. What’s the Difference?
They’re both brief, concise, and give an overview of an aspect of the research paper. So, it’s easy to understand why many new researchers get the two confused. However, a research summary and abstract are two very different things with individual purpose. To start with, a research summary is written at the end while the abstract comes at the beginning of a research paper.
A research summary captures the essence of the paper at the end of your document. It focuses on your topic, methods, and findings. More like a TL;DR, if you will. An abstract, on the other hand, is a description of what your research paper is about. It tells your reader what your topic or hypothesis is, and sets a context around why you have embarked on your research.
Getting Started with a Research Summary
Before you start writing, you need to get insights into your research’s content, style, and organization. There are three fundamental areas of a research summary that you should focus on.
- While deciding the contents of your research summary, you must include a section on its importance as a whole, the techniques, and the tools that were used to formulate the conclusion. Additionally, there needs to be a short but thorough explanation of how the findings of the research paper have a significance.
- To keep the summary well-organized, try to cover the various sections of the research paper in separate paragraphs. Besides, how the idea of particular factual research came up first must be explained in a separate paragraph.
- As a general practice worldwide, research summaries are restricted to 300-400 words. However, if you have chosen a lengthy research paper, try not to exceed the word limit of 10% of the entire research paper.
How to Structure Your Research Summary
The research summary is nothing but a concise form of the entire research paper. Therefore, the structure of a summary stays the same as the paper. So, include all the section titles and write a little about them. The structural elements that a research summary must consist of are:
It represents the topic of the research. Try to phrase it so that it includes the key findings or conclusion of the task.
The abstract gives a context of the research paper. Unlike the abstract at the beginning of a paper, the abstract here, should be very short since you’ll be working with a limited word count.
Introduction
This is the most crucial section of a research summary as it helps readers get familiarized with the topic. You should include the definition of your topic, the current state of the investigation, and practical relevance in this part. Additionally, you should present the problem statement, investigative measures, and any hypothesis in this section.
Methodology
This section provides details about the methodology and the methods adopted to conduct the study. You should write a brief description of the surveys, sampling, type of experiments, statistical analysis, and the rationality behind choosing those particular methods.
Create a list of evidence obtained from the various experiments with a primary analysis, conclusions, and interpretations made upon that. In the paper research paper, you will find the results section as the most detailed and lengthy part. Therefore, you must pick up the key elements and wisely decide which elements are worth including and which are worth skipping.
This is where you present the interpretation of results in the context of their application. Discussion usually covers results, inferences, and theoretical models explaining the obtained values, key strengths, and limitations. All of these are vital elements that you must include in the summary.
Most research papers merge conclusion with discussions. However, depending upon the instructions, you may have to prepare this as a separate section in your research summary. Usually, conclusion revisits the hypothesis and provides the details about the validation or denial about the arguments made in the research paper, based upon how convincing the results were obtained.
The structure of a research summary closely resembles the anatomy of a scholarly article . Additionally, you should keep your research and references limited to authentic and scholarly sources only.
Tips for Writing a Research Summary
The core concept behind undertaking a research summary is to present a simple and clear understanding of your research paper to the reader. The biggest hurdle while doing that is the number of words you have at your disposal. So, follow the steps below to write a research summary that sticks.
1. Read the parent paper thoroughly
You should go through the research paper thoroughly multiple times to ensure that you have a complete understanding of its contents. A 3-stage reading process helps.
a. Scan: In the first read, go through it to get an understanding of its basic concept and methodologies.
b. Read: For the second step, read the article attentively by going through each section, highlighting the key elements, and subsequently listing the topics that you will include in your research summary.
c. Skim: Flip through the article a few more times to study the interpretation of various experimental results, statistical analysis, and application in different contexts.
Sincerely go through different headings and subheadings as it will allow you to understand the underlying concept of each section. You can try reading the introduction and conclusion simultaneously to understand the motive of the task and how obtained results stay fit to the expected outcome.
2. Identify the key elements in different sections
While exploring different sections of an article, you can try finding answers to simple what, why, and how. Below are a few pointers to give you an idea:
- What is the research question and how is it addressed?
- Is there a hypothesis in the introductory part?
- What type of methods are being adopted?
- What is the sample size for data collection and how is it being analyzed?
- What are the most vital findings?
- Do the results support the hypothesis?
Discussion/Conclusion
- What is the final solution to the problem statement?
- What is the explanation for the obtained results?
- What is the drawn inference?
- What are the various limitations of the study?
3. Prepare the first draft
Now that you’ve listed the key points that the paper tries to demonstrate, you can start writing the summary following the standard structure of a research summary. Just make sure you’re not writing statements from the parent research paper verbatim.
Instead, try writing down each section in your own words. This will not only help in avoiding plagiarism but will also show your complete understanding of the subject. Alternatively, you can use a summarizing tool (AI-based summary generators) to shorten the content or summarize the content without disrupting the actual meaning of the article.
SciSpace Copilot is one such helpful feature! You can easily upload your research paper and ask Copilot to summarize it. You will get an AI-generated, condensed research summary. SciSpace Copilot also enables you to highlight text, clip math and tables, and ask any question relevant to the research paper; it will give you instant answers with deeper context of the article..
4. Include visuals
One of the best ways to summarize and consolidate a research paper is to provide visuals like graphs, charts, pie diagrams, etc.. Visuals make getting across the facts, the past trends, and the probabilistic figures around a concept much more engaging.
5. Double check for plagiarism
It can be very tempting to copy-paste a few statements or the entire paragraphs depending upon the clarity of those sections. But it’s best to stay away from the practice. Even paraphrasing should be done with utmost care and attention.
Also: QuillBot vs SciSpace: Choose the best AI-paraphrasing tool
6. Religiously follow the word count limit
You need to have strict control while writing different sections of a research summary. In many cases, it has been observed that the research summary and the parent research paper become the same length. If that happens, it can lead to discrediting of your efforts and research summary itself. Whatever the standard word limit has been imposed, you must observe that carefully.
7. Proofread your research summary multiple times
The process of writing the research summary can be exhausting and tiring. However, you shouldn’t allow this to become a reason to skip checking your academic writing several times for mistakes like misspellings, grammar, wordiness, and formatting issues. Proofread and edit until you think your research summary can stand out from the others, provided it is drafted perfectly on both technicality and comprehension parameters. You can also seek assistance from editing and proofreading services , and other free tools that help you keep these annoying grammatical errors at bay.
8. Watch while you write
Keep a keen observation of your writing style. You should use the words very precisely, and in any situation, it should not represent your personal opinions on the topic. You should write the entire research summary in utmost impersonal, precise, factually correct, and evidence-based writing.
9. Ask a friend/colleague to help
Once you are done with the final copy of your research summary, you must ask a friend or colleague to read it. You must test whether your friend or colleague could grasp everything without referring to the parent paper. This will help you in ensuring the clarity of the article.
Once you become familiar with the research paper summary concept and understand how to apply the tips discussed above in your current task, summarizing a research summary won’t be that challenging. While traversing the different stages of your academic career, you will face different scenarios where you may have to create several research summaries.
In such cases, you just need to look for answers to simple questions like “Why this study is necessary,” “what were the methods,” “who were the participants,” “what conclusions were drawn from the research,” and “how it is relevant to the wider world.” Once you find out the answers to these questions, you can easily create a good research summary following the standard structure and a precise writing style.
You might also like

Consensus GPT vs. SciSpace GPT: Choose the Best GPT for Research

Literature Review and Theoretical Framework: Understanding the Differences

Types of Essays in Academic Writing - Quick Guide (2024)
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- QuestionPro

- Solutions Industries Gaming Automotive Sports and events Education Government Travel & Hospitality Financial Services Healthcare Cannabis Technology Use Case NPS+ Communities Audience Contactless surveys Mobile LivePolls Member Experience GDPR Positive People Science 360 Feedback Surveys
- Resources Blog eBooks Survey Templates Case Studies Training Help center
Home Surveys Academic Research
Research Summary: What is it & how to write one

The Research Summary is used to report facts about a study clearly. You will almost certainly be required to prepare a research summary during your academic research or while on a research project for your organization.
If it is the first time you have to write one, the writing requirements may confuse you. The instructors generally assign someone to write a summary of the research work. Research summaries require the writer to have a thorough understanding of the issue.
This article will discuss the definition of a research summary and how to write one.
What is a research summary?
A research summary is a piece of writing that summarizes your research on a specific topic. Its primary goal is to offer the reader a detailed overview of the study with the key findings. A research summary generally contains the article’s structure in which it is written.
You must know the goal of your analysis before you launch a project. A research overview summarizes the detailed response and highlights particular issues raised in it. Writing it might be somewhat troublesome. To write a good overview, you want to start with a structure in mind. Read on for our guide.
Why is an analysis recap so important?
Your summary or analysis is going to tell readers everything about your research project. This is the critical piece that your stakeholders will read to identify your findings and valuable insights. Having a good and concise research summary that presents facts and comes with no research biases is the critical deliverable of any research project.
We’ve put together a cheat sheet to help you write a good research summary below.
Research Summary Guide
- Why was this research done? – You want to give a clear description of why this research study was done. What hypothesis was being tested?
- Who was surveyed? – The what and why or your research decides who you’re going to interview/survey. Your research summary has a detailed note on who participated in the study and why they were selected.
- What was the methodology? – Talk about the methodology. Did you do face-to-face interviews? Was it a short or long survey or a focus group setting? Your research methodology is key to the results you’re going to get.
- What were the key findings? – This can be the most critical part of the process. What did we find out after testing the hypothesis? This section, like all others, should be just facts, facts facts. You’re not sharing how you feel about the findings. Keep it bias-free.
- Conclusion – What are the conclusions that were drawn from the findings. A good example of a conclusion. Surprisingly, most people interviewed did not watch the lunar eclipse in 2022, which is unexpected given that 100% of those interviewed knew about it before it happened.
- Takeaways and action points – This is where you bring in your suggestion. Given the data you now have from the research, what are the takeaways and action points? If you’re a researcher running this research project for your company, you’ll use this part to shed light on your recommended action plans for the business.
LEARN ABOUT: Action Research
If you’re doing any research, you will write a summary, which will be the most viewed and more important part of the project. So keep a guideline in mind before you start. Focus on the content first and then worry about the length. Use the cheat sheet/checklist in this article to organize your summary, and that’s all you need to write a great research summary!
But once your summary is ready, where is it stored? Most teams have multiple documents in their google drives, and it’s a nightmare to find projects that were done in the past. Your research data should be democratized and easy to use.
We at QuestionPro launched a research repository for research teams, and our clients love it. All your data is in one place, and everything is searchable, including your research summaries!
Authors: Prachi, Anas
MORE LIKE THIS

Customer Communication Tool: Types, Methods, Uses, & Tools
Apr 23, 2024

Top 12 Sentiment Analysis Tools for Understanding Emotions

QuestionPro BI: From Research Data to Actionable Dashboards
Apr 22, 2024

21 Best Customer Experience Management Software in 2024
Other categories.
- Academic Research
- Artificial Intelligence
- Assessments
- Brand Awareness
- Case Studies
- Communities
- Consumer Insights
- Customer effort score
- Customer Engagement
- Customer Experience
- Customer Loyalty
- Customer Research
- Customer Satisfaction
- Employee Benefits
- Employee Engagement
- Employee Retention
- Friday Five
- General Data Protection Regulation
- Insights Hub
- Life@QuestionPro
- Market Research
- Mobile diaries
- Mobile Surveys
- New Features
- Online Communities
- Question Types
- Questionnaire
- QuestionPro Products
- Release Notes
- Research Tools and Apps
- Revenue at Risk
- Survey Templates
- Training Tips
- Uncategorized
- Video Learning Series
- What’s Coming Up
- Workforce Intelligence
- Research Summary: What Is It & How To Write One

Introduction
A research summary is a requirement during academic research and sometimes you might need to prepare a research summary during a research project for an organization.
Most people find a research summary a daunting task as you are required to condense complex research material into an informative, easy-to-understand article most times with a minimum of 300-500 words.
In this post, we will guide you through all the steps required to make writing your research summary an easier task.
What is a Research Summary?
A research summary is a piece of writing that summarizes the research of a specific topic into bite-size easy-to-read and comprehend articles. The primary goal is to give the reader a detailed outline of the key findings of a research.
It is an unavoidable requirement in colleges and universities. To write a good research summary, you must understand the goal of your research, as this would help make the process easier.
A research summary preserves the structure and sections of the article it is derived from.
Research Summary or Abstract: What’s The Difference?
The Research Summary and Abstract are similar, especially as they are both brief, straight to the point, and provide an overview of the entire research paper. However, there are very clear differences.
To begin with, a Research summary is written at the end of a research activity, while the Abstract is written at the beginning of a research paper.
A Research Summary captures the main points of a study, with an emphasis on the topic, method , and discoveries, an Abstract is a description of what your research paper would talk about and the reason for your research or the hypothesis you are trying to validate.
Let us take a deeper look at the difference between both terms.
What is an Abstract?
An abstract is a short version of a research paper. It is written to convey the findings of the research to the reader. It provides the reader with information that would help them understand the research, by giving them a clear idea about the subject matter of a research paper. It is usually submitted before the presentation of a research paper.
What is a Summary?
A summary is a short form of an essay, a research paper, or a chapter in a book. A research summary is a narration of a research study, condensing the focal points of research to a shorter form, usually aligned with the same structure of the research study, from which the summary is derived.
What Is The Difference Between an Abstract and a Summary?
An abstract communicates the main points of a research paper, it includes the questions, major findings, the importance of the findings, etc.
An abstract reflects the perceptions of the author about a topic, while a research summary reflects the ideology of the research study that is being summarized.
Getting Started with a Research Summary
Before commencing a research summary, there is a need to understand the style and organization of the content you plan to summarize. There are three fundamental areas of the research that should be the focal point:
- When deciding on the content include a section that speaks to the importance of the research, and the techniques and tools used to arrive at your conclusion.
- Keep the summary well organized, and use paragraphs to discuss the various sections of the research.
- Restrict your research to 300-400 words which is the standard practice for research summaries globally. However, if the research paper you want to summarize is a lengthy one, do not exceed 10% of the entire research material.
Once you have satisfied the requirements of the fundamentals for starting your research summary, you can now begin to write using the following format:
- Why was this research done? – A clear description of the reason the research was embarked on and the hypothesis being tested.
- Who was surveyed? – Your research study should have details of the source of your information. If it was via a survey, you should document who the participants of the survey were and the reason that they were selected.
- What was the methodology? – Discuss the methodology, in terms of what kind of survey method did you adopt. Was it a face-to-face interview, a phone interview, or a focus group setting?
- What were the key findings? – This is perhaps the most vital part of the process. What discoveries did you make after the testing? This part should be based on raw facts free from any personal bias.
- Conclusion – What conclusions did you draw from the findings?
- Takeaways and action points – This is where your views and perception can be reflected. Here, you can now share your recommendations or action points.
- Identify the focal point of the article – In other to get a grasp of the content covered in the research paper, you can skim the article first, in a bid to understand the most essential part of the research paper.
- Analyze and understand the topic and article – Writing a summary of a research paper involves being familiar with the topic – the current state of knowledge, key definitions, concepts, and models. This is often gleaned while reading the literature review. Please note that only a deep understanding ensures efficient and accurate summarization of the content.
- Make notes as you read – Highlight and summarize each paragraph as you read. Your notes are what you would further condense to create a draft that would form your research summary.
How to Structure Your Research Summary
- Title – This highlights the area of analysis, and can be formulated to briefly highlight key findings.
- Abstract – this is a very brief and comprehensive description of the study, required in every academic article, with a length of 100-500 words at most.
- Introduction – this is a vital part of any research summary, it provides the context and the literature review that gently introduces readers to the subject matter. The introduction usually covers definitions, questions, and hypotheses of the research study.
- Methodology –This section emphasizes the process and or data analysis methods used, in terms of experiments, surveys, sampling, or statistical analysis.
- Results section – this section lists in detail the results derived from the research with evidence obtained from all the experiments conducted.
- Discussion – these parts discuss the results within the context of current knowledge among subject matter experts. Interpretation of results and theoretical models explaining the observed results, the strengths of the study, and the limitations experienced are going to be a part of the discussion.
- Conclusion – In a conclusion, hypotheses are discussed and revalidated or denied, based on how convincing the evidence is.
- References – this section is for giving credit to those who work you studied to create your summary. You do this by providing appropriate citations as you write.
Research Summary Example 1
Below are some defining elements of a sample research summary.
Title – “The probability of an unexpected volcanic eruption in Greenwich”
Introduction – this section would list the catastrophic consequences that occurred in the country and the importance of analyzing this event.
Hypothesis – An eruption of the Greenwich supervolcano would be preceded by intense preliminary activity manifesting in advance, before the eruption.
Results – these could contain a report of statistical data from various volcanic eruptions happening globally while looking critically at the activity that occurred before these events.
Discussion and conclusion – Given that Greenwich is now consistently monitored by scientists and that signs of an eruption are usually detected before the volcanic eruption, this confirms the hypothesis. Hence creating an emergency plan outlining other intervention measures and ultimately evacuation is essential.
Research Summary Example 2
Below is another sample sketch.
Title – “The frequency of extreme weather events in the UK in 2000-2008 as compared to the ‘60s”
Introduction – Weather events bring intense material damage and cause pain to the victims affected.
Hypothesis – Extreme weather events are more frequent in recent times compared to the ‘50s
Results – The frequency of several categories of extreme events now and then are listed here, such as droughts, fires, massive rainfall/snowfalls, floods, hurricanes, tornadoes, etc.
Discussion and conclusion – Several types of extreme events have become more commonplace in recent times, confirming the hypothesis. This rise in extreme weather events can be traced to rising CO2 levels and increasing temperatures and global warming explain the rising frequency of these disasters. Addressing the rising CO2 levels and paying attention to climate change is the only to combat this phenomenon.
A research summary is the short form of a research paper, analyzing the important aspect of the study. Everyone who reads a research summary has a full grasp of the main idea being discussed in the original research paper. Conducting any research means you will write a summary, which is an important part of your project and would be the most read part of your project.
Having a guideline before you start helps, this would form your checklist which would guide your actions as you write your research summary. It is important to note that a Research Summary is different from an Abstract paper written at the beginning of a research paper, describing the idea behind a research paper.

Connect to Formplus, Get Started Now - It's Free!
- abstract in research papers
- abstract writing
- action research
- research summary
- research summary vs abstract
- research surveys
- Angela Kayode-Sanni

You may also like:
Action Research: What it is, Stages & Examples
Introduction Action research is an evidence-based approach that has been used for years in the field of education and social sciences....

The McNamara Fallacy: How Researchers Can Detect and to Avoid it.
Introduction The McNamara Fallacy is a common problem in research. It happens when researchers take a single piece of data as evidence...
Research Questions: Definitions, Types + [Examples]
A comprehensive guide on the definition of research questions, types, importance, good and bad research question examples
How to Write An Abstract For Research Papers: Tips & Examples
In this article, we will share some tips for writing an effective abstract, plus samples you can learn from.
Formplus - For Seamless Data Collection
Collect data the right way with a versatile data collection tool. try formplus and transform your work productivity today..
- IRB-SBS Home
- Contact IRB-SBS
- IRB SBS Staff Directory
- IRB SBS Board Members
- About the IRB-SBS
- CITI Training
- Education Events
- Virginia IRB Consortium
- IRB-SBS Learning Shots
- HRPP Education & Training
- Student Support
- Access iProtocol
- Getting Started
- iProtocol Question Guide
- iProtocol Management
- Protocol Review Process
- Certificate of Confidentiality
- Deception and/or Withholding Information from a Participant
- Ethnographic Research
- IRB-SBS 101
- IRB-SBS Glossary
- Participant Pools
- Paying Participants
- Research in an Educational Setting
- Research in an International Setting and/or Location
- Risk-Sensitive Populations
- Student Researchers and Faculty Sponsors
- Study Funding and the IRB
- Understanding Risk in Research
- Vulnerable Participants
- IRB-SBS PAM & Ed
- Federal Regulations
- Ethical Principals
- Partner Offices
- Determining Human Subjects Research
- Determining HSR or SBS

Participant Summary
The Participant Summary section assembles all of the created Participant Groups and combines the number of participants provided in each group into a grand total. In addition it provides an opportunity to highlight the experience of the principal investigator and the research team (where applicable), as well as describe the relationship that the principal investigator and any other individuals associated with the study have with the participants.
Researcher experience
Researcher experience is an important factor when the IRB-SBS weighs the risks of the study against its potential benefits. An experienced researcher who is familiar with a population and knows how to work sensitively with them is far more likely to yield generalizable results while understanding how to best protect a participant as compared with an undergraduate student embarking on his or her first study. That said, it doesn’t mean that the undergraduate’s researcher isn’t valuable but rather the researcher may need to design a study that has reduced risk and also needs to demonstrate that he or she has adequate supervision through their faculty sponsor. Providing a good description of the principal investigator’s experience as well as the rest of the research team (where applicable) can help the IRB-SBS have a better understanding of the overall experience level of the group and how prepared they are to work with their proposed participants.
Researcher relationships
Providing consent free of coercion is a central tenant of the ethical foundation for human subjects research. Even though a researcher may assure a participant that they can participate without pressure, simply being a subordinate of the researcher may make the participant feel compelled to participate. The IRB-SBS will want to know if anyone on the research team holds any authority over their participants or if there is any other type of relationship between participants and researchers. Being in a position of authority doesn’t necessarily preclude a researcher from including a participant, but the IRB-SBS may require specific steps be put in place to create layers of separation between the researcher and participant.
In addition, the final question asks about financial relationships. It is important for the IRB-SBS to understand any relevant financial relationships that may create a conflict of interest for researchers.
- The questions have been answered adequately.
- The researcher will take appropriate precautions to protect participants from coercion.
- The researcher’s experience is appropriate for the study.
- Funding and the IRB-SBS
Bulk Content Generator
Brand Voice
AI Text Editor
Research Summary Generator
Craft a detailed research synopsis utilizing the given data for an all-encompassing understanding.
Try Research Summary for free →
Research Summary
Learn how to provide the key points, main findings, and any other relevant information for the research you want to summarize
1 variation
What is a Research Summary?
Have you ever found yourself drowning in a sea of research articles, struggling to make sense of it all? Well, fear not! A research summary is here to save the day. But what exactly is a research summary, and how can it help you navigate the vast ocean of information?
A research summary is a concise and informative overview of a research article, report, or thesis. It aims to provide the reader with a clear understanding of the study's purpose, methods, results, and conclusions without having to read the entire document. Think of it as a mini-version of the original work that highlights its most important aspects.
Now that we know what a research summary is let's dive into why they're so beneficial.
The Benefits of Research Summaries
Research summaries offer several advantages for both readers and writers:
Time-saving : Reading a well-written research summary can save you hours of sifting through dense academic papers. It allows you to quickly grasp the key points and decide if you want to explore the full document further.
Improved comprehension : By breaking down complex ideas into digestible chunks, research summaries make it easier for readers to understand the material. This is particularly helpful for those who are new to a topic or have limited knowledge in the field.
Enhanced communication : Research summaries enable researchers to share their findings with a wider audience, including non-experts and industry professionals. This can lead to increased collaboration and knowledge exchange across disciplines.
Better decision-making : For professionals who rely on evidence-based practices, research summaries provide an accessible way to stay informed about the latest developments in their field. This enables them to make better decisions based on up-to-date information.
With these benefits in mind, let's explore some tips for writing an effective research summary.
Tips for Writing a Great Research Summary
Creating an engaging and informative research summary doesn't have to be a daunting task. Here are some tips to help you craft the perfect summary:
Know your audience : Consider who will be reading your summary and tailor your language and content accordingly. If you're writing for a general audience, avoid jargon and technical terms. If your readers are experts in the field, focus on the most relevant and novel aspects of the research.
Be concise : A research summary should be brief yet informative. Aim to capture the essence of the study without getting bogged down in unnecessary details.
Use clear language : Write in simple, straightforward sentences that are easy to understand. Avoid flowery language or complex sentence structures that may confuse readers.
Highlight key points : Focus on the main elements of the study, such as its purpose, methods, results, and conclusions. Make sure these points stand out by using headings, bullet points, or bold text.
Stay objective : Present the information in a neutral tone and avoid expressing personal opinions or biases. Stick to the facts and let your readers draw their own conclusions.
Proofread : Before submitting your research summary, take the time to proofread it carefully for grammar, spelling, and punctuation errors. A polished summary will make a better impression on your readers.
Generate the Perfect Research Summary with Our Research Summary Generator
Now that we've covered what a research summary is, its benefits, and tips for writing one – wouldn't it be great if there was a tool that could generate a perfect research summary every single time? Well, guess what? There is!
With our Research Summary Generator, you can create an engaging and informative summary in just a few clicks. Say goodbye to hours spent poring over dense academic papers and hello to quick, easy-to-understand summaries tailored to your needs.
Give it a try today and see how our Research Summary Generator can revolutionize your research process!
Example outputs
This Research Summary Generator saves you time and effort by summarizing your research findings in a clear and concise manner, allowing you to easily communicate your results to others.
The Effects of Exercise on Mental Health
A study was conducted to investigate the effects of exercise on mental health. Participants were randomly assigned to either an exercise group or a control group. The exercise group engaged in moderate-intensity aerobic exercise for 30 minutes, three times per week for eight weeks. The results showed that the exercise group had significantly lower levels of depression and anxiety compared to the control group.
Keywords: exercise, mental health, depression, anxiety
The impact of social media on body image.
This study aimed to examine the impact of social media on body image. A sample of young adults completed surveys assessing their use of social media and their perceptions of their own body image. Results indicated that individuals who spent more time on social media reported greater dissatisfaction with their bodies. Additionally, exposure to images of thin and fit individuals on social media was associated with increased body dissatisfaction.
Keywords: social media, body image, young adults, body dissatisfaction
The benefits of meditation for stress reduction.
This meta-analysis aimed to evaluate the effectiveness of meditation for stress reduction. A total of 18 randomized controlled trials were included in the analysis. Results showed that meditation was effective in reducing perceived stress, with larger effect sizes observed for mindfulness-based interventions. Furthermore, the benefits of meditation appeared to be maintained over time.
Keywords: meditation, stress reduction, mindfulness, meta-analysis
What other amazing things can this template help you create.
✔ Meta Title
✔ Meta Description
✔ Extract keywords
✔ Feature Image
✔ Soon Internal Linking
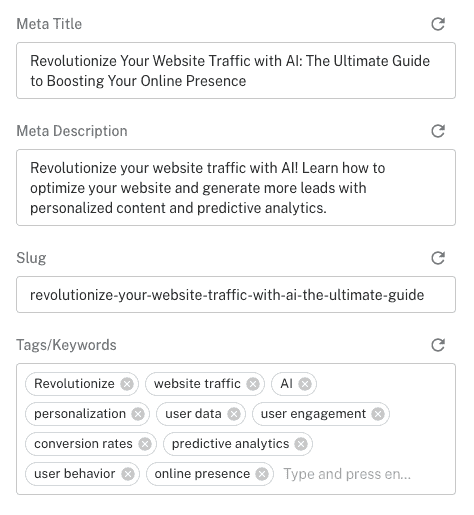
Who needs Research Summary Generator?
Researchers
Graduate students
Business professionals
Frequently asked questions
- How does the Research Summary Generator work? Simply input your research findings into the generator, and it will automatically summarize them in a clear and concise manner.
- Can I customize the generated summary? Yes, you can edit the summary as needed to ensure it accurately reflects your research findings.
- Is the Research Summary Generator free to use? Yes, the generator is completely free to use with no limitations.
This site uses session cookies and persistent cookies to improve the content and structure of the site.
By clicking “ Accept All Cookies ”, you agree to the storing of cookies on this device to enhance site navigation and content, analyse site usage, and assist in our marketing efforts.
By clicking ' See cookie policy ' you can review and change your cookie preferences and enable the ones you agree to.
By dismissing this banner , you are rejecting all cookies and therefore we will not store any cookies on this device.
Communicating study findings to participants: guidance
This guidance is about communicating study findings (results) to participants. As well as making study findings public , findings should be shared directly to those who participated in the study. Communicating study findings is different to communicating individual health-related findings. Study findings refer to interim or overall results of a study, whereas individual health-related findings refer to results specific to a participant. We have separate guidance for communicating individual health-related findings to participants.
This guidance is for researchers, chief investigators, funders, and sponsors who are responsible for sharing findings to participants. All types of research studies should consider communicating study findings to participants. The UK Policy Framework for Health and Social Care Research states that study findings should be published as well as being summarised and shared with those who took part.. This guidance will help you to plan the best way to do this.
This guidance covers:
- why it’s important to communicate findings to participants
- why it’s important to plan it early on
- what to consider when communicating findings to participants
- useful resources to help you
Why it’s important to communicate findings
Research participants have a right to know the findings of the research study they took part in. Sharing research findings should go beyond publishing results in registries or academic journals, where technical or academic language is used that participants may not understand. Sharing findings directly with participants helps to build trust, show respect, and helps participants to feel valued.
Why it’s important to plan it early on
How you’ll share findings with your participants should form part of the study plan. You may need to factor it into your funding application. You’ll also need to include information about your proposed approach in your application for approvals and participant information sheets . When planning, you’ll need to consider the following:
a) Public Involvement
You should involve relevant people early in the design of your study. This may mean involving patients, service users, carers, other advocates or members of the public. Engaging with relevant people early on will help you to decide how to share findings with participants. Our public involvement webpages have lots of information and guidance to help you.
Sharing findings with participants may require additional funding and resources. You may need to include these in grant or fund applications. You may prefer to consider low cost options, such as sharing information electronically. Discuss this with your public involvement group.
c) Including information in your participant information sheets and approval application
Though the material used for sharing study findings does not need to be submitted for regulatory review, your research application should include an outline about how you plan to share findings to participants.
You should set out your plans to communicate findings to participants in the participant information . Make participants aware of the likely timing of communication about findings so that they know when and how to expect this information.
It is important that you comply with UK GDPR and data protection legislation. To meet the transparency principle, you should explain to participants how you will collect, store and use their contact details, and how and when you will communicate findings.
Participants should not be surprised by receiving study findings or how they have been sent to them. To comply with the legislation, you should make sure you minimise the amount of personal data you use. That means you should only use another organisation to manage communication if other methods are not feasible. If you are using another organisation to manage communication to participants, you should make sure that this is explained to participants. This should include clear information about how and why the other organisation will use their data, and what controls are in place.
You should also make participants aware of their right to object to the processing of their personal data for the communication of research findings.
Participants should have a choice as to whether they receive findings and should be allowed to record if they change their mind during the study.
If you later change your plan for communicating findings to participants, or if you didn’t include details in your initial application but later decide to, then you’ll need to submit an amendment. The amendment should include:
- details of how and when you plan to communicate findings,
- a process to record participants’ choice about receiving findings or not,
- if the study is still recruiting, then you will also need to update the general consent form and participant information.
What to consider when communicating findings to participants
The following information outlines the different aspects to consider when planning your communication of findings. Discuss these aspects with your public involvement contributors at the planning and design stage of your study.
a) who will receive the findings
You should offer a summary of study findings directly to participants. This is in addition to any findings made publicly available.
In some instances, it may be expected that participants could die or lose capacity during the study. In these cases, it may be appropriate to share findings with participants’ relatives or carers. Give immediate family members/carers the choice. Plan early for this to ensure you receive the appropriate consent for storing contact details for this purpose or have put other appropriate mechanisms in place to allow you to share results with participants.
If your participants are children you should also consider how you’ll adapt your communication to ensure it is accessible for them and their family members/carers.
b) how you’ll communicate the findings
There are various ways to share findings with participants. It’s valuable to discuss the options with your public involvement group. Commonly used methods are:
- end of study information sheet or newsletter
- verbal information provided at study visits
- study websites
- study social media accounts
The way you communicate will depend on the type of study and your audience. For example, digital options may not be accessible for everyone – some participants may not be computer literate or may not want to use technology to access findings. Where using a digital option, it may be beneficial for participants if this is also supplemented with hard copy information.
You should consider who your audience is so that you can tailor your communication to be effective. To ensure the findings will be understood by your audience, consider using different versions for different audiences, such as children or adults lacking capacity. You might want to adjust how much detail you provide on your findings depending on the audience.
Where your research study does not collect contact details of participants, it may not be possible to share findings directly to participants. You may wish to include details in the participant information sheet about where findings will be published, so participants can choose to read the findings if they want to. For other types of studies, it may be appropriate to collect email addresses and provide updates that way. Alternatively, you may, with appropriate consent, arrange for a third party to hold contact details solely for the purpose of communicating study updates and findings. For in-person studies, you may decide to share updates and findings during study visits.
Regardless of the type of study and your audience, write your findings in lay language. We provide further guidance on how to write a plain language summary of your research findings .
c) giving participants a choice
While most participants/their relatives or carers will want to receive study findings, some may not. Those who might not wish to receive findings or updates should be given a choice at the start of the study and again during it. You should plan for how people can indicate their choice during the study.
Your participant-facing information should include details about how and when you’ll share study findings. Use the consent form to record the choice participants/their relatives or carers make about receiving findings. You’ll need to take this into consideration when deciding your method for communicating findings.
You need to take three different aspects into account when thinking about how participants or their relatives or carers can make choices about receiving study findings.
- Common law duty of confidentiality – people’s contact details are part of their health and care data when used alongside information about their health or care. That means that you need to get their consent to use these details for sending information about study findings.
- UK GDPR and other legislation – you do not need to obtain GDPR-compliant consent, because your organisation can rely on public task or legitimate interests to send out research findings. That means that the consent for sending study findings can be kept simple and does not need to meet all the legal requirements for consent under GDPR. Communicating findings to individuals about research they have been involved in is not direct marketing if such an activity falls within your organisation’s task or function. Therefore, you don’t need to comply with the direct marketing consent requirement or other rules under the Privacy and Electronic Communications Regulations ( PECR ).
- Ethics – it’s important that people feel that they can make choices, and that they can change their mind at any time.
The choices about receiving study findings should be separate from the choices about participating in the study. You should consider whether to offer people choices about how they will be contacted, for example by text, email or post as appropriate.
Participants might change their minds as the study progresses. It’s important that you plan to give participants another opportunity to either receive study findings or not. You should make it clear how people can change their choices about receiving study findings, and you should make sure that you can add or remove people from your communication system in accordance with their wishes. Consider also what you’ll do if a participant withdraws during your study. They may decide that they no longer want to receive study findings.
d) responsibility for communicating findings
This will depend on the way you’re sharing the findings. If sharing findings in-person during a study visit, the site team or usual care team may need to take responsibility for this process. If sharing findings via email or post, we recommend that the central study team takes responsibility. When planning your study, decide who is taking responsibility for sharing findings and how this will be funded and resourced. Where you will rely on local teams to provide findings to participants, you should discuss the logistics with representative parties. You can formalise arrangements in study site agreements and contracts.
Your public involvement group may have a preference about who is best placed to communicate study findings. Some findings could be upsetting to participants, for example if they find out that the intervention had no effect, or that study findings were negative or inconclusive. You should still offer to share these findings to participants, explaining that their contribution was important and worthwhile. If you’re communicating findings that may distress or upset participants, consider communicating more individually, such as through a discussion with a research nurse or doctor. When you communicate findings, make sure to include the details of someone that participants can get in touch with if they have any questions or want to discuss the findings in more detail. That person should also be able to point people directly to the sponsor’s Data Protection Officer, or the latter’s details should be provided at the bottom of each communication (along with a reminder of how to withdraw consent from receiving future communications of the same type).
e) exceptions
There may be occasions where it isn’t possible to share study findings directly to participants. For example, a study using anonymised tissue samples or a study using patient identifiable data without consent (approved by the Confidentiality Advice Group under section 251 of the NHS Act 2006) may not be able to share findings to participants. Where you cannot provide findings to participants directly, you should make this clear in your participant-facing information or patient notification materials . Where it’s not possible to share findings directly to participants, you should still publish findings. It may be possible to provide a website link to where the findings will be shared, and include this in participant-facing information at the start of the study.
f) when to communicate findings
When and how often you communicate findings depends on the type of research. For some studies, it will be possible to share interim findings or updates throughout the duration of the study, whereas other studies will only have findings right at the end. For a study with several phases or lots of endpoints, you may want to share findings for each of these as the study progresses. If doing so, it’s important to consider how sharing of results at multiple timepoints may influence the overall study design.
You might want to stagger information, giving participants some information when their involvement in the study is over and some at the end of the study. Keeping in contact with participants in this way helps them feel connected to the research even though their involvement has ended. You should discuss this with your public involvement group.
g) evaluate your communication
More research is needed to evaluate communication of findings strategies and determine best practice. Where possible, you should evaluate your communication methods (ideally in a randomised Study Within a Trial) to establish how effective your strategy is, and whether it has had the impact that you wanted. Depending on when you feedback results to participants, your strategy could become a retention intervention in and of itself.
Useful Resources
- Parkinson’s UK has a research communications toolkit developed with the HRA and our Research Ethics Committees. The Staying Connected Toolkit is a resource for research teams to use to achieve gold standard practices for communicating with participants throughout the course of the study.
This toolkit covers:
- general principles and timelines for communicating with participants throughout their research journey
- how to plan research communications ahead of time
- simple standardised tools and templates to help researchers to easily communicate with participants during the study
- guidance on how to build a sustainable relationship with the research participant community
2. The RECAP project provides stakeholder-informed guidance on sharing summaries of trial findings
3. The Show RESPECT study looks at the best ways to share study findings with participants
4. The Knowledge Mobilisation Alliance have created an infographic sharing 10 recommendations for communicating research findings to patients and the public
5. Our guidance on developing a plain language summary of your research findings
6. Our guidance on consent and UK GDPR
7. Guidance on What is and isn’t direct marketing? by NHS Transformation Directorate (england.nhs.uk).
Acknowledgements
We would like to thank Professor Katie Gillies from the University of Aberdeen, Professor Matt Sydes and Annabelle South from University College London and Amelia Hursey from Parkinson's Europe, as well as all our public contributors, Anne-Laure Donskoy, Dianna Moylan and Mary Nettle, for their invaluable contribution to creating this guidance.
- Privacy notice
- Terms & conditions
- Accessibility statement
- Feedback or concerns

- How it works
Online Usability Testing
Understand user behavior on your website.
Mobile & Tablet UX Testing
Test usability on mobile devices and tablets.
Moderated Testing
Conduct live, guided user testing sessions.
Information Architecture Testing
Design or refine your information architecture.

Unmoderated Testing
Allow users to test without assistance.
True Intent Studies
Understand visitors’ goals and satisfaction.
A/B Testing
Determine which design performs better.
UX Benchmarking
Analyze your website against competitors.
Prototype Testing
Optimize design before development.
Search Engine Findability
Measure ease of finding your online properties.
Explore all Loop11 features →
Reporting Features
AI Insights
Gain deeper insights using the power of AI.
Clickstream Analytics
Track users’ clicks and navigation patterns.
Heatmap Analysis
Visualize user engagement and interaction.
User Session Recording & Replay
Capture user interactions for usability analysis.
Get access to all Loop11 features for free. Start free trial

- Clients & Testimonials
Written by Bridgette Hernandez
3 September, 2020

Writing good user research summaries can be hard.
They’re supposed to communicate complex, detailed user testing findings in a really concise and simple way. This can be a bit of a challenge for non-writer folks like UX designers.
Are you one of them?
If yes, you’re in the right place. This article is here to help you write clearly, so you don’t end up with a huge summary that no one really wants to read.
Keep reading to know how to write a great research summary everyone will want to get credit for.
How to Write a User Research Summary: Step-by-Step Instructions
Now, let me walk you through the nine steps of writing that epic user research summary.
Step 1. Go Over Research Findings Once Again
Used-based testing is complex. To make sense of the insights from testers, you have to pay attention to every single detail. Not to mention that some critical thinking skills are necessary to read between the lines and make meaningful recommendations.
How to make sure to cover all bases? Read/analyze/watch research materials once again before writing. Not only does this refresh your memory, but it also gives one more chance to spot something important.
So, go over the results and make notes for yourself. The goal would be to summarize the results and make it easier for yourself to structure the summary.
Step 2. Make an Outline
With the research findings and notes fresh in your mind, proceed to outline your summary. It’ll be helpful to structure your thoughts and present everything in a logical order.
There’s no magical formula for the best summary structure, but you can go for this one. It ensures a logical flow of information and covers all important areas.
Report Outline Example
- Research goals and objectives (research questions)
- Summary of the most important findings
- Methodology + participants
- The findings in more detail
- Bugs and other issues
- Recommendations.
Sounds good? If yes, read more about each section next.
Step 3. Research Goals and Objectives (Research Questions)
The first section of your report should give a quick project background. It will give context to the goals and objectives. Describing them will be the most important part to help readers understand how the project contributed to making a better product.
For example:
“For this user testing project, our team was looking to understand the user’s impressions and perceptions of ABC app.”
Consider using a bullet list to describe your goals. This format clarifies writing and is easy to spot and read.
Pro tip: Include a sentence describing the goal of your summary. It can be something like:
“This report describes the user testing process, how the data was collected, the most important results, and recommendations.”
Related: User Testing a Mobile App Prototype: Essential Checklist.
Step 4. Summary of the Most Important Findings
“So what did they find? What do I need to know?
This section should answer these questions. The findings you need to describe are the themes that occurred across more than one tester, e.g., three users struggling to understand how to complete a certain action.
Struggling to keep the sentences you’re writing short and clear? Consider getting professional writing help from tools like Hemingway Editor . Remember that a clear description is critical to making the entire summary useful.
One way to give a clear explanation is to group the findings by themes, e.g., “Navigation.” If you wish to introduce more structure, also consider giving each finding a priority value (low, medium, and high). For example, the findings that point to the most severe issues can be given a “high” priority.
Step 5. Methodology + Participants
Describe the methods that were used to complete the testing.
Say, you invited 30 people between the ages of 20 and 40 to your office and several coffee houses around the city. You asked them to test your new app and tell you their thoughts.
After they “played” with your app for about 20 minutes, you sat down with each tester and talked. So, the primary method of collecting data was an interview.
There were two parts to it. During the first part, the tester shared their experiences with the interviewer. The second part had the interviewer asking the tester a series of pre-written questions, e.g., “ Did you find it difficult to book a breakfast via our app?”
To describe this plausible UX research methodology, you can use this structure:
- Interview plan + questions . Here, list the structure of the interview, e.g., “ The interview started with a quick introduction…”
- Most important interview questions . Describe the questions in a bullet list and add your reasoning to each (see the next point)
- The reasoning behind questions. Include a short explanation of why a particular question was asked, e.g., “ With this question, we were trying to learn how to present information about additional hotel features in a way that even skeptical app users would click to see more”
- Participant description . Let the readers know how many testers participated, and give some demographic details like age, gender, and why they were chosen.
Pro tip : Consider giving the participants fake names in the summary. It’ll make them easier to remember compared to generic “Participant I” or “Participant II.” To make this process more fun, use the famous Fake Name Generator .
Related: Top 10 Questions When Recruiting Participants for User Tests.
Step 6. Test Findings, in More Detail
You’ve given some idea of test findings already, but now it’s time to really go in detail. In this section, you don’t just state the findings, but also provide your explanation of why the test ended this way.
Here’s how you can write the explanation (a very concise one, go for more details):
“The testers weren’t interested in viewing the extra booking features on the app’s home page. According to them, they rarely got to the bottom of the page where the banner was placed. To engage more users, we need to move it up close to the search feature.”
Jenny Amendola, a UX writer from SupremeDissertations , advises to “Differentiate the results by assigning values like ‘Good’ and ‘Bad’ to them. This way, you can make it easier to understand the results.”
Let the readers know how you organized, analyzed, and grouped the results into themes, too.
Step 7. Bugs and Other Issues
In this section, provide the description of problems discovered during the test that affected the results. Feel free to make it into a bullet list where each bug/issue comes with an explanation.
Categorizing them is also a good idea to clarify the text. For example…
Important! Be sure to include screenshots and images to visualize each issue. It’ll help UX designers understand what you mean.
Step 8. Recommendations
It’s time for your critical thinking genius to shine. In this section, list the ideas for improvement, from most important to least important.
Feel free to follow the structure we’ve used so far: the themes, categories, and bullet lists with explanations. Also, consider supporting each recommendation with a quote from a tester.
“Recommendation 1: We need to focus more on making extra booking options visible above the fold on the home page:
Tester review: ‘I rarely scroll down to the bottom of the home page, so I didn’t see that banner.’”
Some visuals with your recommendations could also be useful even if you create something really simple in an image processing app.
Need some help with spotting improvement opportunities and coming up with useful recommendations? Read a beginner-friendly, simple guide below to get started.
Read the Guide: A Beginner’s Guide to User Experience Testing .
Just One More Thing…
Put your name on that awesome summary.
As a UX researcher or someone involved in doing user research, you’ll be writing many more summaries in the future. Keep this outline to make the next one easier.
Happy writing!
- Recent Posts
- How to Write Actionable User Research Summaries -
Were sorry to hear about that, give us a chance to improve.
Article contents
Related articles

How to Use Rating Scale Questions in Your UX Research
Are you trying to figure out what your audience really wants? How can you improve the customer experience (CX) unless you know what they prefer? Doing user experience (UX) research can take on many different tools and tactics. One excellent way to figure out what your customers need is by conducting some rating scale question […]

How To Track The Value Of UX In Your Project
User experience projects are often ambiguous and challenging, especially in terms of tracking value. Remember the three constraints: cost, scope, and time. Stakeholders are unlikely to consider a project if it costs them much for too little value. Tracking value becomes a bit hard for UX projects for numerous reasons. *Enter UX benchmarking.* UX benchmarking […]

Screener Questions to Find Great Research Participants
Recruiting participants for user experience research is a formidable hurdle in any burgeoning project. It can be a lengthy, tedious process — full of rejections, reschedules, and no-shows. But, one foolproof way to guarantee your UX research study proceeds smoothly with little fuss is to filter out the bad apples early on. That’s where screener […]
Create your free trial account
- Free 14-day trial. Easy set-up. Cancel anytime
- No UX or coding experience required
We love sharing interesting UX topics and work by creatives out there. Follow us for weekly UX posts, inspirations and reels!
Follow us on Instagram
Maybe next time!
Research summary template
Synthesize and summarize research findings Analyze and frame problems

How to create a Research summary template
Get started with this template right now.
Research summary template frequently asked questions
Template by

Mural is the only platform that offers both a shared workspace and training on the LUMA System™, a practical way to collaborate that anyone can learn and apply.
More Research & analysis templates
.jpeg)
Trend card deck

Persona grid
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on the Return of Individual-Specific Research Results Generated in Research Laboratories; Downey AS, Busta ER, Mancher M, et al., editors. Returning Individual Research Results to Participants: Guidance for a New Research Paradigm. Washington (DC): National Academies Press (US); 2018 Jul 10.

Returning Individual Research Results to Participants: Guidance for a New Research Paradigm.
- Hardcopy Version at National Academies Press
Biospecimens from research participants are an essential resource for a broad range of studies, from exploratory, basic science inquiries to clinical trials using well-validated tests. These types of research have been enormously valuable in advancing knowledge about almost every aspect of human health and disease. The conduct of research with human volunteers is dependent on a collaborative, productive relationship between participants who give their time and samples and the investigators and research teams that conduct the research. This complex relationship has many elements, but in the past the communication of individual research results to participants has generally not been one of them.
In the last several decades, questions have been raised about the practice of not returning test results generated in a research study to the study's participants; early on, much of the discussion was focused on returning results from imaging studies, while more recently the focus has moved more to the disciplines of genetics and environmental research. At the same time, the push for increased community and participant engagement across the research study life cycle and the rise of technology-enabled open science and data-sharing movements have added further momentum to the issue. Recent significant changes to federal regulations have promoted transparency and allowed individuals greater access to their clinical and research test results. These changes include the elimination of the laboratory exclusion from the Health Insurance Portability and Accountability Act (HIPAA) privacy rule and revisions to the Common Rule that require prospective participants to be told during the consent process whether clinically relevant individual research results will be returned. On the other hand, the Clinical Laboratory Improvement Amendments of 1988 (CLIA) bars laboratories that are not CLIA certified from reporting individual research results. This creates a dilemma when research results that are clinically relevant or otherwise valuable to participants, particularly those that might not otherwise be discovered, are generated in research laboratories that are not CLIA certified. See Box S-1 for a brief description of these federal regulations. ( Box 6-1 in Chapter 6 , from which Box S-1 is adapted, includes additional laws and regulations relevant to the return of individual research results.)
HIPAA, CLIA, and the Common Rule.
Over the last couple of decades expert groups have written position statements supporting the return of individual research results and secondary findings 2 under certain conditions, such as when the results are clinically actionable, valid, and reliable. However, participant demand for individual research results is driven not just by the potential benefits that individuals could gain by learning about clinically actionable information, but also by their desire to learn about themselves from information that they would not otherwise obtain. More specific guidance is needed on how stakeholders should consider the benefits, risks, and costs associated with the return of individual research results, including the broad spectrum of results which may not be accurate, medically actionable, or have clear meaning.
Seeking guidance on these issues from a consensus body of experts representing diverse perspectives, the Centers for Medicare & Medicaid Services (CMS), the Food and Drug Administration (FDA), and the National Institutes of Health (NIH) asked the National Academies of Sciences, Engineering, and Medicine to conduct a study and generate a report that reviews and evaluates the ethical, societal, regulatory, and operational issues related to the return of individual-specific research results generated from research on human biospecimens. The full Statement of Task for the committee is presented in Box S-2 .
Statement of Task for the Committee on the Return of Individual-Specific Research Results Generated in Research Laboratories.
- SCOPE AND KEY TERMINOLOGY
The topic of the return of research results is exceptionally broad in scope and encompasses all fields of human research (e.g., biomedical, psychological, behavioral). During its first meeting on July 19, 2017, the committee had an opportunity to clarify the scope of the study with representatives of the three sponsoring federal agencies. In the course of that discussion, the study sponsors clarified that the committee was intended to focus on research results that are generated from the analysis of human biospecimens (samples of material collected from the human body, such as urine, blood, tissue, and cells). The committee was not to consider the return of results from imaging, behavioral, or cognitive tests, for example. Of note, the committee's charge was not limited to the return of genetic test results, as many other kinds of research are performed on human biospecimens. These include, for example, basic science studies using tumor biopsies to identify a new biomarker for colon cancer, clinical trials that evaluate blood samples for antibody levels induced by a new malaria vaccine, and epidemiological studies measuring the level of a suspected toxin in urine samples for an environmental exposure study—all of these involve laboratory tests on human biospecimens and are in the scope of this report.
In recent years, this topic has generated immense interest and debate among bioethicists and scientists, particularly in the fields of genetics and environmental exposure research. In the field of genetics, much of the debate has been focused on the return of clinically actionable secondary findings —results that were not the primary objective of the research. This is an important issue in the broader context of returning information generated in the course of research to individual participants, but the sponsors clarified that it was not intended to be a central focus of this committee's report. Instead, in this report the committee uses the term individual research results to refer to results that are generated in the course of a study to help answer the research question or otherwise support the study objectives (e.g., to determine clinical trial inclusion/exclusion) and that are specific to one participant. Distinctions can also be made between different types of individual research results according to the kind of information provided—i.e., uninterpreted data versus interpreted findings. In the genetics field there is also an ongoing discussion about the return of sequencing information, which is generally referred to as “raw data.” For the purposes of this report, all of these types of information are included in the term “individual research results.” Chapter 5 discusses ways to facilitate the understanding of different types of individual research results.
While not a primary focus of the committee's deliberations, there was recognition that secondary findings remain an important part of the discussion about returning research results, given that many sequencing and other “omics” research studies have no primary target. Moreover, the issue of returning secondary findings has a long history (e.g., in the context of returning results from imaging tests), and the committee recognized that the lessons learned from those experiences might be relevant to the committee's task. Furthermore, the committee acknowledged that the recommendations in this report may have impact beyond their application to results generated from biospecimens. In addressing its charge, the committee considered three general scenarios in which consideration of the return of individual research results is relevant:
the planned offer of anticipated individual research results to participants,
the return of individual research results upon the request of participants, and
the offer of unanticipated research results to an individual participant.
For the purposes of this report, anticipated results are those results that are actively sought or are expected to arise when using a particular research test on human biospecimens. This includes results that are not the primary objective of the test or study. Unanticipated results are those that are unexpected either because they could not have been anticipated given the current state of scientific knowledge or because the research team did not consider the potential of generating them using a particular research test. In designing a study, investigators can anticipate several types of results and possible outcomes that may arise from the tests and analyses used over the course of investigation, and very few results should be unanticipated. However, despite investigators' ability to predict the possible outcomes of their research, unanticipated results cannot be entirely avoided, as the state of the science may change over the course of a study or a participant may have an unknown or undiagnosed condition that becomes apparent over the course of biospecimen analysis, thus leading to unforeseen results.
Frequently, the considerations that stakeholders will need to take into account for the return of individual-specific research results to research participants for any of the three scenarios described above will be identical. Therefore, throughout the report the committee uses the shorthand phrase “return of results” to refer to the practice of returning individual research results in any of the three scenarios described above when it is not important to make a distinction between them.
During the discussion of the charge at the committee's first meeting, the following additional areas were identified by the study sponsors as falling outside the study scope, although it should be noted that only some of these are explicitly excluded in the Statement of Task:
- Specific assays or test results (i.e., the committee was not asked to generate a list, for example, of specific genes associated with disease susceptibility that, when tested for, should or should not be returned);
- The return of aggregate research data or study-level results;
- The return of results from anonymized or de-identified specimens that investigators cannot link back to the contributing participant, as well as the role or obligation of biobanks that retain identifiers that would enable the return of individual research results generated by investigators using de-identified biobank specimens (e.g., for secondary research);
- The infrastructure and policies needed for the implementation of a system to return results from secondary research; and
- Laboratory developed tests (LDTs) and the associated LDT regulations.
In discussions with the sponsors, the committee also clarified the scope as it applies to CLIA. The sponsors indicated to the committee that it would be appropriate to include in its description of the current regulatory environment for the return of individual research results CMS's current interpretation of the scope and applicability of CLIA, which is that “only those facilities performing research testing on human biospecimens that do not report patient-specific results may qualify to be excepted from CLIA certification.” Although CMS's current interpretation has been questioned by some legal scholars, the committee was advised that making any comments, analysis, or conclusions regarding the appropriateness of that interpretation would be beyond what was intended in the Statement of Task. Furthermore, the committee was not asked to make recommendations to Congress regarding changes to the CLIA law. However, recommendations on changes to the CLIA regulations were within the study scope if the committee felt that such changes were needed to better align the regulatory environment with the risks and benefits of the return of research results. Chapter 6 addresses the committee's recommendations on clarifying and revising federal regulations.
- BENEFITS AND RISKS OF RETURNING INDIVIDUAL RESEARCH RESULTS
We know from research and shared experience that participants often want and value their individual research results. Participants may benefit from the return of individual research results that inform clinical decision making, life or reproductive planning, and other decisions that may affect health and quality of life. Additionally, results may have personal value to participants by providing a newfound understanding about a health condition. Participants, patients, and their advocates want to be active contributors to the research process and are at the forefront of the movement to get participants involved in the research process from planning to completion. Many advocates consider the practice of withholding results based on concerns about participant welfare to be paternalistic, and they question the notion that participants cannot understand the distinction between clinical results and individual-specific results generated in a research context. Because of its potential to increase public engagement and trust in the research enterprise, the return of individual research results could have multiple positive effects, including possible improvements in the efficiency, generalizability, and participant-centeredness of research. These considerations suggest that the return of individual research results should be an important element of research in this transition toward more transparency and more robust participant engagement in the conduct of research.
On the other hand, important countervailing considerations have been raised concerning whether and how research results should be returned to participants. For instance, research participants do not have the same relationship with investigators as clinicians do with their patients. This means the ethical obligation to return results is less clear (see Appendix D ). Furthermore, we know that research participants may conflate the research and clinical care relationships, having what has been termed a “therapeutic misconception.” The problem here is that some participants may misinterpret the goals of clinical care (individual benefit) with the goals of research (generalizable knowledge) and mistakenly assume that a research study will yield reliable results with clinical value. By its very nature research often produces results that are of uncertain value and, depending on the stage of research, may not be analytically or clinically valid. 3 The return of uncertain, poorly validated, or poor-quality results poses a risk that participants will make important clinical or life decisions based on information that subsequently proves to be wrong or is misinterpreted.
The risks associated with returning individual research results may be minimized by improving result validity through the adoption of an externally accountable quality system by research laboratories. Furthermore, the use of effective communication strategies can minimize the risk of misinterpretation or over-interpretation of research results. However, implementing such strategies may be a significant challenge for many research laboratories, which often operate with little in the way of formal quality assurance processes and often with constrained resources as well. In addition, few investigators have been specifically trained and have the resources needed to communicate results to participants in an effective manner. Clearly, this expanded activity will require additional resources, including resources devoted to planning, ensuring laboratory quality, and the time, effort, and expertise necessary for engaging participants. The cost and feasibility of any additional requirements or expectations on investigators, research laboratories, and institutions is a serious concern, especially when the level of funding for research from government bodies is uncertain. To the extent that additional resources cannot be found to address these costs, a central question is how to balance the value of return of individual research results with the costs, including the opportunity costs, and how to use existing resources. Careful consideration must be given to how the return of individual research results could more broadly affect the research enterprise, health care, and society.
The committee carefully considered the potential benefits, risks, and competing ethical justifications of investigators to return, or not to return, individual research results. Strong justifications can be made for returning results in many circumstances beyond traditional and current practices. The committee identified situations with compelling reasons for the return of individual results to participants, as well as situations with reasons to limit or constrain the returning of results. In determining whether to return results for any given study, ethical principles must be balanced, and the benefits and risks must be carefully considered based on the specific context of the study.
Recommendation 1: Determine the Conditions Under Which Individual Research Results Will Be Returned to Participants. When conducting research involving the testing of human biospecimens, investigators and their institutions should routinely consider whether and how to return individual research results on a study-specific basis through an informed and thoughtful decision-making process.
Investigators, with oversight from their IRBs and institutions, will ultimately be responsible for making decisions on a case-by-case basis regarding whether and how to return individual research results, as the decisions require the careful consideration of many factors, which are described below. However, research sponsors and funding agencies also have an important role in developing policies to support reasonable consistency across research studies and institutions. Although these oversight mechanisms are no guarantee against harm, the committee believes that at this time institutional review is the most practical and reasonable approach to support decision making regarding the return of individual research results. Chapter 4 presents the committee's framework that can support investigators and IRBs in their decision making. The committee recognizes that it will be challenging for IRBs to foster the return of results and to assess the risks and benefits of this practice in the near future before experience and an evidence base has fully developed. In the meantime, we encourage IRB professionals to approach the issue reflectively, regularly engage stakeholders, attend to accumulating data and institutional experiences, and share experiences, data, and protocols with colleagues through professional meetings and publications. Current practices and research into the return of results taking place in NIH-funded research like the All of Us Research Program, the Clinical Sequencing Evidence-Generating Research (CSER) consortium, and the Electronic Medical Records and Genomics (eMERGE) Network can be used to develop initial guidance for IRBs. NIH could also assist IRBs by convening a workshop or working group with other research funders to examine current practices regarding the return of results from biospecimens and explore lessons learned from biomonitoring programs and other domains such as radiology, imaging, and social and behavioral health research. As the evidence base expands, there may be a further role for government agencies to develop guidance to support investigators and their IRBs in their decision-making process.
- GUIDING PRINCIPLES FOR RETURNING INDIVIDUAL RESEARCH RESULTS
The purpose of research is to create generalizable information for the benefit of society, and, unlike clinical care, research is not primarily focused on providing personal benefit for individual participants. Given this perspective, what is the nature of the relationship and expectations between investigators and participants and does our evolving conception of the relationship and expectations require more transparency with respect to individual research results? To what extent should the established ethical obligations to research participants, as codified in international and national guidelines, such as those laid out by the Council for International Organizations of Medical Sciences, the Declaration of Helsinki, the Belmont Report, and the Common Rule, be extended to entail obligations or responsibilities to promote the return of individual research results?
The complexity of these broad questions is substantial because of the sometimes competing, deeply held values involved. The research enterprise has been criticized for its lack of transparency and for the transactional nature of taking from participants without creating value when the results are too often not published or shared. This lack of transparency and true collaboration may factor into the contemporary concerns regarding current difficulties with research participant recruitment and retention, which in turn contribute to the escalating costs of conducting clinical studies. Amid growing consumer expectations for user-centeredness, engagement, and value, these criticisms have led to calls for a paradigm shift. At the same time, the productivity of research in an era of uncertain resources is dependent on making prudent decisions in the allocation of those resources in the pursuit of valuable ends.
Efforts to transform the culture of the research enterprise involve actions and attitude changes along many fronts. One such change involves important and powerful modifications in terminology. Throughout this report—and consistent with use in the broader research community—the committee refers to human research volunteers as “participants” rather than research “subjects,” terminology we recommend for adoption by federal regulators in guidance and new regulations. The use of such language goes beyond semantics; it represents a conceptual move from the passive language of subjects to the active language of participants, it is in accordance with the ethical principles of autonomy and respect for persons, and it reflects the growing movement for participants to be engaged more robustly in the design and conduct of biomedical research.
Two general themes should be evident in this report. First, through its findings, conclusions, and recommendations, the committee is encouraging more frequent return of individual results than is currently the practice in research involving human biospecimens. While careful consideration on a study-by-study basis is important, the committee believes that if the return of individual research results becomes a more common practice, it will demonstrate respect for participants and support transparency and the development of trust with participants, in turn bringing benefits to participants, investigators, sponsors, funding agencies, research institutions, and society. Second, because this is a relatively unfamiliar practice to many investigators, sponsors, funding agencies, and institutions, and because it is a practice that requires the mobilization of resources, we do not expect our recommendations to change standards and practices immediately. We understand that accomplishing the goals articulated in this report will take time and resources and that best practices in terms of when and how to return results will emerge with experience and with new research focused on these very questions. Our hope is that this report will motivate stakeholders in ways that will ultimately transform research practices in parallel with other changes that promote transparency and trust, participant engagement, higher research quality, and improved reproducibility of research findings.
Taking into account the complex ethical and societal considerations underpinning the movement to increase the return of individual research results, the committee formulated the following six principles to help guide its deliberations and the development of the recommendations presented in this report:
- Principle 1: Participants bring essential and valuable information to the research enterprise without which research cannot be conducted. Because research results have value to many participants, as a matter of reciprocity, respect, transparency, and trust, the return of results should be routinely considered in the design of research protocols involving human participants.
- Principle 2: Research has significant societal value. The potential value of returning individual research results must be carefully considered along with the trade-offs for research participants, investigators, research institutions, and society.
- Principle 3: When individual research results are offered, participants have the right to decide whether to receive or to share their results.
- Principle 4: When individual research results are returned, the process of communication is important to promote understanding of the meaning, potential uses, and limitations of the information.
- Principle 5: The value of research results to investigators, participants, and society depends on the validity and reliability of the result. High standards of laboratory quality, from the acquisition of specimens to the communication of results, enhance the validity and reliability of the results generated in research laboratories.
- Principle 6: The conduct of high-quality, generalizable, and equitable research involves the inclusion of diverse populations and requires investigators to return individual research results in a manner that accommodates the full spectrum of community needs and preferences, regardless of participant social or economic status. The potential value of results, which is best assessed with input from the participant, community, or trusted proxy, should be considered.
- QUALITY MANAGMEMENT SYSTEMS FOR LABORATORIES TESTING HUMAN BIOSPECIMENS
Establishing laboratory processes to give all stakeholders (investigators, institutions, regulators, and participants) confidence in the validity of the individual research results being returned is critical to ensuring the accuracy of information provided to research participants as well as the quality of the science. However, many research laboratories without CLIA certification currently do not have the systems in place to provide confidence in the validity of individual participants' research results. Certainly, many research laboratories produce high-quality science, but without the documentation of practices under a quality management system (QMS), 4 it is difficult to know which laboratories can generate accurate and reliable individual research results with proper assignment of the individual results to the correct research participants. Questions about validity and thus quality of individual research results pose a barrier to the responsible return of research results to participants. More broadly, the lack of established quality processes poses a problem for the rigor and reproducibility of the science.
When individual research results are intended for use in clinical decision making in the study protocol, tests must be performed in laboratories that are CLIA certified. When the study protocol does not call for individual research results to be used in clinical decision making (see Box S-3 ), CLIA certification may not always be an appropriate or necessary mechanism to ensure that the quality of the research test results is sufficient to permit the return of results. While CLIA has significantly improved the quality of clinical laboratory results used in clinical decision making, its requirements are not always a good fit with the kinds of testing performed in the research context, such as tests relevant to biomonitoring for environmental contaminants. Moreover, current CLIA regulatory requirements have not kept up with the rapid pace of technological innovation (e.g., genetic sequencing technologies). For example, current CLIA requirements do not address the complexity of the required informatics analyses, interpretation, and reporting required with next-generation sequencing technologies or other omics testing. Furthermore, while the direct cost of CLIA certification may not be prohibitory, meeting the requirements to obtain the certification by compliance with all of the regulatory requirements would come with significant costs for most research laboratories, although the extent of the burden would depend on the infrastructure and processes already in place in the laboratory (see Chapter 3 for more detail).
Results Not Intended for Clinical Decision Making in the Study Protocol.
However, if investigators plan to return individual research results to participants, it is essential that the quality of the laboratory analysis is sufficient to provide confidence in the result to be returned. Currently, there is no accepted QMS for research laboratories that could serve as an alternative to CLIA certification. For these reasons, the committee recommends that NIH lead an effort with the Centers for Disease Control and Prevention, FDA, CMS, and other relevant federal agencies and nongovernmental organizations, including patient and community groups, to develop a QMS with external accountability 5 for research laboratories that perform tests on human biospecimens. Outside the United States, several governmental and nongovernmental organizations are already working in this arena, including ongoing initiatives in Europe aimed at producing guidance and recommendations to assist investigators in meeting quality essentials in laboratory practice. Prior to the development of the recommended QMS for research laboratories, or in the event that results are generated over the course of a study that may be valuable to a participant but were not anticipated by the investigator, IRB review should serve as an alternative pathway for determining if certain conditions have been met and if the return of results not intended for use in clinical decision making is permissible (see Recommendation 3 and Figure S-1 ).
Determining whether laboratory quality is sufficient for investigators to return individual research results. a CLIA-certified includes tests run in a CLIA-certified, -accredited, or -waived laboratory. b See Recommendation 2.
Recommendation 2: Develop a Quality Management System for Research Laboratories Testing Human Biospecimens. NIH should lead an interagency effort including nongovernmental stakeholders to develop an externally accountable quality management system for non-CLIA-certified research laboratories testing human biospecimens. Recommendation 3: Ensure the High Quality of Individual Research Results That Are Returned to Participants. To provide confidence in the quality of research test results disclosed to participants, institutions and their IRBs should permit investigators to return individual research results if A. testing is conducted in a CLIA-certified laboratory; or B. results are not intended for clinical decision making in the study protocol (as defined in Box S-3 ) and testing is conducted under the externally accountable quality management system for research laboratories once established (see Recommendation 2 ); or C. results are not intended for clinical decision making in the study protocol (as defined in Box S-3 ) and the IRB determines that 1. the probability of value to the participant is sufficiently high and the risks of harm are sufficiently low to warrant return; 2. the quality of the laboratory analysis is sufficient to provide confidence in the result to be returned, as determined by a review process independent of the laboratory; and 3. information will be provided to the participant(s) regarding limits on test validity and interpretation (see Recommendation 10 ). B and C will require changes to the CLIA regulations, embodied in Recommendation 12 , or changes to the interpretation of the CLIA regulations.
Quality management systems have been shown to make work more efficient, facilitate the training of new staff, improve reproducibility, increase patient safety, and enhance data integrity. However, putting a QMS in place will have multiple impacts on research laboratories, in terms of both their research processes and resource requirements. Adoption of the quality system will likely require changes to the laboratory operations and the training environment. Additional resources may be needed for the analytical and clinical validation of testing procedures, equipment maintenance standards, and more stringent staffing and staff training and competency assessment requirements. To minimize the burden for research laboratories, sponsors, funding agencies, and research institutions need to facilitate access to resources and support quality management system training and the development of the necessary laboratory infrastructure to ensure that human biospecimen testing is performed under high-quality standards. The initial training, cost, and time commitment will likely be significant, but the value added will be considerable, both for participants and for science.
Recommendation 4: Ensure Adequate Resources and Infrastructure to Generate High-Quality Research Results. Research institutions and funding agencies should develop and provide access to the resources and infrastructure needed to ensure that investigators conducting testing on human biospecimens can meet the necessary standards for quality, so that research test results can be returned to participants (see Recommendation 3 ). This may include assisting investigators and their research laboratories in A. training and access to resources to prepare for the future adoption of the externally accountable quality management system for research laboratories (see Recommendation 2 ); B. adopting the externally accountable quality management system for research laboratories once established for relevant laboratories (see Recommendation 2 ); or C. becoming CLIA certified or facilitating access to core, affiliated, or third-party CLIA-certified laboratories for sample testing, retesting, or a confirmatory testing process when research results are for use in clinical decision making in a study protocol.
- A DECISION-MAKING FRAMEWORK FOR THE RETURN OF INDIVIDUAL RESEARCH RESULTS
Decisions about whether and how to return individual research results are influenced by many factors. These include the potential value of the information to the participant; the nature of the relationship, if any, between the participant and investigator; the analytic and clinical validity of the research result; and the feasibility of return. Benefits to the participants and to the research enterprise have to be weighed against risks, including potential harms to individuals, the diversion of resources and investigator efforts from conducting research, liabilities, risks of privacy breach, and discrimination. Furthermore, investigators may be legally required to disclose a result if a participant makes a request under HIPAA, which ensures individuals a right to access any personal health information contained within the DRS of a HIPAA-covered entity.
A small number of well-defined cases present clear and broadly accepted rationales for when the return of results should be obligated or discouraged (see Box S-4 ). But, for the majority of scenarios, decisions have to be made on a case-by-case basis by weighing several factors. As the potential value of the result to participants and the feasibility of return increase, the justification for returning results becomes stronger (see Figure S-2 for a conceptual framework). Value in this context means the value of a result from the perspective of the participant and might entail clinical utility or personal utility as well as personal meaning (e.g., lineage information). This participant-centric approach recognizes that the value of a result is not necessarily tied to its use. To clarify, defining value in this way is not meant to imply that each participant needs to be queried regarding the results that would be meaningful to him or her, but it does require the investigator to consider value from the participant perspective rather than from the more traditional clinical perspective. Feasibility is also determined by multiple factors, including potential challenges, the costs and burdens of returning results, whether biospecimens can be linked to a specific participant, and the resources available to communicate the results effectively and appropriately.
Individual Research Results That Should and Should Not Be Returned to Participants.
A conceptual framework for decisions on returning individual research results. NOTES: This figure demonstrates that as the potential value of the result to participants and the feasibility of return increase, the justification for returning results becomes (more...)
Ascertaining Participant Needs, Preferences, and Values
Investigators, institutions, and research sponsors and funding agencies need to be cautious about making assumptions regarding the kinds of results that participants may find meaningful. Expert-identified criteria do not always reflect participant preferences and values, as the value of a research result to participants will be influenced by both their perspectives and the contexts in which they are participating in the research. Incorporating the needs, preferences, and values of community representatives and advocacy groups into decision making regarding the return of individual research results is important for helping investigators to better understand what participants value and to weigh the benefits and risks of disclosure.
Ascertaining and incorporating participant needs, preferences, and values into decision-making processes regarding whether or not to return individual research results can be undertaken at the study level but also in the development of policy or guidance. Both are critical to advancing a more participant-centric research paradigm. For some kinds of studies—particularly those that will involve significant interactions between researchers and participants—obtaining representative input from relevant and representative community members in the study design phase (e.g., through advocacy groups or community advisory boards) can help ensure that decisions on whether and how to return results are aligned with participant values and needs. For other types of studies (e.g., when biospecimens have been de-identified or if investigators can reasonably rely on existing documentation of participant needs, preferences, and values in the literature or from past experiences working with community groups), engagement may not be as important.
Many investigators will be new to participant and community engagement activities and will need to rely on existing models, guidance, and informational resources as they develop study protocols and consider return of individual research results. Investigators may need to be made aware of the existence of these resources or receive training in order to effectively engage participants in discussions of their preferences for the return of individual research results. To minimize the burdens on individual investigators, research sponsors and institutions can help investigators understand the preferences and needs of their prospective participants by leveraging their core resources (e.g., Clinical and Translational Science Awards Program cores, community advisory boards) and by engaging community and participant representatives to develop policies and guidance (see Chapter 4 for additional information on the range of engagement in the return of individual research results).
Recommendation 5: Incorporate Participant Needs, Preferences, and Values in Decision Making About the Return of Individual Research Results. Research stakeholders should ensure that participant needs, preferences, and values are incorporated into decision making regarding the return of individual research results. To facilitate this, A. investigators should seek information through various mechanisms, including reviewing published literature, leveraging experiences from similar studies, consulting participant or community advisory boards, and engaging community and participant groups and advocacy organizations in the development of the research protocols; B. research institutions and sponsors should enable and facilitate investigator access to the relevant community and participant networks, resources, and training; and C. research sponsors should engage community and participant representatives in the development of policy and guidance related to the return of individual research results.
- PLANNING FOR THE RETURN OF INDIVIDUAL RESEARCH RESULTS
The development of a plan at the design phase of a study that addresses whether, when, and how results will be offered to participants as part of the study protocol, or provided in response to a participant request or upon discovery of an unanticipated but potentially valuable result, can help maximize the benefits and prevent or mitigate the potential harms associated with the return of research results. Incorporating the plan into the research protocol ensures transparency and appropriate budgeting, while IRB review ensures that the risks and benefits to participants are carefully considered in a peer-review process.
The planning process should consider the types of results that might be shared (such as routine clinical results generated in the course of research, test results generated in a research laboratory, or urgent findings) and when in the study life cycle they might be shared without threatening the scientific integrity of the study. By requiring and reviewing plans and providing support for the return of individual research results, research institutions and sponsors can help foster a culture in which the return of individual research results is more routinely considered and practiced.
Recommendation 6: Include Plans for the Return of Individual Research Results in Research Protocols. For all studies using human biospecimens, investigators should routinely address their plans regarding the return of individual research results in their funding application or research protocol. The investigator's plan should describe A. whether individual research results will be offered to participants and, if so, when and how. The plan should also provide the rationale for these decisions, including how participant needs, preferences, and values were considered; B. how the consent process will reflect transparency and effective communication with participants regarding whether and, if so, how individual results will be offered; C. how investigators and their institutions will respond if participants request their results, including how information in the designated record set will be released to participants when they have a right to access their individual research results under HIPAA; and D. the budget and resources for the return of individual research results, when appropriate. Recommendation 7: Ensure Planning for the Return of Individual Research Results in Applications for Funding. Research sponsors and funding agencies should ensure that investigators are considering whether and how individual research results will be returned to participants, by A. requiring that applications for research funding consistently address the return of individual research results, indicating whether, and if so, when and how individual research results will be offered to research participants, as well as the rationale for these decisions; B. including in the scientific review process for funding applications an assessment of plans for the return of individual research results; and C. building funding into grants and contracts or providing administrative supplements for the return of individual research results. Recommendation 8: Develop Policies and Procedures to the Support Review of Plans Regarding the Return of Individual Research Results. Research institutions and their IRBs should develop policies and procedures that support the assessment of plans for the return of individual research results. Policies and procedures should ensure that A. the IRB has, or has access to, the necessary expertise to review the return of individual research results plans; B. appropriate consideration has been given to participant needs, preferences, and values (see Recommendation 5 ); C. the research teams have access to the appropriate expertise (e.g., a scientific review committee) to consider the factors relevant to decisions on returning individual research results, including analytic validity, clinical validity, and the value of the results to participants; D. the consent process is aligned with the return of individual research results plan (see Recommendation 9 ); and E. the investigators have access to the necessary resources (e.g., core resources) and expertise to enable the communication of individual research results in an effective manner (see Recommendation 10 ).
- EFFECTIVELY COMMUNICATING INDIVIDUAL RESEARCH RESULTS TO PARTICIPANTS
The return of individual research results to participants is relatively uncommon in the research enterprise. As a result, few standardized practices or even guidance on how to accomplish this challenging communication task have been developed. Different communication approaches may be appropriate in different contexts and may be associated with different costs or burdens to investigators. Given the scientific community's general lack of experience with returning individual research results to participants, as well as the complexity and uncertainty inherent in results generated through research, the development of guidance and best practices may help address inconsistency in practices and minimize the risk of harm from the return of research results.
To establish an empirical evidence base for the development of best practices, the research community will need to develop a learning system in which processes for returning research results are evaluated for benefits and harms and communication practices are refined. This will require the accumulation of experience over time. In the absence of such empirically derived best practices, applying existing principles for clear communication, such as considering audience characteristics and needs and having a clearly defined communication objective, represents a clear strategy for improving the quality of return-of-results practices now. Being clear and transparent during the consent process regarding whether, under what circumstances, and how investigators will offer and return research results can help to set appropriate expectations and build trust. The use of established communication principles is also important in order to enhance the likelihood of participants understanding research results and the appropriate use of that information.
The ability of participants to understand and make use of research results depends on the provision of relevant contextual information that clarifies what is known or unknown about the meaning of a specific result. When relevant contextual information (such as reference standards) for a result is not known, studies should weigh the benefits and risks of return and consider whether the return of only aggregate results would be more appropriate than the return of individual results. Understanding is also facilitated by providing a clear takeaway message that includes a statement regarding actionability. For more complex studies, it can be challenging to effectively communicate to research participants the degree of uncertainty that the research results entail, especially in contrast to the more familiar context of clinical testing. As a result, the return of individual research results should often be accompanied by caveats and qualifiers that address potential inaccuracies and uncertainties.
The appropriate return of individual research results requires investment and careful forethought regarding the necessary contextualizing information, takeaway messages, and caveats. It also requires a consideration of the need to communicate in ways appropriate for participants with different needs, resources, and backgrounds. However, upfront investments to improve investigator access to resources, training, and expertise can be scalable, and the development of best practices over time will improve the consistency and quality of the process of returning individual research results.
Recommendation 9: Ensure Transparency Regarding Return of Individual Research Results in the Consent Process. In the consent process, investigators should communicate in clear language to research participants A. which individual research results participants can access, if requested, including any results participants have a legal right to access under HIPAA, and how to request these results; and B. which individual research results, if any, will be offered to participants and why, and the participant's option to decline to receive their research results. C. If results are going to be offered the following elements should also be communicated during the consent process: 1. the risks and benefits associated with receiving individual research results; 2. conditions under which researchers will alert participants of urgent results; 3. at what time and through what process results will be communicated to participants; 4. whether the results will be placed in the participant's medical record and whether the results will be communicated to the participant's clinician; and 5. when relevant to the research protocol, the participant's option to have results shared with family members in the event the participant becomes incapacitated or deceased. Recommendation 10: Enable Understanding of Individual Research Results by Research Participants. Whenever individual research results are communicated to participants, investigators and institutions should facilitate understanding of both the meaning and the limitations of the results by A. ensuring that there is a clear takeaway message and necessary reference information to convey what is known and not known about both the meaning of the result and potential clinical implications; B. communicating effectively the level of uncertainty in the result validity; C. providing mechanisms for participants to obtain additional information and answers to questions when appropriate and feasible; D. providing guidance for follow-up actions/consultations when appropriate; E. aligning the communication approaches to the particular needs and preferences of the participants and the context of the study; F. providing a written summary of the results and other information communicated to participants for future reference by participants and investigators; and G. leveraging existing and emerging health information technologies to enable tailored, layered, and large-scale communications when appropriate. Recommendation 11: Expand the Empirical Evidence Base Relevant to the Return of Individual Research Results. To expand the empirical evidence base relevant to the return of individual research results, sponsors and funding agencies should support additional research to better understand the benefits and harms of the return of results as well as participant needs, preferences, and values and to enable the development of best practices and guidance.
When it comes to funding empirical research for the return of individual research results, NIH is the obvious, and likely primary, sponsor who would fund such an endeavor. However, this responsibility should not fall to NIH alone. The return of research results will soon become an integral part of the research enterprise—it is a global endeavor and all sponsors of research using human biospecimens should direct resources to addressing the needs of investigators and participants through the funding of empirical research in the practice. The development of unified guidance on returning individual research results will help prevent dramatic variability in practice between institutions and will aid IRBs in making informed decisions. Funding agencies have a responsibility to ensure that processes for return are both feasible and implemented appropriately.
- RESHAPING THE REGULATORY ENVIRONMENT
The legal and regulatory requirements and restrictions pertaining to the return of individual results are currently uncertain, thus causing variable interpretation and action across IRBs and research sites. As currently written and implemented, the laws and regulations governing access to laboratory results, both clinical and research, are not harmonized; they afford inconsistent and inequitable access for participants to their individual research results, and regulatory conflicts create dilemmas for laboratories. Specifically, CMS's interpretation of CLIA blocks any laboratory from returning a test result if the laboratory is not CLIA certified, but HIPAA requires the return of results upon a request by the patient if the results are part of their DRS. In some cases regulations are too restrictive, while in others they are not restrictive enough, allowing for the return of results of poor or unsubstantiated quality without appropriate caveats or context. Moreover, the regulations governing the protection of human participants do not address the return of results, meaning that the guidance available to research participants and investigators in inadequate. Overall, the current regulatory environment for the return of individual research results is not well aligned with the benefits and risks associated with the practice.
The current absolute prohibition of the return of results from non-CLIA-certified laboratories fails to account for several factors, including the high quality maintained by some research laboratories, the value that many participants place on results despite uncertain validity, and the access rights afforded by HIPAA to individual results regardless of quality standards. Additionally, there is little evidence of harm from the return of research results, although the overall body of evidence is limited and may reflect a lack of evidence rather than conclusive evidence of a lack of effect. Accordingly, the committee believes that, in certain circumstances, results can be provided to participants when laboratories have not achieved CLIA certification. However, the committee is cognizant of the potential harms to participants and the research enterprise if laboratory quality systems are not in place in research laboratories or if loopholes are created that can be abused to perform clinical testing in a research laboratory without CLIA certification. Therefore, the Office for Civil Rights of the Department of Health and Human Services should limit access to individual research results under HIPAA to those generated in a CLIA-certified laboratory or in a laboratory that has adopted the QMS for research laboratories recommended by the committee (see Figure S-3 ). Through its recommendations, the committee promotes an approach that requires high-quality standards when investigators plan to return results, but also supports a thorough peer review and approval process for the potential return of valuable results generated in laboratories not meeting CLIA requirements.
Determining whether participants have the right to access their individual research results under HIPAA. a CLIA-certified includes tests run in a CLIA-certified, -accredited, or -waived laboratory. b See Recommendation 2.
Recommendation 12: Revise and Harmonize Regulations to Support the Return of Individual Research Results. Regulators and policy makers should revise and harmonize the relevant regulations in a way that respects the interests of research participants in obtaining individual research results and appropriately balances the competing considerations of safety, quality, and burdens on the research enterprise. Specific actions that should be taken include the following: A. Because the designated record set (DRS) is intended to include information used to make decisions about individuals, those decisions should be based on test results that are of sufficient quality to be valuable for decision making. Accordingly, the Office for Civil Rights (OCR) of the Department of Health and Human Services (HHS) should define the DRS to include only individual research results generated in a CLIA-certified laboratory or under the externally accountable quality management system for research laboratories (see Recommendation 2 ); B. OCR should require all HIPAA-covered entities that conduct research on human biospecimens to develop a plan that is reviewed and approved by the IRB for the release of individual research results in the designated record set to participants in a responsive manner when requested under HIPAA; C. CMS should revise CLIA regulations such that when there is a legal obligation under the HIPAA access right to return individual research results, a laboratory will not be considered in violation of CLIA and need not obtain CLIA certification before satisfying this legal obligation; D. CMS should revise CLIA regulations to allow research results to be returned from a non-CLIA-certified laboratory when they are not intended for clinical decision making in the study protocol (as defined in Box S-3 ) and the laboratory conducts its testing under the quality management system with external accountability or the IRB has approved the return of results (as described in Recommendation 3 ); E. CMS and OCR should harmonize the definitions of the following terms, providing a clear explanation and justification for any differences or discrepancies: “test report” and “completed test report” (CLIA), and “PHI in the designated record set” (HIPAA); F. OCR, OHRP, and NIH should harmonize the definitions of the following terms, providing a clear explanation and justification for any differences or discrepancies: “de-identified” (HIPAA), “non-identified” (Common Rule), and “identifiable sensitive information” (21st Century Cures Act regarding certificates of confidentiality); G. HHS (including CMS, FDA, NIH, OHRP) should ensure that all regulations, policies, and guidance relevant to human research refer to research “participants” rather than research “subjects,” in accordance with the ethical principles of autonomy and respect for persons; and H. FDA should clarify and provide additional guidance that if a device is not exempt from investigational device exemption (IDE) regulations, disclosure of results in many circumstances, including to healthy volunteers, will not necessarily entail significant risk, and FDA should clarify when it will consider the return of individual research results to entail significant risk. Additionally, FDA should provide guidance to IRBs on how to determine significant risk if the device is not exempt from IDE regulations.
- FINAL THOUGHTS
The recommendations in this report, if followed, will result in substantial and potentially controversial changes to the research regulations and the research enterprise involving research with human biospecimens. The opportunity for change has arisen in response to the evolving relationship between investigators and participants and is supported by an assessment of the potential benefits and risks of returning individual research results. The need for higher standards of quality in many research laboratories is clearly illustrated in this report (see Chapter 3 ). Yet, despite the inherent limitations in the validity and interpretability of some research results, our assessment is that the risks associated with the communication of results have been overstated, particularly for the many research projects that are unlikely to yield highly sensitive or clinically meaningful results. Furthermore, the potential benefits of results disclosure to individual participants and to the research enterprise have been understated.
Therefore, we are recommending that the current absolute standard—that all disclosed results must be generated in a CLIA-certified laboratory—should be replaced with a process-oriented standard, meaning that a peer-review process can be used in some circumstances to weigh competing considerations regarding the return of individual results. We recommend that such a process take into account, on a case-by-case basis, the values of the participants, the risks and benefits of the return of particular results, the quality of the research laboratory and test performance, and the feasibility for investigators to pursue this course. There are risks to moving away from an absolute standard, but we believe that the risks can be mitigated through improvements in laboratory quality, a case-by-case assessment of the risks and benefits, and the promotion and development of communication strategies to help place results in the proper context for participants. The committee believes that the benefits of this more nuanced approach will greatly exceed the adverse impacts and costs.
The committee is well aware that more frequent return of individual research results will create new demands on the research enterprise. Many institutions and researchers currently lack the experience and resources to return individual research results in a deliberate and effective manner. The committee does not expect that a more widespread return of results will happen immediately. However, the committee foresees an evolving set of responsibilities and offers recommendations that it believes will help stakeholders prepare for these added responsibilities and develop the necessary expertise over time.
At a broader level, the justification for fundamental changes in the research landscape can be found in our changing understanding of the ethics of human participant research as well as in our recognition that failures to support transparency and to earn respect and trust from individuals in the community are hampering the conduct of science. The vision is that a dedicated commitment to collaboration will better honor participants, benefit science, and promote the welfare of society. While the standards and practices related to the return of individual results are but one set of elements in this evolving landscape, the return of research results is a tangible, measurable piece that we know is valued by participants and is feasible in many more circumstances than are reflected in current practice. Our hope is that this report will promote the practice through selected changes in research regulations, the use of quality management systems that ensure the quality of research results, and the commitment of all stakeholders (see Table S-1 ) to innovative, collaborative processes in the planning and conduct of research.
Recommendations by Stakeholder .
This Summary does not include references. Citations for the discussion presented in this Summary appear in subsequent report chapters.
Secondary findings are results that are not the primary objective of the research. Such findings are referred to in the literature by a variety of terms, such as “additional,” “secondary,” “incidental,” “ancillary,” “supplemental,” etc., and these terms can be combined with additional clarifiers such as “unanticipated” and “anticipated.”
Analytic validity indicates how well a test measures the property or characteristic it was intended to measure, whereas clinical validity is a measure of how consistently and accurately a test detects or predicts the intermediate or final outcomes of interest.
Quality management systems (QMSs) are defined by the World Health Organization, the International Organization for Standardization, and the Clinical and Laboratory Standards Institute as “coordinated activities to direct and control a laboratory with regard to result validity and reliability.”
External accountability means that a research laboratory's compliance with defined QMS standards is assessed by an entity independent of the laboratory.
- Cite this Page National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on the Return of Individual-Specific Research Results Generated in Research Laboratories; Downey AS, Busta ER, Mancher M, et al., editors. Returning Individual Research Results to Participants: Guidance for a New Research Paradigm. Washington (DC): National Academies Press (US); 2018 Jul 10. Summary.
- PDF version of this title (3.7M)
In this Page
Recent activity.
- Summary - Returning Individual Research Results to Participants Summary - Returning Individual Research Results to Participants
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Research Summary

A research paper analyzes a perspective or argues a point. It is an expanded essay based on your interpretation, evaluation or argument about a certain topic.
According to Sunny Empire State College , “When you write a research paper you build upon what you know about the subject and make a deliberate attempt to find out what experts know. A research paper involves surveying a field of knowledge in order to find the best possible information in that field.” Whatever type of research paper you choose to write, it should present your own ideas backed with others’ (especially experts on the field) information and data.
Every research paper has a research summary. A research summary is a brief overview of what the whole research is about. It is a professional piece of writing that describes your research to the readers. It concisely yet perfectly captures the essence of the research as a whole. You may also see What Should Be in an Executive Summary of a Report?

Fundamentals of a Research Summary
Having a good template for a research summary is nothing if you don’t know its importance and basic function. Before you start writing your research summary, you should first know its fundamentals on the areas you need to pay attention to such as its content, style and organization.
- The content of your research summary must briefly discuss the techniques and tools used in the research and the importance of the research as a whole. Explain how the research can be of benefit for the people.
- To organize your research summary, each topic must be discussed in separate paragraphs. How you came up with a factual research must be briefly explained in a separate paragraph.
- If you have a lengthy research paper, try not to write not more than 10% of the entire paper. If it’s not as lengthy, you should not write more than 300 words in your summary.
However, rules may vary according to your research professor’s standards. This is just the basic fundamentals on how to write your research summary. Also see Thesis Outline Examples
How to Write a Research Summary
It is apparent that a research summary is a condensed version of the main idea of your research paper. Because of this, it is advised that the summary of your paper is written after you are done with your entire research. This is to ensure that all the added information in your research can be written in your summary as well and all of those that removed can be edited out. Here are a few steps on how to write a research summary:
Read your paper
It should be a fact you should know beforehand; the importance of reading your entire research paper thoroughly to write an effective research summary. Along the way, take notes of the important details and key findings that you want to highlight in your paper. This will help you organize your summary better. Remember that your research summary is a mini-paper of your study and it should contain the main ideas of your entire research.
Write a draft
For your first draft, focus on the content rather than the length of your summary. Your draft is your first outline on what to include in the final summary. Writing a draft ensures you write a clear, thorough and coherent summary of your research paper. Also see How to Write a Rough Outline
Identify main points
Within your research paper, you must identify the major points that will encourage prospective readers to go through your research paper. These major points must thoroughly and completely explain what the paper is trying to convey.
Separate sections
Identify the differences of the main section in your paper. Write a few sentences describing the main ideas of each section. In short, you should be able to present and thoroughly describe what each main section is focused on. It should have these basic sections:
- Introduction, brief opening statement
- Purpose of the study
- Data gathering method
- Summary of findings
- Description of recommendations with actual justification.
Combine Information
All the information you have gathered must be then used to make your summary. Remember that your summary is just an overview of your research paper as a whole. It should be not be more than 10% of your whole paper. Also see 5 Summary Writing Examples and Samples
Making The First Draft
After establishing the basic way of writing a research summary, it is a must to write a first draft. It should follow the flow of the original paper. Here’s a few steps on how to make a first draft:
First, state the research question in the introduction of your summary. This holds the ground as to the summary’s direction. Provide an explanation why your research is interesting and how it can help your target recipients.
Second, state the hypothesis you wish to prove. This will help you and your readers stay grounded on the topic at hand.
Third, briefly discuss the methodology used in your research. Discuss and describe the procedure, materials, participants, design, etc. The analysis of your data must also be included. You may also see How to Write a Successful Thesis Proposal
Fourth, describe the results and significance of your research. And lastly, briefly discuss the key implications of your research. The results and its interpretation should directly coincide with your hypothesis.

Editing your Research Summary
A research paper is a formal piece of writing. Your summary should be tailored to your expected readers. Say for example the prospective readers are your classmates, so the style of your paper should be clearly understood by them.
Eliminate wordiness. Avoid using unnecessary adjectives and adverbs. Write in a way it would be easier for your readers to understand. It is common for research papers to establish a word count. Avoid elongating your sentences when it has shorter versions.
Being vague in describing and explaining the points of your paper might lead to confusion in your readers part. Use specific, concrete language when presenting results. Use reliable and specific examples and references as well. You should also use scientifically accurate language to help support your claims. Avoid informal words and adjectives to describe the results of your research.
Paraphrase the information you want to include in your research paper. Direct quoting the information you have read from a different source is not oftenly used in formal writings. To give the exact credit for the information you paraphrased, follow the citation format required by your professor.
Reread your paper and let others read it as well. This way minor errors you were not able to notice can be quickly pointed out and corrected.
Research Summary Writing Tips
Your research summary should not be more than 10 pages long or not more than 10% of your original document. This keeps your research summary concise and compact. It should be short enough for your readers to read through but long enough for you to clearly explain your study.
Copy and paste
Avoid simply copy and pasting different parts of your paper into your summary. You should paraphrase parts that you want to include. As most research advisers read through all of your paper, it can easily be identified if you have copy-pasted parts from your research and might give you a bad grade.
Consider the readers
Although not a requirement from your professor, catering your summary to what the readers need is sometimes required. As some studies are given out to different influential people in the field, writing a summary that caters to the readers’ necessities might be required.
Research Article Summary Template

- Google Docs
Size: 158 KB
Research Report Executive Summary Template

Size: 120 KB
Research Summary Example
Size: 130 KB
Research Summary Sample

Size: 486 KB
Research Writing Summary Tips (continuation)
Clarity and organization.
One of the common mistakes in writing a research is publishing an unclear and unpolished summary. Bear in mind that your readers are likely reading about the topic of your research for the first time, avoid unclear and uncertain explanations and a disorganized summary.
Use strong and positive language
Use precise and strong words to help strengthen the foundation of your summary. Your summary should be able to stand alone despite it being a part of the research paper. Once you have convinced your readers with the recommendations regarding the topic of your paper, the readers should be able to find concrete evidence and explanations within your summary. Avoid pleas and biased statements in your summary, but make sure you are able to relay the sense of urgency for the recommendations you have given.
Divide into parts
To make things easier for you, divide your paper into different sections and headings, much like creating an outline. With this in mind, every point should be explained limited to its essence. In this way, you avoid writing too much information about your paper in your summary.
AI Generator
Text prompt
- Instructive
- Professional
10 Examples of Public speaking
20 Examples of Gas lighting

- Bioenergy Technologies Office
- Accomplishments
- National Biotechnology & Biomanufacturing Initiative
- Bold Goals for U.S. Biotechnology & Biomanufacturing
- 2023 Multi-Year Program Plan
- Bioproduct Production
- CO2 Utilization
- Deconstruction & Fractionation
- Synthesis & Upgrading
- Conversion Technologies Related Links
- Sustainability
- Data, Modeling, & Analysis Related Links
- Feedstock-Conversion Interface R&D
- Feedstock Technologies Related Links
- Algal Logistics
- Algal Production
- Algal Related Links
- Development
- Infrastructure
- Systems Development & Integration Related Links
- Sustainable Aviation Fuels
- Sustainable Marine Fuels
- Waste-to-Energy
- CO2 Reduction & Upgrading for e-Fuels Consortium
- Consortium for Computational Physics & Chemistry
- Co-Optimization of Fuels & Engines
- Feedstock-Conversion Interface Consortium
- Bioenergy FAQs
- Biofuel Basics
- Biopower Basics
- Bioproduct Basics
- Biomass Feedstocks
- Career Exploration Wheel
- Career Grid
- Workforce Training Opportunities Map
- Algae Technology Educational Consortium
- Workforce Development Strategy
- Bioenergy Jobs at BETO
- BRIDGES Events
- BRIDGES Webinar Recordings and Presentations
- Internships & Fellowships
- Teacher & Classroom Resources
- BioenergizeME Infographic Challenge
- Wood Heater Design Challenge
- Funding Opportunities
- Analytical Tools
- Bioenergy Publications
- Bioenergy Related Links
- State Biomass Contacts
- BioProse: Bioenergy R&D Blog
- Bioenergy News
- Bioeconomy 2020
- Bioeconomy 2018
- Bioeconomy 2017
- Bioenergy 2016
- Bioenergy 2015
- Biomass 2014
- Biomass 2013
- Biomass 2012
- Biomass 2011
- Inaccessible
- Biomass 2009
- Peer Review 2023
- Peer Review 2021
- Peer Review 2019
- Peer Review 2017
- Peer Review 2015
- Peer Review 2013
- Peer Review 2011
- Past Meetings & Workshops
- Bioenergy Newsletter

The U.S. Department of Energy (DOE) Bioenergy Technologies Office (BETO) hosted the 2023 Project Peer Review in April 2023, in Denver, Colorado. During the event, new research and development (R&D) projects from 11 technology areas within BETO’s portfolio were presented to external subject-matter experts from industry, academia, and federal agencies. Experts reviewed the research and provided critical feedback.
A comprehensive summary report detailing the results of stakeholder and expert feedback on BETO’s latest R&D projects is available below.
Download full 2023 Project Peer Review Report
Individual Report Sections
- Introductory Letter, Executive Summary, Introduction
- Advanced Algal Systems Program
- Biochemical Conversion and Lignin Utilization
- Agile BioFoundry Consortium
- Catalytic Upgrading
- Carbon Dioxide Utilization
- Data, Modeling, and Analysis Program
- Performance-Advantaged Bioproducts, Bioprocessing Separations, and Plastics
- Organic Waste Conversion
- Feedstock Technologies Program
- Systems Development & Integration Program: Emerging and Supporting Technologies
- Systems Development & Integration Program: Scale-Up
The next Project Peer Review will be scheduled for Spring 2025.
RESEARCH ASST I (TEMP)
This is an entry level position that provides administrative and coordination support for multiple projects in the conduct of clinical research projects. This position may assist with study coordination but will not be assigned clinical trials independently. This position may provide coordination for clinical research projects such as registries, retrospective data reviews, studies in long-term follow-up, and other non-interventional studies.
Assist with screening participants for study eligibility & enroll with accuracy in various databases; May complete simple study reimbursement with partners; May provide lab results, not interpretation, to participants)
Responsibilities*
- Demonstrates ability to screen participants for study eligibility & enroll with accuracy in various databases.
- Performs simple study procedures with accuracy.
- Understands protocol structure and how to interpret study requirements to ensure study compliance.
- Understands proper documentation techniques as outlined in the ICH-GCP guidelines.
- May complete simple study reimbursement with partners such as the Human Subjects Incentive Program (HSIP) and the CTSU finance team.
- Demonstrates the ability to find and utilize information from EMR and databases/CTMS/EDC.
- May mark visits as planned/occurred in OnCore.
- May provide lab results, not interpretation, to participants.
- May maintain essential regulatory documents.
- May assist research coordinator in conduct of SIV; attends SIV.
- May assist in and attends monitor visits and or audits.
- Demonstrates the ability to complete simple data collection during study visits (e.g., basic demographic information).
- Enters data to complete forms (CRFs) on paper, databases, or EDCs.
- Assists with collection of external medical records, CLIAs, CAPs, and radiology CDs as assigned.
- May administer minimal risk consents, surveys, and questionnaires.
- Checks own work and confirms accuracy.
- Review of consents for signatures
- Demonstrates understanding of the clinical research objectives associated with the program.
- May communicate with study participants such as sending study correspondence via mail or email
Required Qualifications*
High school diploma or GED is necessary.
Desired Qualifications*
Graduation from a college or university or an equivalent combination of education and experience.
Background Screening
Michigan Medicine conducts background screening and pre-employment drug testing on job candidates upon acceptance of a contingent job offer and may use a third party administrator to conduct background screenings. Background screenings are performed in compliance with the Fair Credit Report Act. Pre-employment drug testing applies to all selected candidates, including new or additional faculty and staff appointments, as well as transfers from other U-M campuses.
Application Deadline
Job openings are posted for a minimum of seven calendar days. The review and selection process may begin as early as the eighth day after posting. This opening may be removed from posting boards and filled anytime after the minimum posting period has ended.
U-M EEO/AA Statement
The University of Michigan is an equal opportunity/affirmative action employer.
Research on speech in autism: Paid Volunteer Opportunity
Announcement details, employee login.
Sign in with your SFUSD Google account to view employee content.
Login for non-SFUSD users
Announcement Message
UCSF is currently recruiting pediatric participants ages 8-16 for a study examining voice, speech and communication in children with autism and children with typical development. Participants will be compensated $400 for completion of all study procedures, which include diagnostic screening, neuropsychological evaluation, and MEG and MRI scans.
For more information please contact [email protected]
SFUSD neither endorses nor sponsors the organization or activity described in this announcement. This distribution is provided as a community service.
Announcement Links
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Health and social care
- Research and innovation in health and social care
- Extreme cold temperatures in domiciliary care
- UK Health Security Agency
Extreme cold temperatures in domiciliary care: executive summary
Published 25 April 2024

© Crown copyright 2024
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/extreme-cold-temperatures-in-domiciliary-care/extreme-cold-temperatures-in-domiciliary-care-executive-summary
Background to the research
In the UK mortality is significantly higher during the winter months (December to March) than in other seasons. On average there are around 35,000 excess winter deaths ( EWDs ) each year in England and Wales. Excess mortality attributed to cold is significantly higher than that attributed to heat, and cold-related mortality is a considerable health burden, particularly in deprived areas. There is also an appreciable increase in risk of mortality during extreme cold for people aged 65 and over across a range of studies and settings. People with long-term conditions (more common among the ‘oldest old’) are also more vulnerable ( 1 ).
While the focus of this research was on people receiving care, carers themselves are also at risk during periods of cold weather alerts. So the aim of this study was to explore both the client and care worker risks as well as general preparedness for cold weather, with a view to identifying mitigations and incorporating these into advice and cold weather planning.
Methodology
The UK Health Security Agency ( UKHSA ) commissioned Discovery Research, an independent research agency, to carry out this study to understand the attitudes and actions of social care practitioners working in domiciliary care during winter months as well as their experiences of planning for cold weather. A qualitative approach was used to explore the research objectives.
Key findings
Risk perceptions of cold weather.
Domiciliary carers had an accurate idea of what to look out for and understood what actions they should be taking to support the health needs of their clients. They identified where some adults receiving care were at more risk from the cold than others and these considerations were built into their preparations.
Planning and preparedness for cold weather
While preparations for cold weather were commonplace, the extent to which plans were formalised as opposed to carers carrying out behaviours they felt were ‘common sense’ was variable. Larger care organisations had more formal plans than the smaller organisations.
Impact of sudden cold weather
Dealing with the impacts of sudden cold weather was a key issue facing the sector. Enabling the safe and efficient transport of carers to clients’ homes in the context of disruption to travel routes was a particular challenge. Activating full community support involving local authorities, a network of care organisations in the area and local charities was felt to be the best course of action to ensure all clients were seen but this was not happening consistently across local authorities.
Impact of increased energy cost
The cost of energy was a particular issue in the winter of 2022 to 2023. Some clients turned their heating down or off, so risking their health, while others left it on continuously, so risking getting into financial hardship. Carers also found this situation difficult to manage and were unsure whether they could or should intervene. They were also keen to ensure they knew how clients could take advantage of the financial support available to them.
Wellbeing and vulnerability
Poor mental health and feelings of isolation were a concern for many clients, particularly during prolonged periods of cold. Some domiciliary carers also felt isolated and unsupported over the course of the winter months and did not feel their personal needs were always considered by their employer.
Information needs
The research identified 3 areas in which information could be improved for domiciliary carers:
1. More tailored and practical formal guidance from UKHSA .
2. Information to enable consistent coordination and communication within agencies and local authorities.
3. Greater clarity about what information to provide to clients to ensure they are getting all the support available.
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. We’ll send you a link to a feedback form. It will take only 2 minutes to fill in. Don’t worry we won’t send you spam or share your email address with anyone.
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

IMAGES
VIDEO
COMMENTS
Research Summary. Definition: A research summary is a brief and concise overview of a research project or study that highlights its key findings, main points, and conclusions. It typically includes a description of the research problem, the research methods used, the results obtained, and the implications or significance of the findings.
So, follow the steps below to write a research summary that sticks. 1. Read the parent paper thoroughly. You should go through the research paper thoroughly multiple times to ensure that you have a complete understanding of its contents. A 3-stage reading process helps.
A research article usually has seven major sections: Title, Abstract, Introduction, Method, Results, Discussion, and References. The first thing you should do is to decide why you need to summarize the article. If the purpose of the summary is to take notes to later remind yourself about the article you may want to write a longer summary ...
research participation. A Narrative Summary is a written summary of your study's research findings. It is a useful way to succinctly summarize the purpose, main findings, and impact of your research study that is shared with research participants. Crafting a Narrative Summary can accompany writing a scientific manuscript and abstract.
A research summary is a piece of writing that summarizes your research on a specific topic. Its primary goal is to offer the reader a detailed overview of the study with the key findings. A research summary generally contains the article's structure in which it is written. You must know the goal of your analysis before you launch a project.
Table of contents. When to write a summary. Step 1: Read the text. Step 2: Break the text down into sections. Step 3: Identify the key points in each section. Step 4: Write the summary. Step 5: Check the summary against the article. Other interesting articles. Frequently asked questions about summarizing.
Hence, the research procedures for selecting participants, analyzing data, and ensuring research rigor differ from those for quantitative research. The following sections address these approaches. table 1 provides a comparative summary of methodological approaches for quantitative and qualitative research.
A research summary is a piece of writing that summarizes the research of a specific topic into bite-size easy-to-read and comprehend articles. The primary goal is to give the reader a detailed outline of the key findings of a research. It is an unavoidable requirement in colleges and universities. To write a good research summary, you must ...
E. Research Methodology (Summary) In paragraph form, describe the basic elements of your research design. Use words that lay practitioners will understand. Word Limit: About 200 words for each separate experiment or study that is described (up to 500 words if three or more experiments or separate studies were conducted).
Participant Summary. The Participant Summary section assembles all of the created Participant Groups and combines the number of participants provided in each group into a grand total. In addition it provides an opportunity to highlight the experience of the principal investigator and the research team (where applicable), as well as describe the ...
The summary section of your paper shows that you understood the basic facts of the research. The analysis shows that you can evaluate the evidence presented in the research and explain why the research could be important. Summary. The summary portion of the paper should be written with enough detail so that a reader would not have to look at ...
A research summary is a concise and informative overview of a research article, report, or thesis. It aims to provide the reader with a clear understanding of the study's purpose, methods, results, and conclusions without having to read the entire document. ... Participants were randomly assigned to either an exercise group or a control group ...
A research summary or a research article comprehensively covers a topic. It is a brief overview of a study typically from a peer-reviewed journal. Many universities and colleges give such assignments and assess the students' performance based on how they interpret the scientific knowledge and data presented in the written academic article. If ...
The Staying Connected Toolkit is a resource for research teams to use to achieve gold standard practices for communicating with participants throughout the course of the study. This toolkit covers: general principles and timelines for communicating with participants throughout their research journey; how to plan research communications ahead of ...
Step 1: Listen to the research interview again. Step 2: Write a transcription of notes (I use Excel) of the entire conversation while listening. Step 3: Tag each (relevant) data point with a need, pain point, motivation, or goal. Step 4: Do a mini-affinity diagram with the session, where you group all the needs, pain points, motivations, and goals.
Participant observation is a research method where the researcher immerses themself in a particular social setting or group, observing the behaviors, interactions, and practices of the participants. This can be a valuable method for any research project that seeks to understand the experiences of individuals or groups in a particular social ...
The goal would be to summarize the results and make it easier for yourself to structure the summary. Step 2. Make an Outline. With the research findings and notes fresh in your mind, proceed to outline your summary. It'll be helpful to structure your thoughts and present everything in a logical order.
Participants: 2-10. 11-25. 25+ Prep Time: Time to run: Research summary template. Synthesize and summarize research findings Analyze and frame problems. ... Research summary template frequently asked questions. Template by , A Twitter icon in black. A LinkedIn icon in black. An Instagram icon in black. ...
Biospecimens from research participants are an essential resource for a broad range of studies, from exploratory, basic science inquiries to clinical trials using well-validated tests. These types of research have been enormously valuable in advancing knowledge about almost every aspect of human health and disease. The conduct of research with human volunteers is dependent on a collaborative ...
Like an abstract in a published research article, the purpose of an article summary is to give the reader a brief, structured overview of the study. To write a good summary, identify what information is important and condense that information for your reader. The better you understand a subject, the easier it is to explain it thoroughly and ...
Every research paper has a research summary. A research summary is a brief overview of what the whole research is about. It is a professional piece of writing that describes your research to the readers. ... Discuss and describe the procedure, materials, participants, design, etc. The analysis of your data must also be included. You may also ...
To structure your methods section, you can use the subheadings of "Participants," "Materials," and "Procedures.". These headings are not mandatory—aim to organize your methods section using subheadings that make sense for your specific study. Note that not all of these topics will necessarily be relevant for your study.
Sharing research findings with participants is recognized as an ethical imperative for the research community. However, most discourse on this topic in mainstream public health takes a paternalistic approach, with researchers retaining the power to choose if, when, and how research findings are shared.
Being Asian in America. In the fall of 2021, Pew Research Center undertook the largest focus group study it had ever conducted - 66 focus groups with 264 total participants - to hear Asian Americans talk about their lived experiences in America. The focus groups were organized into 18 distinct Asian ethnic origin groups, fielded in 18 ...
The U.S. Department of Energy (DOE) Bioenergy Technologies Office (BETO) hosted the 2023 Project Peer Review in April 2023 in Denver, Colorado. During the event, new research and development (R&D) projects from 11 technology areas within BETO's portfolio were presented to external subject-matter experts from industry, academia, and federal agencies. Experts reviewed the research and provided ...
Summary. This is an entry level position that provides administrative and coordination support for multiple projects in the conduct of clinical research projects. This position may assist with study coordination but will not be assigned clinical trials independently. This position may provide coordination for clinical research projects such as ...
Alaska Hatchery Research Program Science Panel meeting January 26, 2024 . Hybrid meeting (in-person meeting in Anchorage and other virtual guests, connected via Microsoft Teams) Summarized meeting notes and decision points . Attendees. Science Panel . Milo Adkison, ADF&G . John Burke, ADF&G and Southern Southeast Regional Aquaculture Association
UCSF is currently recruiting pediatric participants ages 8-16 for a study examining voice, speech and communication in children with autism and children with typical development. Participants will be compensated $400 for completion of all study procedures, which include diagnostic screening, neuropsychological evaluation, and MEG and MRI scans ...
The UK Health Security Agency ( UKHSA) commissioned Discovery Research, an independent research agency, to carry out this study to understand the attitudes and actions of social care practitioners ...
Start Preamble AGENCY: Environmental Protection Agency (EPA). ACTION: Notice of workshop. SUMMARY: The Center for Public Health and Environmental Assessment (CPHEA) within U.S. EPA's Office of Research and Development is announcing a workshop entitled "Workshop to Discuss Policy-Relevant Science to Inform EPA's Integrated Plan for the Review of the Ozone National Ambient Air Quality ...