- Case Report
- Open access
- Published: 15 October 2008

Chronic tophaceous gout presenting as acute arthritis during an acute illness: a case report
- Abhijeet Dhoble 1 ,
- Vijay Balakrishnan 1 &
- Robert Smith 1
Cases Journal volume 1 , Article number: 238 ( 2008 ) Cite this article
15k Accesses
5 Citations
Metrics details
Gout is a metabolic disease that can manifest as acute or chronic arthritis, and deposition of urate crystals in connective tissue and kidneys. It can either manifest as acute arthritis or chronic tophaceous gout.
Case presentation
We present a 39-year-old male patient who developed acute arthritis during his hospital course. Later on, after a careful physical examination, patient was found to have chronic tophaceous gout. The acute episode was successfully treated with colchicines and indomethacin.
Gout usually flares up during an acute illness, and should be considered while evaluating acute mono articular arthritis. Rarely, it can also present with tophi as an initial manifestation.
Gout is a metabolic disease, which is characterized by acute or chronic arthritis, and deposition of monosodium urate crystals in joint, bones, soft tissues, and kidneys [ 1 – 4 ]. In 18 th century, Garrod proposed a causative relationship between elevated uric acid and urate crystal formation, which is underlying pathology for gout [ 4 ]. Gout can either manifest as acute arthritis or chronic arthropathy, which is also called tophaceous gout [ 1 , 2 , 5 ].
A 39-year-old African American male patient was admitted with one-day history of acute left lower quadrant pain, and was diagnosed with acute uncomplicated diverticulitis, confirmed by computed tomography (CT) of the abdomen. His medical and surgical history was unremarkable, and he denied any medication use. He denied smoking or illicit drug use, but admitted occasional alcohol use on every other weekend. He did not follow any particular diet. He had an average built with BMI of 29.6. He was started on intravenous antibiotics and pain medication, which led to significant clinical improvement within two days.
On the third day of hospitalization, he developed acute, severe pain and swelling of the left elbow. Within next few hours, pain worsened and he was unable to move the elbow joint, which was tender, erythematous, and swollen on examination (figure 1 ). Never investigated in the past, we also noted a firm 4 × 6 cm mass on each elbow, and another one surrounding the proximal inter-phalangeal joint of right middle finger (figure 2 ). There was no overlying edema or cellulitis. There were no other swellings or tophi noted especially on toes or ears. When asked particularly, he denied similar episodes in the past. He also denied any episode of swelling of great toe in the past.
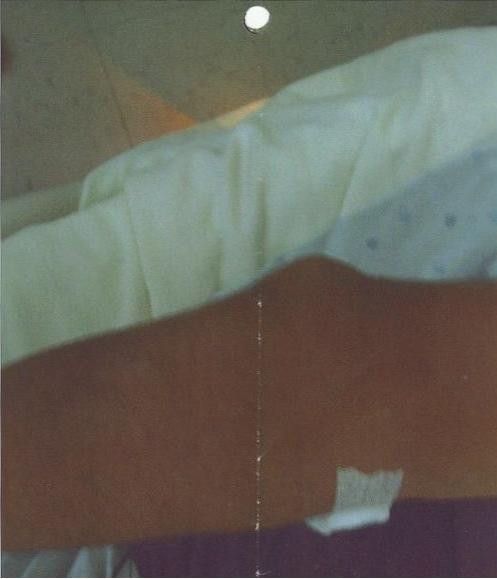
Tophus at the back of right elbow.
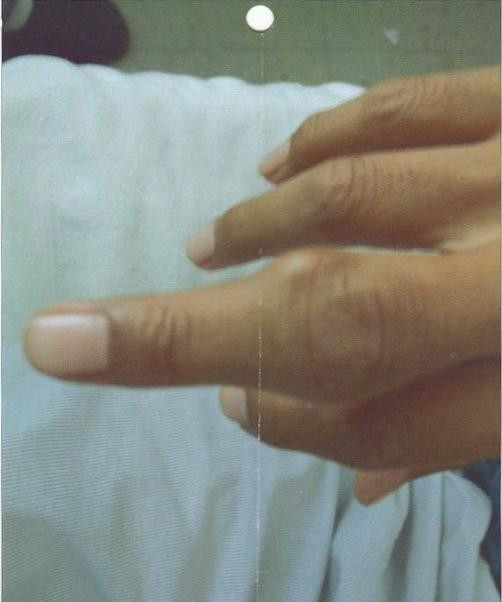
Tophi/tophus around the proximal inter-phalangeal joint of right middle finger.
Plain radiography of left elbow showed joint effusion, and soft tissue swelling. Radiography of other joints including hands and feet was not performed. Laboratory values on the third day are given in table 1 . Liver function test was also performed, and the results were unremarkable. Diagnostic arthocentesis was performed on both the elbows, and revealed negatively birefringent needle-shaped crystals using polarized microscopy in both samples. Detailed analysis of synovial fluid is given in table 2 . The swelling on the right elbow was aspirated to determine the etiology because patient had that swelling for a long time.
The patient responded partially to colchicine, but later had great relief with indomethacin. Colchicine was used at the dose of 0.6 mg every two hourly. He received total of six doses, but it was stopped because he developed severe nausea and vomiting. He admitted that his pain was reduced to 4/10 in intensity from 9/10 before treatment, but swelling was persistent. We initiated indomethacin at 50 mg every eight hourly, and his pain and swelling was relieved to great extent in 48 hours.
Gout is a metabolic disease that can manifest as acute or chronic arthritis, and deposition of urate crystals in connective tissue and kidneys. All patients have hyperuricemia at some point of their disease. But, either normal or low serum uric acid levels can occur at the time of acute attack; and asymptomatic hyperuricemic individuals may never experience a clinical event resulting from urate crystal deposition [ 1 – 4 ]. Low to normal uric acid concentration can be due to excessive excretion of uric acid, crystal formation, or systemic inflammatory state [ 6 , 7 ]; however, exact mechanism is still not completely understood. A diagnosis of gout is most accurate when supported by visualization of uric acid crystals in a sample of joint or bursal fluid, or demonstrated histologically in excised tissue. Synovial fluid analysis of our patient was consistent with inflammatory arthritis. Mild leucocytosis in this patient was due to systemic inflammatory response.
Visible or palpable tophi, as this patient exhibited, are usually noted only among those patients who are hyperuricemic and have had repeated attacks of acute gout, often over many years. However, presentation of tophaceous deposits in the absence of gouty arthritis is also reported [ 5 , 8 ]. Pain and inflammation are manifested when uric acid crystals activate the humoral and cellular inflammatory processes [ 9 ].
During an acute illness, if systemic inflammatory state prevails, such as in an acute infection, cytokines and chemokines triggers inflammation and cause arthritis in the presence of urate crystals [ 10 , 11 ]. Phagocytosis of these crystals by macrophages in the synovial lining cells precedes influx of neutrophils in the joint [ 9 – 11 ]. This process releases various mediators of inflammation locally [ 12 , 13 ].
Hyperuricemia is often present in patients with tophaceous gout, and they can benefit from uric acid lowering therapy early during the course [ 14 , 15 ]. In our patient, serum uric acid and 24-hour urine uric acid level was within normal limits when measured in the hospital before his discharge from the hospital. It was decided to follow him up in the clinic in two weeks, and measure these values again during 'interval gout' before deciding to start him on any particular medication to prevent further attacks of acute arthritis.
Our patient presented with tophi as an initial presentation of gout, which is very rare, but has been reported [ 5 , 8 ]. Investigational studies due to acute elbow joint pain deciphered the underlying mystery of chronic swelling. Systemic inflammatory response secondary to diverticulitis exposed the joints to the effects of urate.
First-line treatments for an acute flare are either oral colchicine and/or non-steroidal anti-inflammatory agents. Systemic or intra-articular corticosteroids can also be used, and are equally effective, but with more side effects [ 16 , 17 ]. Interleukin-1 inhibitors are still under investigation, and are not approved for an acute attack of gout [ 18 ].
Gout usually flares up during an acute illness, and should always be considered while evaluating acute mono articular arthritis in hospitalized patients. Gout can present with tophi as an initial manifestation of the disease process.
Written informed consent was obtained from the patient for publication of this case report and accompanying images in Journal of Medical Case Reports. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Campion EW, Glynn RJ, DeLabry LO: Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987, 82: 421-10.1016/0002-9343(87)90441-4.
Article CAS PubMed Google Scholar
Hall AP, Barry PE, Dawber TR, McNamara PM: Epidemiology of gout and hyperuricemia: A long term population study. Am J Med. 1967, 42: 27-10.1016/0002-9343(67)90004-6.
Logan JA, Morrison E, McGill PE: Serum uric acid in acute gout. Ann Rheum Dis. 1997, 56: 696-7.
Article PubMed Central CAS PubMed Google Scholar
Garrod AB: The Nature and Treatment of Gout and Rheumatic Gout. 1863, 2
Google Scholar
Wernick R, Winkler C, Campbell S: Tophi as the initial manifestation of gout. Report of six cases and review of literature. Arch intern med. 1992, 152: 873-10.1001/archinte.152.4.873.
Urano W, Yamanaka H, Tsutani H, Nakajima H, Matsuda Y, Taniguchi A, Hara M, Kamatani N: The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol. 2002, 29 (9): 1950-3.
CAS PubMed Google Scholar
Simkin PA: The pathogenesis of podagra. Ann Intern Med. 1977, 86: 230.
Hollingworth P, Scott JT, Burry HC: Nonarticular gout: hyperuricemia and tophus formation without gouty arthritis. Arthritis Rheum. 1983, 26: 98-101. 10.1002/art.1780260117.
Beutler A, Schumacher HR: Gout and 'pseudogout': when are arthritic symptoms caused by crystal disposition?. Postgrad Med. 1994, 95: 103-6.
Schumacher HR, Phelps P, Agudelo CA: Urate crystal induced inflammation in dog joints: sequence of synovial changes. J Rheumatol. 1974, 1: 102.
Gordon TP, Kowanko IC, James M, Roberts-Thomson PJ: Monosodium urate crystal-induced prostaglandin synthesis in the rat subcutaneous air pouch. Clin Exp Rheumatol. 1985, 3: 291.
Malawista SE, Duff GW, Atkins E, Cheung HS, McCarty DJ: Crystal-induced endogenous pyrogen production. A further look at gouty inflammation. Arthritis Rheum. 1985, 28: 1039-10.1002/art.1780280911.
Falasca GF, Ramachandrula A, Kelley KA, O'onnor CR, Reginato AJ: Superoxide anion production and phagocytosis of crystals by cultured endothelial cells. Arthritis Rheum. 1993, 36: 105-10.1002/art.1780360118.
Sutaria S, Katbamna R, Underwood M: Effectiveness of interventions for the treatment of acute and prevention of recurrent gout – a systematic review. Rheumatology (Oxford). 2006, 45: 1422-10.1093/rheumatology/kel071.
Article CAS Google Scholar
Wallace SL, Singer JZ: Therapy in gout. Rheum Dis Clin North Am. 1988, 14: 441.
Janssens HJ, Janssen M, Lisdonk van de EH, van Riel PL, van Weel C: Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 371 (9627): 1854-60. 10.1016/S0140-6736(08)60799-0. 2008 May 31
Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Lioté F, McCarthy G, Netter P, Nuki G, Perez-Ruiz F, Pignone A, Pimentão J, Punzi L, Roddy E, Uhlig T, Zimmermann-Gòrska I, EULAR Standing Committee for International Clinical Studies Including Therapeutics: EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006, 65 (10): 1312-24. 10.1136/ard.2006.055269.
So A, De Smedt T, Revaz S, Tschopp J: A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007, 9: R28-10.1186/ar2143.
Article PubMed Central PubMed Google Scholar
Download references
Acknowledgements
We thank patient for giving us consent for the publication of the case report.
Author information
Authors and affiliations.
Department of Internal Medicine, Michigan State University, East Lansing, Michigan, USA
Abhijeet Dhoble, Vijay Balakrishnan & Robert Smith
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Abhijeet Dhoble .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
All authors contributed equally in collecting patient data, chart review, and editing medical images. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Dhoble, A., Balakrishnan, V. & Smith, R. Chronic tophaceous gout presenting as acute arthritis during an acute illness: a case report. Cases Journal 1 , 238 (2008). https://doi.org/10.1186/1757-1626-1-238
Download citation
Received : 09 October 2008
Accepted : 15 October 2008
Published : 15 October 2008
DOI : https://doi.org/10.1186/1757-1626-1-238
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diverticulitis
- Serum Uric Acid
- Uric Acid Level
Cases Journal
ISSN: 1757-1626
- Case Report
- Open access
- Published: 06 January 2014
Gout in a 15-year-old boy with juvenile idiopathic arthritis: a case study
- Hallie Morris 2 ,
- Kristen Grant 1 ,
- Geetika Khanna 3 &
- Andrew J White 4
Pediatric Rheumatology volume 12 , Article number: 1 ( 2014 ) Cite this article
18k Accesses
14 Citations
10 Altmetric
Metrics details
Joint pain is a common complaint in pediatrics and is most often attributed to overuse or injury. In the face of persistent, severe, or recurrent symptoms, the differential typically expands to include bony or structural causes versus rheumatologic conditions. Rarely, a child has two distinct causes for joint pain. In this case, an obese 15-year-old male was diagnosed with gout, a disease common in adults but virtually ignored in the field of pediatrics. The presence of juvenile idiopathic arthritis (JIA) complicated and delayed the consideration of this second diagnosis. Indeed, the absence of gout from this patient’s differential diagnosis resulted in a greater than two-year delay in receiving treatment. The patients’ BMI was 47.4, and he was also mis-diagnosed with osteochondritis dissecans and underwent medical treatment for JIA, assorted imaging studies, and multiple surgical procedures before the key history of increased pain with red meat ingestion, noticed by the patient, and a subsequent elevated uric acid confirmed his ultimate diagnosis. With the increased prevalence of obesity in the adolescent population, the diagnosis of gout should be an important consideration in the differential diagnosis for an arthritic joint in an overweight patient, regardless of age.
In the pediatric population, there are numerous causes of joint pain, stiffness, and swelling. Many can be attributed to minor activity or overuse-related injury, especially in the overweight and obese populations [ 1 ], but in the face of persistent, severe, or recurrent symptoms, other diagnoses must be considered. These typically fall into two categories in children and adolescents: bony or structural causes [ 2 ], or rheumatologic conditions [ 3 ]. Careful history and physical examination, along with use of imaging and laboratory studies, can often distinguish between the two [ 4 , 5 ]; however, when a complete work-up is performed and no clear answer emerges, the differential must be expanded [ 6 ]. In the rare case in which a firm primary diagnosis has been made, it is more difficult still to consider additional, secondary, causes of joint pain.
The following case describes an adolescent young man with severe ankle pain, as well as multiple other joint complaints, who was correctly diagnosed and treated for polyarticular juvenile idiopathic arthritis. While the majority of his joints improved, his ankle continued to be extremely tender and swollen. After two years of aggressive medical treatment, surgical procedures, and multiple imaging studies, it was careful probing into his history and classic physical examination findings that ultimately led to the additional diagnosis of gout.
Case presentation
A 15-year-old obese Hispanic young man presented to the orthopedic surgery service with right-sided ankle pain. His pain began after a sports injury approximately one year prior to presentation but did not respond to immobilization, physical therapy, or prolonged rest. The pain was located at the medial side of the ankle, was worse with activity, and was accompanied by intermittent swelling. He took ibuprofen at night occasionally that provided modest relief.
His past medical history was unremarkable. Family history was negative for autoimmune disease including JIA. His only medication was occasional ibuprofen, and his immunizations were up to date.
On examination, the patient was heavyset, weighing 142.8 kg and standing 173.6 cm tall, with a BMI of 47.4. His vital signs were normal and he was in no distress. Musculoskeletal exam revealed bilateral limited ankle dorsiflexion, worse on the right. He had tenderness to palpation over the medial aspect of his ankle just anterior to the medial malleolus. All other joints were normal.
His initial work-up consisted of radiographs of his right ankle, showing evidence of a healing osteochondritis dissecans (OCD) lesion. He was instructed to continue physical therapy and follow up in three months with the possibility of arthroscopy of the affected joint if there was no improvement.
At follow-up, his pain was unchanged. Radiographs showed the previously seen presumed OCD lesion, now with a sclerotic border. He was scheduled for an MRI of the ankle, which was read as more consistent with a chondroblastoma versus an inflammatory process.
Several months later, he began complaining of left shoulder pain and decreased range of motion of gradual onset. He denied any trauma causing this new complaint. He was again seen by the orthopedic service and found to have significantly decreased range of motion and AC joint tenderness. Radiographs of the shoulder did not show any abnormalities. He was diagnosed with adhesive capsulitis and instructed to perform stretching exercises and take acetaminophen for pain. An MRI of the shoulder was performed given the unusual age of presentation for adhesive capsulitis, which revealed evidence of inflammation and synovitis consistent with juvenile idiopathic arthritis. He was referred to the rheumatology service.
The patient described daily pain and some morning stiffness for several weeks at a time, which would then subside for several weeks before returning. The pain and stiffness involved the right elbow, left knee, right wrist and several fingers in addition to the left shoulder and right ankle previously described. Examination by the pediatric rheumatologist noted improvement of his range of motion of the left shoulder with his home exercises and was back within normal limits. He did have moderate pain with extension of the shoulder. His left wrist was also tender with limited range of motion. His range of motion was limited bilaterally in the ankles and multiple PIP joints were painful and swollen. Considering the symmetric joint distribution, involvement of PIPs, and an elevated rheumatoid factor of 24.7 IV/mL (nl 0.0-13.9), the patient’s presentation was felt to be most consistent with early onset rheumatoid arthritis, or RF + polyarticular JIA. Subsequently, an anti-CCP antibody test was positive. He was prescribed naproxen and methotrexate. Laboratory studies from this initial visit were also notable for an elevated CRP 6.7 mg/dL, WBC 12.6 K/uL, Hgb of 12.3 g/dL, and ESR of 61 mm/hr. ANA, dsDNA, and HLA-B27 were negative.
The patient presented for follow-up approximately 2.5 months after beginning the naproxen and methotrexate regimen. At that time, the pain in the majority of his joints had improved substantially, however, the right ankle had become more tender and swollen. The pain was worse in the morning, to the point that he began using crutches. He was started on twice weekly etanercept injections, but when he experienced no relief, was subsequently switched to adalimumab and then to rituximab.
Given the lack of response of the patient’s ankle to this therapeutic regimen (Figure 1 ) over the course of the next year, despite improvement in his other joints, arthroscopic exploration of the right ankle with synovectomy and potential OCD curettage was performed. During surgery, the patient was found to have “significant debris” including a “crystalline white substance” within the joint space, which was attributed by the surgeons to “the previous steroid injection.” The fluid was not sent for culture, cell count or crystal studies, despite the fact that the patient had not ever received a steroid injection in that joint. The debris was removed, but not sent for pathology.
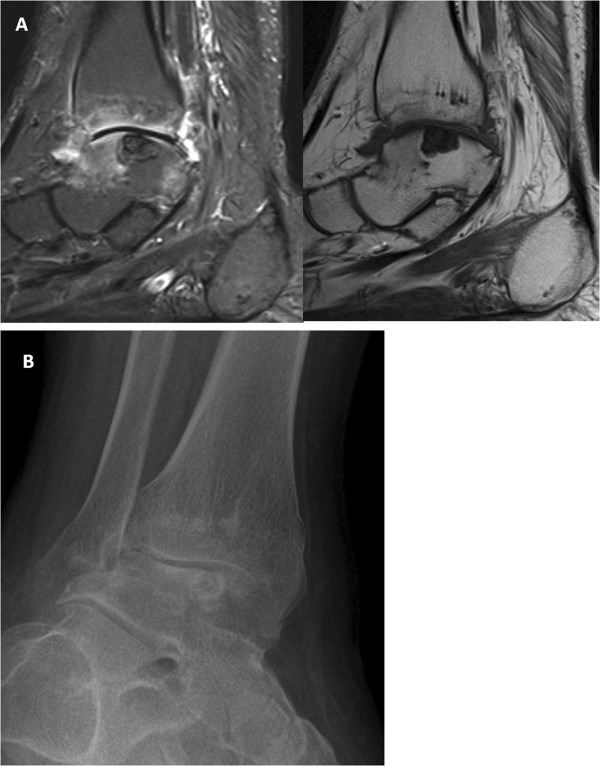
Imaging of gouty joint in an adolescent. (A) Synovitis on MRI with and without IV contrast of the right ankle with osteitis in the distal tibia and talar dome. Talar dome lesion was thought to represent an osteochondritis dissecans/osteonecrosis. (B) Radiographs performed 15 months later show marked joint space loss with persistent talar dome lesion which likely represents an intraosseous tophus.
After recovery from surgery, the patient returned to the rheumatology service for further management of his JIA. He continued to have pain and stiffness of his right ankle as well as several other joints that was difficult to manage. He was eventually started on a low dose of prednisone, previously avoided given his weight, which did offer moderate symptomatic relief. Ultimately the medication regimen of prednisone, etanercept, methotrexate, naproxen, and sulfasalazine had the greatest impact on his JIA symptoms, although his right ankle continued to be the most painful joint.
2.5 years after he first presented, the patient developed multiple non-tender scattered subcutaneous nodules over the extensor surface of his bilateral forearms, his left elbow, and his right knee (Figure 2 ). Their etiology was unclear, but attributed to rheumatoid nodules. Four months later, however, the patient spontaneously noted that his ankle pain seemed to worsen following the ingestion of red meat. With this new data in hand, a uric acid level was sent and was extremely elevated at 13.3 mg/dL. The nodules were deemed to be consistent with tophi, virtually pathognomonic for gout. The patient was started on colchicine and then allopurinol and improved. Over the course of time he did begin to complain of pain in his big toe, a more classic presentation of gout. Uric acid levels however, remained high, running between 11.7 and 13.5 mg/dL over the following year.

X-ray imaging of gouty tophus in an adolescent. Lateral view of the forearm shows subcutaneous nodules along the dorsal aspect of the proximal forearm.
At the time of the patient’s transition to adult care, he continued to exhibit active symptomatology, with joint pain in the knees, elbows, and ankles, limited range of motion in the aforementioned joints as well as his wrists, MCP’s and PIP’s, and difficulty ambulating. He continues to be treated for both JIA and gout.
Joint pain is a common complaint in pediatrics and in the face of persistent, severe, or recurrent symptoms, the differential typically expands to include bony or structural causes versus rheumatologic conditions. In this case, however, diagnosis was complicated because the child had two distinct etiologies causing joint pain. Moreover, the patient’s second diagnosis was gout, a very uncommon condition in a pediatric patient, even in the setting of morbid obesity.
Poly-articular juvenile idiopathic arthritis was considered as the unifying diagnosis when the patient presented with involvement of multiple joints. The typical presenting features of JIA are morning stiffness, pain and swelling of the joints, limited range of motion, and joint contractures [ 7 ]. Radiographs may show some soft tissue swelling and osteopenia early, with subchondral sclerosis and erosions evident after long-standing disease, but MRI has been shown to be more sensitive for bone marrow edema and tenosynovitis, as well as bone erosions, cartilage lesions, and synovial hypertrophy [ 8 ]. JIA is most often seen in children of European descent, but affects children throughout the world. The disease is thought to be idiopathic with its cause poorly understood, although there is mounting evidence that autoimmunity may be involved [ 7 ]. There is also thought to be potential genetic susceptibility in affected individuals [ 9 ]. While the majority of the patient’s overall complaints did seem to fit the diagnosis of JIA both in his clinical presentation and laboratory and imaging results, as well as his response to treatment, his right ankle continued to be refractory to treatment.
The acquisition of additional history led to the consideration of gout as a second diagnosis. Historically, gout has affected predominantly older, overweight men but in more recent years the male to female ratio has fallen to 2:1 [ 10 ]. A resurgence of gout across the population has been noted in recent years, and juvenile gout has also begun to be reported, with many of the cases being due solely to known risk factors such as being overweight [ 11 , 12 ]. On imaging, plain films may show little evidence of gout in early stages, but later in the course can show joint effusions, bony erosions, or tophi within the joint. Gout can appear similar to other arthritides on MRI, with mild bone marrow edema, tenosynovitis, and bony erosions, making diagnosis difficult but important to consider, especially in the setting of an overweight or obese patient. However, if tophi are present, MRI is able to detect this as a potentially differentiating characteristic. Other imaging studies may be of more use to differentiate gout from other diagnoses, as ultrasound may be able to detect crystals within the joint space, and can even differentiate between gout and pseudogout [ 13 ]. The gold standard for diagnosing gout remains the acquisition of urate crystals from synovial fluid. In the absence of this data, however, the presence of 2 of the 3 Rome clinical criteria (uric acid >7.0 mg/dL, history of painful joint with abrupt onset and remission within 2 weeks, and presence of tophus), as were existent in our patient, was found to have a positive predictive value of 76.9%, and a specificity of 88.5% [ 14 ].
While it seems likely the patient did have gout, gout alone does not seem most likely given the clinical picture. Gout simulating JIA is a possibility; a patient with untreated gout can develop bony erosions and deformities, leading to the disappearance of the intercritical periods which are usually pathognomonic of gout. Typically, however, one would expect gout to present as an episodic arthritis. Even in untreated individuals, complete resolution of the earliest attacks nearly always occurs within several weeks, which this patient never experienced. Moreover, the symmetric joint distribution with involvement of the PIPs along with a positive rheumatoid factor and CCP antibody point toward the concurrent JIA diagnosis in this case.
There were several missed opportunities to diagnose this patient earlier in his course. First, gout was not considered as a part of the original differential because of its propensity to affect older individuals. As the epidemic of childhood obesity grows, adult conditions usually a result of long term lifestyle consequences are being seen more frequently in the pediatric population, most notably type II diabetes but also musculoskeletal complaints and, as in this case, gout.
Secondly, if gout had been on the differential, the radiologic features may have been recognized as being consistent with this condition. Third, a uric acid level was not sent until after the additional diet history was obtained. And finally, proper examination of joint fluid, from either aspiration or surgical debridement, would have revealed the presence of negatively birefringent crystals, providing a timely diagnosis.
In conclusion, gout was diagnosed in this teenage patient with longstanding juvenile idiopathic arthritis. The diagnosis of gout should therefore be an important element of the differential for a refractory painful joint in an overweight patient regardless of age, and regardless of pre-existing diagnoses. Failing to consider this diagnosis may result in delay of optimal treatment and cause long-term effects of bone erosion and joint destruction. Sending joint fluid for crystalline analysis, checking uric acid levels, and performing imaging studies, specifically non-invasive, cost effective modalities such as ultrasound, are all reasonable parts of a complete work-up in any child with arthritis.
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Deere KC: Obesity is a risk factor for musculoskeletal pain in adolescents: findings from a population-based cohort. Pain. 2012, 153 (9): 1932-1938. 10.1016/j.pain.2012.06.006.
Article PubMed Google Scholar
Amendola A, Panarella L: Osteochondral lesions: medial vs. lateral, persistent pain, cartilage restoration options and indications. Foot Ankle Clin. 2009, 14 (2): 215-227. 10.1016/j.fcl.2009.03.004.
Jennings F, Lambert E, Fredericson M: Rheumatologic diseases presenting as sports-related injuries. Sports Med. 2008, 38 (11): 917-930. 10.2165/00007256-200838110-00003.
Punaro M: Rheumatologic conditions in children who may present to the orthopaedic surgeon. J Am Acad Orthop Surg. 2011, 19 (3): 163-169.
PubMed Google Scholar
Wukich DK, Tuason DA: Diagnosis and treatment of chronic ankle pain. Instr Course Lect. 2011, 60: 335-350.
Choudhary S, McNally E: Review of common and unusual causes of lateral ankle pain. Skeletal Radiol. 2011, 40 (11): 1399-1413. 10.1007/s00256-010-1040-z.
Gowdie PJ, Tse SM: Juvenile idiopathic arthritis. Pediatr Clin North Am. 2012, 59 (2): 301-327. 10.1016/j.pcl.2012.03.014.
Breton S, Jousse-Joulin S, Finel E, Marhadour T, Colin D, de Parscau L, Devauchelle-Pensec V: Imaging approaches for evaluating peripheral joint abnormalities in juvenile idiopathic arthritis. Semin Arthritis Rheum. 2012, 41 (5): 698-711. 10.1016/j.semarthrit.2011.08.004.
Prahalad S, Conneely KN, Jiang Y, Sudman M, Wallace CA, Brown MR: Susceptibility to childhood onset rheumatoid arthritis: investigation of a weighted genetic risk score that integrates cumulative effects of variants at five genetic loci. Arthritis Rheum. 2013, 65 (6): 1663-1667. 10.1002/art.37913.
Article PubMed Central CAS PubMed Google Scholar
Zampogna G, Andracco R, Parodi M, Cutolo M, Cimmino MA: Has the clinical spectrum of gout changed over the last decades?. Clin Exp Rheumatol. 2012, 30 (3): 414-416.
CAS PubMed Google Scholar
Chen SY, Shen ML: Juvenile gout in Taiwan associated with family history and overweight. J Rheumatol. 2007, 34 (11): 2308-2311.
Kedar E, Simkin PA: A perspective on diet and gout. Adv Chronic Kidnet Dis. 2012, 19 (6): 392-397. 10.1053/j.ackd.2012.07.011.
Article Google Scholar
Dalbeth N, Doyle AJ: Imaging of gout: an overview. Best Pract Res Clin Rheumatol. 2012, 26 (6): 823-838. 10.1016/j.berh.2012.09.003.
Malik A, Schumacher HR, Dinnella JE, Clayburne GM: Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid analysis. J Clin Rheumatol. 2009, 15 (1): 22-24. 10.1097/RHU.0b013e3181945b79.
Download references
Author information
Authors and affiliations.
Boston Combined Residency Program, PGY1, Boston, Mass, USA
Kristen Grant
Washington University School of Medicine, WUSM IV, St Louis, Missouri, USA
Hallie Morris
Division of Radiology, Washington University School of Medicine, St Louis, Missouri, USA
Geetika Khanna
Division of Pediatric Rheumatology, Washington University School of Medicine, St Louis, Missouri, USA
Andrew J White
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Andrew J White .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
KG participated in background research and drafting of the manuscript. HM was involved in drafting and revision of the manuscript. GK contributed figures and their impressions. AW conceived of the study, participated in its coordination, and was involved in drafting and revision of the manuscript. All authors read and approved of the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver ( https://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Morris, H., Grant, K., Khanna, G. et al. Gout in a 15-year-old boy with juvenile idiopathic arthritis: a case study. Pediatr Rheumatol 12 , 1 (2014). https://doi.org/10.1186/1546-0096-12-1
Download citation
Received : 14 October 2013
Accepted : 24 December 2013
Published : 06 January 2014
DOI : https://doi.org/10.1186/1546-0096-12-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Juvenile idiopathic arthritis
- Osteochondritis dissecans
- Treatment failure
Pediatric Rheumatology
ISSN: 1546-0096
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Case report
- Open access
- Published: 12 April 2021
Severe erosive lesion of the glenoid in gouty shoulder arthritis: a case report and review of the literature
- Huricha Bao 1 na1 ,
- Yansong Qi 1 na1 ,
- Baogang Wei 1 ,
- Bingxian Ma 1 ,
- Yongxiang Wang 1 &
- Yongsheng Xu 1
BMC Musculoskeletal Disorders volume 22 , Article number: 343 ( 2021 ) Cite this article
7479 Accesses
7 Citations
7 Altmetric
Metrics details
Gout is a metabolic disease characterized by recurrent episodes of acute arthritis. Gout has been reported in many locations but is rarely localized in the shoulder joint. We describe a rare case of gouty arthritis involving bilateral shoulder joints and leading to severe destructive changes in the right shoulder glenoid.
Case presentation
A 62-year-old male was referred for pain and weakness in the right shoulder joint for two years, and the pain had increased in severity over the course of approximately nine months. A clinical examination revealed gout nodules on both feet and elbows. A laboratory examination showed a high erythrocyte sedimentation rate (ESR), high levels of C-reactive protein and hyperuricemia, and an imaging examination showed severe osteolytic destruction of the right shoulder glenoid and posterior humeral head subluxation. In addition, the left humeral head was involved and had a lytic lesion. Because a definite diagnosis could not be made for this patient, a right shoulder biopsy was performed. The pathological examination of the specimen revealed uric acid crystal deposits and granulomatous inflammation surrounding the deposits. After excluding infectious and neoplastic diseases, the patient was finally diagnosed with gouty shoulder arthritis.
Conclusions
Gout affecting the bilateral shoulder joints is exceedingly uncommon, and to our knowledge, severe erosion of the glenoid has not been previously reported. When severe erosion is present, physicians and orthopedic surgeons should consider gouty shoulder arthritis according to previous medical history and clinical manifestations.
Peer Review reports
Gouty arthritis is usually monoarticular and frequently involves the synovial joints of the feet and hands and, rarely, the shoulders [ 1 , 2 , 3 ]. Monosodium urate crystals produce an inflammatory response that generally results in swollen, tender, hot joints. As described in the literature, the manifestations of shoulder gout are tophaceous deposits in the rotator cuff, intraosseous tophi in the humeral head, and tophi in the bursa around the shoulder joint [ 4 , 5 ]. Here, we report a rare case of gout involving bilateral shoulder joints that caused severe erosion of the right shoulder glenoid. In the clinical diagnosis and treatment process, gout may be misdiagnosed as degenerative, infectious arthritis or a malignancy, resulting in delays in diagnosis and treatment. To date, only four cases of shoulder gout have been reported in the English-language literature identified in PubMed, and we provide a brief literature review concerning shoulder gout [ 4 , 5 , 6 , 7 ]. The patient was informed that data concerning the case would be submitted for publication, and he provided consent.
A right-hand-dominant, 62-year-old obese man presented to our department due to progressive right shoulder pain and weakness. There was no history of recent trauma to the right shoulder. He had a 2-year history of intermittent pain in the right shoulder. Nine months prior, he started to experience worsening pain and weakness in the right shoulder with the restriction of active shoulder motion. He had been treated with conservative treatment (acupuncture, physical therapy, and subacromial steroid injections), which provided short periods of relief. The pain did not disappear, and he visited our hospital for further examination and treatment.
His past medical history included right clavicle fracture, hypertension, and gout. Thirty-six years prior, he suffered a right clavicle middle-shaft fracture that was treated conservatively with a figure-of-eight bandage. The patient recalled that there was no abnormality in the right shoulder at that time. He had a 20-year history of long-standing but suboptimally treated gout, and the gout intermittently led to redness and pain in the feet, which occurred 4–5 times a year. The symptoms were relieved by colchicine during acute episodes, and no systemic treatment was given. The patient had a body mass index of 31.9 kg/m 2 and no previous history of tuberculosis. The patient consumed excessive alcohol and had an alcohol consumption history of 250 ml/day for 30 years; additionally, he smoked 20 cigarettes a day for 35 years and followed no particular diet.
A clinical evaluation revealed that his right shoulder joint had a limited active range of motion, and the passive range of motion was nearly normal. On palpation, tenderness was noted in the anterior and posterior aspects of the shoulder joint, and there was no warmth, erythema, swelling, or redness. When the patient’s upper limb was raised, abducted, and externally rotated, the shoulder joint had a sense of movement, and there were a popping sound and a feeling of shoulder reduction. Gouty tophi were observed on the dorsal aspect of the bilateral great toe and extensor aspect of the bilateral elbows; all of the patient’s other joints were clinically normal, and the examination revealed nothing else of note. Plain radiographs of the affected shoulder showed glenohumeral joint space narrowing, erosions of the glenoid, and osteophyte formation on the inferior aspect of the glenoid. At the superolateral point of the humeral head, a lytic lesion (arrow) was detected, and malunion of the right clavicle fracture was also seen (Fig. 1 ). To assess the integrity of the soft tissue of the shoulder joint, we ordered a magnetic resonance imaging (MRI) examination, which revealed that the axial and coronal proton density-weighted, fat-suppressed MRI exhibited an intact rotator cuff, joint effusion, synovial proliferation, effusion within the biceps long head tendon sheath, humoral head superolateral cystic erosion, posterior humeral head subluxation, and severe glenoid erosion (Fig. 2 ). A laboratory examination revealed an elevated uric acid level of 594 µmol/L (normal range 208.00-428.00 µmol/L), erythrocyte sedimentation rate (ESR) of 65 mm/h, C-reactive protein level of 34 mg/L, leukocyte count of 8.35 × 10 9 /L, and hemoglobin level of 12.8 g/dL. The patient had a negative test for rheumatoid factor and anti-citrullinated protein antibodies (ACPAs). The liver and kidney function of the patient were normal. No abnormalities were found on electromyography of the upper extremities. In consideration of the possibility of shoulder joint infection or malignancy, arthrocentesis was performed, and 20 ml of fluid was aspirated. The fluid was macroscopically cloudy and yellow. The synovial analysis revealed an inflammatory cell count with leukocytes 5200/mm 3 , which were predominantly neutrophils. Gram staining of the fluid was negative, and no organisms were cultured. A cytology analysis and the joint fluid Xpert MTB/RIF test were negative. A polarizing microscope was not available in our hospital; therefore, we could not examine the synovial fluid for crystals.
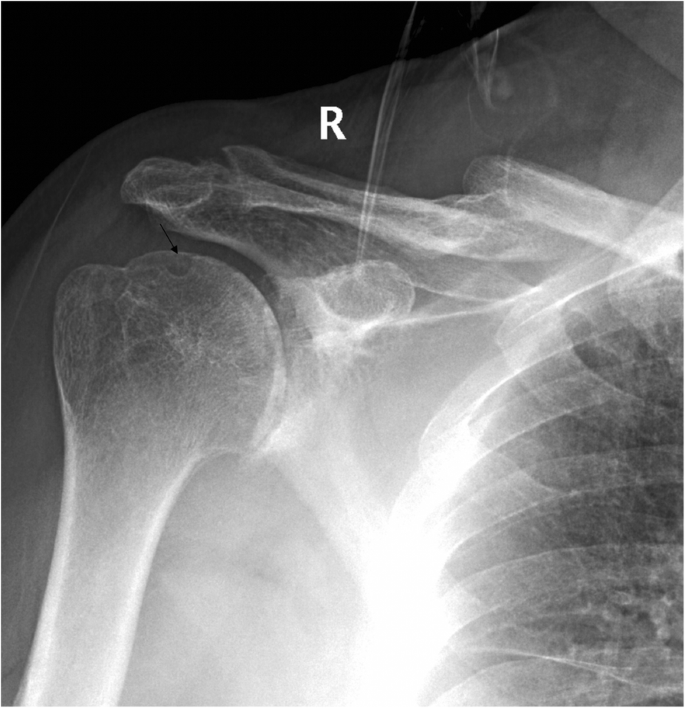
Plain radiographs of the right shoulder showed glenohumeral joint space narrowing and osteophyte formation on the inferior aspect of the glenoid. At the superolateral point of the humeral head, a lytic lesion (arrow) was detected, and malunion of the right clavicle fracture was also seen
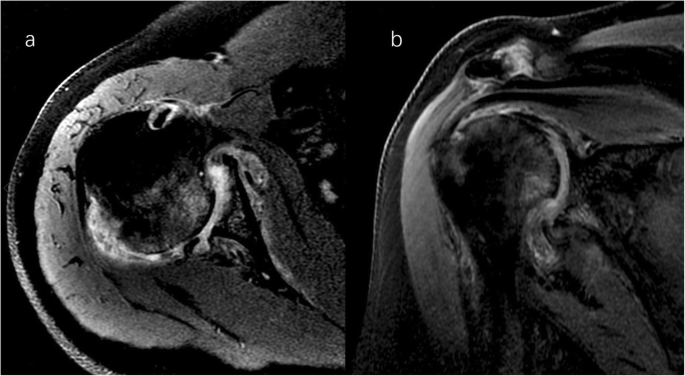
Right shoulder magnetic resonance imaging (proton density-weighted fat-suppressed sequence ). Axial ( a ) and coronal ( b ) views revealed intact rotator cuff, joint effusion, synovial proliferation, effusion within the biceps long head tendon sheath, humoral head superolateral cystic erosion, posterior humeral head subluxation, and severe glenoid erosion
The plain radiograph of the chest showed no neoplastic or tuberculous changes. Serendipitously, the radiograph revealed a round contour lytic lesion in the left humeral head with sclerotic borders near the articular surface (Fig. 3 ). Therefore, a bilateral shoulder joint computed tomography (CT) scan and left shoulder MRI examination were performed. An infectious shoulder etiology appeared unlikely, and differential diagnoses included destructive arthritis or neoplastic lesions. Ultrasound and dual-energy CT imaging of the shoulder joints were not performed on this patient.
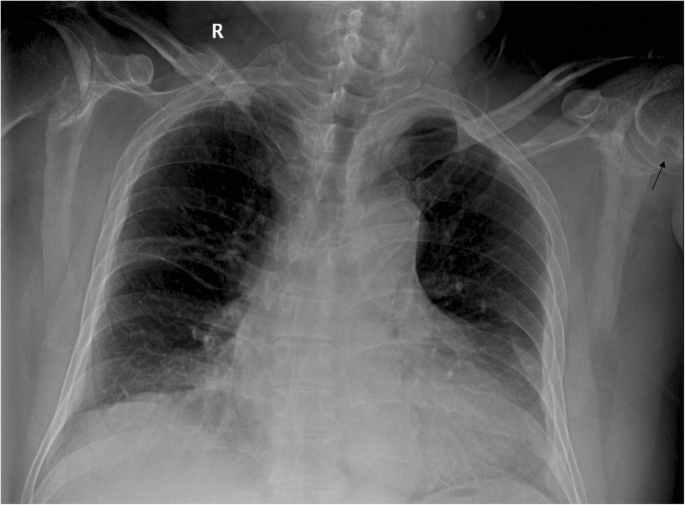
The plain radiograph of the chest showed no neoplastic or tuberculous changes. Serendipitously, the radiograph revealed a round contour lytic lesion (arrow) in the left humeral head with sclerotic borders near the articular surface
The CT scan demonstrated severe destructive lytic changes at the glenoid and erosive lesions and posterior subluxation of the humeral head in the right shoulder. A faint amorphous opacity could also be seen at the posterior capsule of the right shoulder. Circular lytic lesions in the left humeral head with sclerotic borders near the articular surface broke through the articular cartilage (Fig. 4 ). Left shoulder MRI revealed that the axial and coronal proton density-weighted, fat-suppressed MRI showed effusion, lytic destruction of the subchondral bone of the humeral head, erosion, and collapse of the articular cartilage medial to the lesion (Fig. 5 ). However, we still could not determine the cause of the bony destruction of the shoulder joint. Therefore, several biopsies were taken from the capsule and synovial membrane of the right shoulder. The biopsy showed inflammatory cells and gout crystals, and there was no evidence of malignancy or tuberculosis (Fig. 6 ). Based on these findings, we made a diagnosis of gouty arthritis of the bilateral shoulder. Etoricoxib and febuxostat treatment improved the patient’s clinical condition. The patient refused surgical intervention and decided to continue receiving physical therapy and medication for symptom control. After physical therapy and medication, the pain in the right shoulder diminished further but was not eliminated.
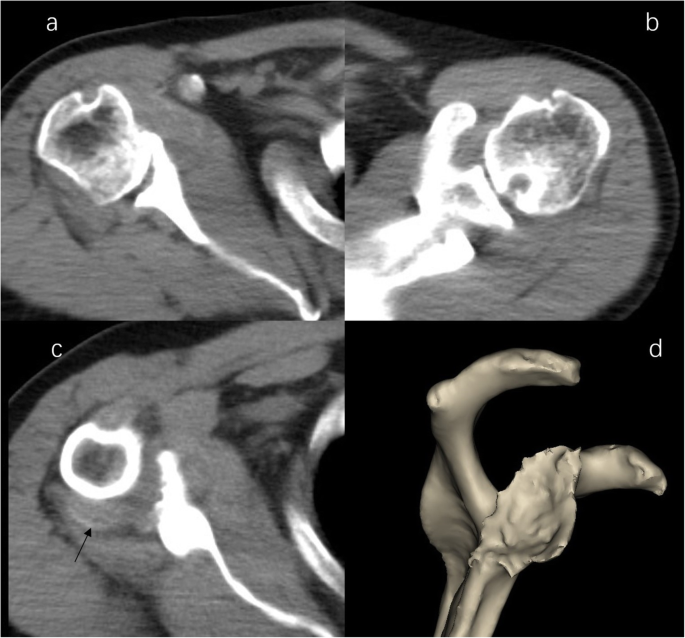
CT image of shoulder. b Right shoulder CT image demonstrated severe destructive lytic changes at the glenoid and erosive lesions and posterior subluxation of the humeral head in the right shoulder. b Left shoulder CT image shows a circular lytic lesion in the left humeral head with sclerotic borders near the articular surface broke through the articular cartilage. c a faint amorphous opacity (arrow) be seen at the posterior capsule of right shoulder. d 3D image shows severe destructive defect of the right shoulder glenoid
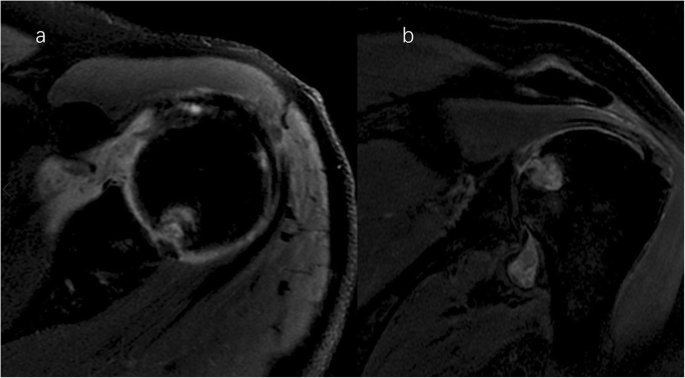
Light shoulder magnetic resonance imaging (proton density-weighted fat-suppressed sequence ). Axial ( a ) and coronal ( b ) MRI image demonstrate effusion, cystic destruction of the subchondral bone of the humeral head, erosion, and collapse of articular cartilage medial to the lesion
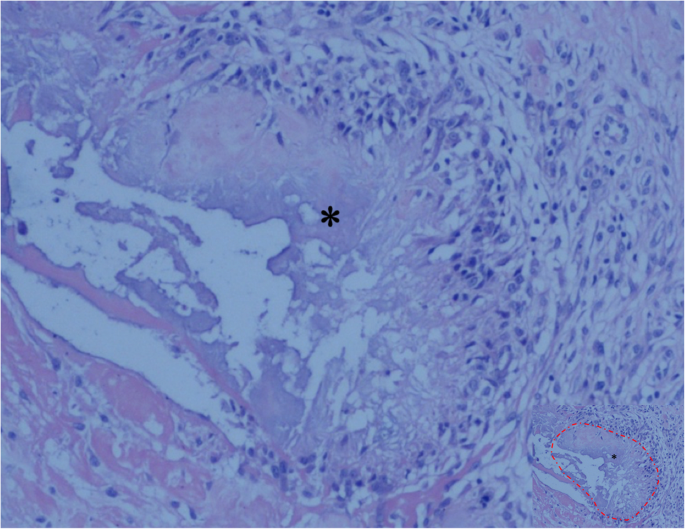
Tissue specimen from the right shoulder showing the deposition of uric acid crystals (asterisk, the part marked with a red dashed line in image with reduced size) surrounded by granulomatous inflammation, and there was no evidence of malignancy or tuberculosis (hematoxylin and eosin; original magnification, ×100)
Discussion and conclusions
Gout is a common inflammatory joint disease characterized by monosodium urate crystal deposition in joints and connective tissue. Gout often affects the feet, hands, elbows, and knees and is relatively uncommon in the shoulder joint. We presented a rare case of gouty arthritis involving bilateral shoulder joints and leading to severe destructive changes in the right shoulder glenoid.
The clinical stage of gout is divided into asymptomatic hyperuricemia, acute gouty arthritis, the intercritical period, and chronic tophaceous gout [ 8 ]. The typical clinical manifestation of acute gouty arthritis is characterized by sudden, severe pain, swelling, redness, and tenderness in the joints. Poorly controlled gout may develop into chronic tophaceous gout, and long-standing gout may present with more atypical symptoms. A chronic deposition of monosodium urate crystals in joints and other body tissues can manifest as a wide array of presentations and can lead to severe joint damage. Such atypical presentations are likely a result of the complexity of reasons. In our case, the patient’s symptoms were pain and weakness in the right shoulder, and the onset of symptoms was relatively insidious, while the patient’s left shoulder joint did not have any clinical symptoms or discomfort. A plain radiograph of the patient’s right shoulder joint revealed degenerative changes, including glenohumeral joint space narrowing, erosion, and osteophyte formation on the glenoid, with a lytic lesion on the humeral head. The left shoulder showed an osteolytic lesion of the humeral head. MRI revealed an intact rotator cuff, inflammatory synovitis, humoral head cystic erosion, posterior humerus subluxation, and a severe glenoid defect. The CT scan further confirmed the severe bone erosive defect of the right shoulder glenoid and posterior humeral head subluxation. From the CT scan, we also observed a round contour lytic lesion with sclerotic borders near the articular surface in the left humeral head. We did not find obvious intra- or extra-articular gout crystal deposition on plain radiographs, CT scans, or MRI images of the bilateral shoulder joints, except in the bilateral humeral head and at the posterior capsule of the right shoulder.
Radiological characteristics of chronic gouty arthritis include punched-out erosions with overhanging cortex and sclerotic margins, preservation of joint space, and dense nodules of soft tissues, which are sometimes calcified [ 9 ]. The typical MRI manifestations of tophaceous gouty arthritis are homogeneous intermediate signal intensity on T1-weighted images and heterogeneous intermediate-to-low signal intensity on T2-weighted images, depending on the calcium concentration within a tophus [ 10 ]. There are several descriptions of gouty shoulder arthritis in the literature, as well as rare cases of coexisting gout and septic shoulder arthritis. Sean et al. first reported a case of subacromial impingement caused by tophaceous gout of the rotator cuff. There was no abnormality on MRI except supraspinatus tendonitis. Arthroscopic findings revealed tophaceous deposits of the supraspinatus and subscapularis tendon [ 4 ]. Chao et al. reported a case of tophaceous gout involving the rotator cuff. In this case, the patient complained of intermittent pain and a limited range of motion of the right shoulder after a shoulder injury. A plain radiograph of the right shoulder demonstrated a faint amorphous opacity above the humeral head. MRI revealed urate crystal deposits in intrasubstance areas and the articular side of the supraspinatus tendon [ 5 ]. Toru et al. reported the coexistence of gouty and septic shoulder arthritis after arthroscopic rotator cuff repair surgery. The patient developed a high fever postoperatively, and at the same time, the left shoulder presented pain, swelling, and warmth. However, the article did not describe the imaging manifestations of the shoulder joint [ 6 ]. Ana et al. reported a case of tophaceous gout of the right shoulder joint. The patient presented with pain in the right shoulder with certain movements and when lying on his affected shoulder at night. A plain radiograph of the shoulder joint showed a punched-out eccentric bony erosion in the clavicle region of the right acromioclavicular joint and irregular opacity occupying the subacromiodeltoid bursa. MRI showed tophi deposits along the upper ridge of the distal end of the clavicle and in the subacromiodeltoid bursa [ 7 ]. Gouty arthritis of the shoulder joint, as described in the literature, is presented as tophaceous gout of the rotator cuff, causing subacromial impingement or rotator cuff tendinitis and shoulder dysfunction. In our case, the patient had no distinct appearance of acute arthritis, and there was severe bone destruction of the glenoid, resulting in severe dysfunction of the right shoulder joint. The gold standard for diagnosing gout is identifying characteristic monosodium urate crystals in the synovial fluid using polarized microscopy. During the patient’s diagnosis process, we considered the possibility of the patient suffering from gouty shoulder arthritis. Since a polarizing microscope was not available in our hospital, the diagnosis of gouty arthritis needed to be confirmed by clinical symptoms or histopathological analysis. The patient’s shoulder bone tissue was severely damaged, and at the same time, there was not a typical presentation of gouty arthritis. Therefore, we focused more attention on the possibility of shoulder neoplasia or infection. After diagnosing the case through histological examination, we carefully observed the patient’s imaging data. We found the typical features of gout: centralized erosions, sclerotic rim, and overhanging edges on the plain radiograph and CT.
In clinical work, gouty arthritis should be considered in patients who present with shoulder pain, weakness, and limited mobility, especially patients who have no distinct appearance of acute arthritis and have severe bone destruction of the shoulder joint. The differentiation of gouty arthritis from infectious arthritis or osteomyelitis is not always easy. It is necessary to exclude other possible etiologies, such as infection, neoplasia, and bone destructive disease. The following conditions must be considered in differential diagnosis: septic shoulder arthritis is associated with acute inflammation, redness, swelling, and pain; laboratory tests have a high percentage of white blood cells and neutrophils, and joint fluid examination will indicate microbes or pus cells. Tuberculous shoulder arthritis manifests as pain, dysfunction, muscular atrophy, and fistula; patients also have systemic symptoms of tuberculosis and are positive for the Xpert test [ 11 ]. Rapid destructive arthropathy of the shoulder joint is described as follows: the course of the disease develops rapidly; and there is bone destruction and tearing of the rotator cuff [ 12 ]. Milwaukee shoulder is a destructive calcium phosphate crystalline arthropathy related to the following factors: trauma or overuse, calcium pyrophosphate dehydrate crystal deposition, neuroarthropathy, dialysis arthropathy, denervation, female sex, and advanced age. The imaging findings are as follows: narrowing of the joint space, subchondral bone sclerosis with cystic changes, subchondral bone destruction, soft tissue swelling, calcification of the joint capsule, free bodies in the joint cavity, rotator cuff tears, and massive haemorrhagic joint effusion [ 13 ].
In this study, the patient’s shoulder joint pathology was insidious, and no apparent acute gout episodes occurred during the entire course of the disease; the condition mainly manifested as chronic arthritis, with right shoulder pain, weakness, and limited mobility. Although the patient had a history of gout, there had been no gout attacks in the shoulder joint; the attacks were mainly manifested in the bilateral feet. Additionally, it was found that the left shoulder joint was also affected by a gouty attack. However, there were no clinical symptoms, which caused some confusion and challenges regarding the diagnosis and treatment. The possibility of gouty arthritis was not initially considered. The patient had a long history of gout, with 2–3 attacks per year, mainly manifested in the bilateral feet, but there were no obvious gout attacks in other parts of the body. Only colchicine was taken orally during the gout attack periods to relieve the symptoms. The patient did not receive an effective and systematic treatment of gout. We believe that the severe destruction of the glenoid was related to an unhealthy diet and the lack of effective and systematic treatment. With effective therapy earlier in the disease process, severe bone destruction of the shoulder bone tissue can be avoided. This condition can lead to significant debilitation in patients if not identified early and managed appropriately.
Gouty arthritis involves the shoulder joint relatively rarely, and cases of osteolytic destruction of the bone tissue of the shoulder joint are rare. Gouty arthritis of the shoulder with severe bone destruction is easily misdiagnosed as a shoulder tumor or infectious disease. In conclusion, although gouty shoulder arthritis is considered unusual, when a patient has a history of gout, atypical manifestations, and severe erosive lesions of the glenoid in the shoulder joint, physicians and orthopedic surgeons should consider the possibility of gout causing severe lesions that mimic infection or neoplastic disease (Table 1 ). This case study aimed to alert physicians to the unusual manifestations and presentations of gouty arthritis, which could be missed if there is no suspicion.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
Computed tomography
Magnetic resonance imaging
Alqatari S, Visevic R, Marshall N, Ryan J, Murphy G. An unexpected cause of sacroiliitis in a patient with gout and chronic psoriasis with inflammatory arthritis: a case report. BMC Musculoskelet Disord. 2018;19(1):126.
Article Google Scholar
Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum. 2020;50(Suppl 3):11–6.
Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16(7):380–90.
O’leary ST, Goldberg JA, Walsh WR. Tophaceous gout of the rotator cuff: a case report. J Shoulder Elbow Surg. 2003;12(2):200–1.
Chang CH, Lu CH, Yu CW, Wu MZ, Hsu CY, Shih TT. Tophaceous gout of the rotator cuff. A case report. J Bone Joint Surg Am. 2008;90(1):178–82.
Ichiseki T, Ueda S, Matsumoto T. Rare coexistence of gouty and septic arthritis after arthroscopic rotator cuff repair: a case report. Int J Clin Exp Med. 2015;8(3):4718–20.
PubMed PubMed Central Google Scholar
Tierra Rodriguez AM, Pantoja Zarza L, Brañanova López P, Diez Morrondo C. Tophaceous gout of the shoulder joint. Reumatol Clin. 2019;15(5):e55-6.
Ragab G, Elshahaly M, Bardin T. Gout: An old disease in new perspective - A review. J Adv Res. 2017;8(5):495–511.
Article CAS Google Scholar
Gentili A. The advanced imaging of gouty tophi. Curr Rheumatol Rep. 2006;8(3):231–5.
McQueen FM, Doyle A, Dalbeth N. Imaging in gout–what can we learn from MRI, CT, DECT and US. Arthritis Res Ther. 2011;13(6):246.
Longo UG, Marinozzi A, Cazzato L, Rabitti C, Maffulli N, Denaro V. Tuberculosis of the shoulder. J Shoulder Elbow Surg. 2011;20(4):e19–21.
Kekatpure AL, Sun JH, Sim GB, Chun JM, Jeon IH. Rapidly destructive arthrosis of the shoulder joints: radiographic, magnetic resonance imaging, and histopathologic findings. J Shoulder Elbow Surg. 2015;24(6):922–7.
Nadarajah CV, Weichert I. Milwaukee shoulder syndrome. Case Rep Rheumatol. 2014;2014:458708.
Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20(1):140.
Download references
Acknowledgements
We thank American Journal Experts for their language editing, which greatly improved the manuscript.
This work was supported by the National Natural Science Foundation of China under Grants 81560374 and 81960399, and the Natural Science Foundation of Inner Mongolia under Grants 2018BS08002 and 2020MS03064.
Author information
Huricha Bao and Yansong Qi contributed equally to this work.
Authors and Affiliations
Department of Orthopedics, Inner Mongolia People’s Hospital, No. 20 Zhao Wu Da Street, Inner Mongolia Autonomous Region, 010017, Hohhot, China
Huricha Bao, Yansong Qi, Baogang Wei, Bingxian Ma, Yongxiang Wang & Yongsheng Xu
You can also search for this author in PubMed Google Scholar
Contributions
HRCB collected the patient’s clinical data and wrote the manuscript. YSQ critically revised the manuscript for intellectual content. BGW was involved in the pathological data collection. BXM and YXW were involved in the clinical case data collection. All authors have read and approved the final version of this manuscript. YSX was responsible for the clinical management of the patient and the drafting and editing of the manuscript. All authors are aware of the manuscript submitted and they all agreed on it.
Corresponding author
Correspondence to Yongsheng Xu .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of Clinical Investigation in the Inner Mongolia People’s Hospital.
Consent for publication
A written informed consent form for participation in the case was obtained from the patient. he is aware of this case report and the possibility of it being published.
Competing interests
We have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Bao, H., Qi, Y., Wei, B. et al. Severe erosive lesion of the glenoid in gouty shoulder arthritis: a case report and review of the literature. BMC Musculoskelet Disord 22 , 343 (2021). https://doi.org/10.1186/s12891-021-04217-5
Download citation
Received : 18 August 2020
Accepted : 03 April 2021
Published : 12 April 2021
DOI : https://doi.org/10.1186/s12891-021-04217-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Musculoskeletal Disorders
ISSN: 1471-2474
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Establishment of a clinical diagnostic model for gouty arthritis based on the serum biochemical profile: A case-control study
Affiliations.
- 1 National Pharmaceutical Engineering Center for Solid Preparation in Chinese Herbal Medicine, Jiangxi University of Traditional Chinese Medicine.
- 2 Department of Pathology, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou.
- 3 State Key Laboratory of Innovative Drug and Efficient Energy-Saving Pharmaceutical Equipment, Nanchang, China.
- PMID: 33879701
- PMCID: PMC8078334
- DOI: 10.1097/MD.0000000000025542
The disease progression of gouty arthritis (GA) is relatively clear, with the 4 stages of hyperuricemia (HUA), acute gouty arthritis (AGA), gouty arthritis during the intermittent period (GIP), and chronic gouty arthritis (CGA). This paper attempts to construct a clinical diagnostic model based on blood routine test data, in order to avoid the need for bursa fluid examination and other tedious steps, and at the same time to predict the development direction of GA.Serum samples from 579 subjects were collected within 3 years in this study and were divided into a training set (n = 379) and validation set (n = 200). After a series of multivariate statistical analyses, the serum biochemical profile was obtained, which could effectively distinguish different stages of GA. A clinical diagnosis model based on the biochemical index of the training set was established to maximize the probability of the stage as a diagnosis, and the serum biochemical data from 200 patients were used for validation.The total area under the curve (AUC) of the clinical diagnostic model was 0.9534, and the AUCs of the 5 models were 0.9814 (Control), 0.9288 (HUA), 0.9752 (AGA), 0.9056 (GIP), and 0.9759 (CGA). The kappa coefficient of the clinical diagnostic model was 0.80.This clinical diagnostic model could be applied clinically and in research to improve the accuracy of the identification of the different stages of GA. Meanwhile, the serum biochemical profile revealed by this study could be used to assist the clinical diagnosis and prediction of GA.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Publication types
- Validation Study
- Area Under Curve
- Arthritis, Gouty / diagnosis*
- Arthritis, Gouty / etiology
- Biomarkers / blood
- Blood Sedimentation
- Blood Urea Nitrogen
- C-Reactive Protein / analysis
- Case-Control Studies
- Clinical Decision Rules*
- Disease Progression
- Hematologic Tests / statistics & numerical data*
- Hyperuricemia / blood
- Hyperuricemia / complications
- Least-Squares Analysis
- Leukocyte Count
- Lipoproteins, HDL / blood
- Middle Aged
- Multivariate Analysis
- Predictive Value of Tests
- Principal Component Analysis
- Regression Analysis
- Reproducibility of Results
- Uric Acid / blood
- Lipoproteins, HDL
- C-Reactive Protein
Grants and funding
- 81560636/Natural Science Foundation of China
- 81760702/Natural Science Foundation of China
- 2019YFC1712300/National Key R&D Program of China
- 20165BCB19009/Jiangxi Province 5511 innovative talent Project
- 2020YBBGWL002/Key R&D project of Jiangxi Province
- 20194AFD45001/Jiangxi Science and Technology Innovation Platform Project
- 20192ZDD02002/Special Project for Central Guidance of Local Science and Technology Development
- GJJ201257/Science and Technology Research Project of Education Department of Jiangxi Province
- 202110124/Science and Technology Project of Health Commission of Jiangxi Province
- 2020BSZR016/Doctoral Research Foundation of Jiangxi University of Traditional Chinese Medicine
- Research article
- Open access
- Published: 17 September 2013
Efficacy of anakinra in gouty arthritis: a retrospective study of 40 cases
- Sébastien Ottaviani 1 ,
- Anna Moltó 2 ,
- Hang-Korng Ea 2 ,
- Séverine Neveu 3 ,
- Ghislaine Gill 1 ,
- Lauren Brunier 1 ,
- Elisabeth Palazzo 1 ,
- Olivier Meyer 1 ,
- Pascal Richette 2 ,
- Thomas Bardin 2 ,
- Yannick Allanore 4 ,
- Frédéric Lioté 2 ,
- Maxime Dougados 3 &
- Philippe Dieudé 1
Arthritis Research & Therapy volume 15 , Article number: R123 ( 2013 ) Cite this article
7130 Accesses
16 Altmetric
Metrics details

Introduction
Gout is a common arthritis that occurs particularly in patients who frequently have associated comorbidities that limit the use of conventional therapies. The main mechanism of crystal-induced inflammation is interleukin-1 production by activation of the inflammasome. We aimed to evaluate the efficacy and tolerance of anakinra in gouty patients.
We conducted a multicenter retrospective review of patients receiving anakinra for gouty arthritis. We reviewed the response to treatment, adverse events and relapses.
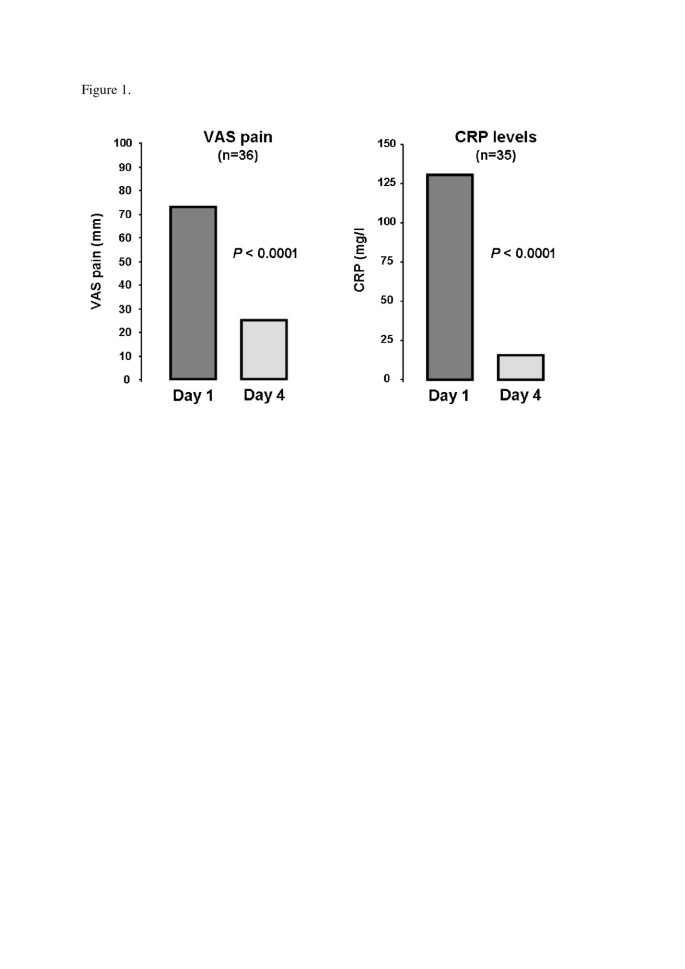
We examined data for 40 gouty patients (32 men; mean age 60.0 ± 13.9 years) receiving anakinra. Mean disease duration was 8.7 ± 8.7 years. All patients showed contraindications to and/or failure of at least two conventional therapies. Most (36; 90%) demonstrated good response to anakinra. Median pain on a 100-mm visual analog scale was rapidly decreased (73.5 (70.0 to 80.0) to 25.0 (20.0 to 32.5) mm, P <0.0001), as was median C-reactive protein (CRP) level (130.5 (55.8 to 238.8) to 16.0 (5.0 to 29.5) mg/l, P <0.0001). After a median follow-up of 7.0 (2.0 to 13.0) months, relapse occurred in 13 patients after a median delay of 15.0 (10.0 to 70.0) days. Seven infectious events, mainly with long-term use of anakinra, were noted.
Conclusions
Anakinra may be efficient in gouty arthritis, is relatively well tolerated with short-term use, and could be a relevant option in managing gouty arthritis when conventional therapies are ineffective or contraindicated. Its long-term use could be limited by infectious complications.
Gout is a common arthritis caused by deposition of monosodium urate (MSU) crystals within and around joints secondary to chronic hyperuricemia. It affects 1% to 2% of adults in developed countries and may be increasing in prevalence [ 1 ]. Acute gouty arthritis may be associated with high inflammatory clinical and biological symptoms. Thus, one of the goals of management is rapid relief of inflammation [ 2 , 3 ].
Acute gouty attacks are usually treated with nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine and corticosteroids [ 3 ]. Gouty patients often have concomitant renal, cardiovascular and gastrointestinal diseases as well as diabetes mellitus [ 4 ]. These comorbidities and associated treatments can lead to increased frequency of side effects or contraindications to conventional therapies for gouty arthritis [ 4 ]. We have abundant evidence of side effects from the use of colchicine (for example, for diarrhea) [ 5 ] and NSAIDs (for example, for gastrointestinal bleeding, cardiovascular events including myocardial infarction, renal impairment) [ 6 , 7 ], so care must be taken when prescribing such drugs. Thus, alternative therapies are needed for these 'difficult-to-treat' cases.
The main mechanism of crystal-induced inflammation is interleukin 1β (IL-1β) production by activation of the NLRP3 inflammasome [ 8 ], which strengthens the relevance of targeting IL-1β in patients with crystal-induced arthritis. Anti-IL-1 agents, such as anakinra, have been evaluated in gouty arthritis, for treating acute attacks or for preventing gouty attacks while initiating urate-lowering therapy [ 9 – 14 ]. To date, only two small open studies have evaluated the efficacy of anakinra in acute gouty arthritis [ 13 , 14 ] although anakinra has been labeled for rheumatoid arthritis treatment for more than 10 years. Other IL-1 inhibitors, canakinumab and rilonacept, appear to be effective in reducing pain and signs of inflammation in randomized controlled trials, which validate IL-1 as playing a pivotal role in gout inflammation [ 9 , 10 , 12 , 15 ].
Here, we aimed to evaluate the efficacy and safety of anakinra in patients with acute and chronic gouty arthritis but with contraindications to or failure of conventional therapies.
This was a multicenter retrospective review of charts for patients who received anakinra for gouty arthritis. Patients were identified by treating rheumatologists and by searching available electronic medical records with the keyword 'anakinra' or 'Kineret ® '. Patients receiving anakinra who had concomitant connective tissue diseases were not included. Inclusion criteria were diagnosis of gouty arthritis defined as recommended by the identification of MSU crystals in synovial fluid [ 16 ] and at least one documented visit after the acute gouty arthritis requiring anakinra. The study was approved by the local institutional review board of Paris North Hospitals (No. 12-081) and all patients provided informed written consent to their physician to receive anakinra.
We retrospectively assessed response to anakinra at baseline and at the first documented visit following the acute gouty arthritis according to the following items: swollen joint count (SJC) and tender joint count (TJC), patient evaluation of pain by a visual analog scale (VAS pain, 0 to 100 mm) and C-reactive protein (CRP) levels (mg/L). We collected data on demographics (age, gender), clinical variables (tophus, localization of arthritis, comorbid conditions, and disease and flare duration), radiologic features of gouty arthropathy and biological variables (serum uric acid levels (SUA), CRP and creatinine). The outcome of anakinra treatment was classified as good, partial, or no response. A good response was arbitrarily defined as an improvement >50% in VAS pain or CRP level and/or documentation in the chart of the word 'good' response after anakinra treatment. A partial response was defined as a report of improvement in joint symptoms but not a 'good' response (20% to 50% improvement). No response was defined as the absence of symptom relief (<20% improvement).
Adverse events were defined as diarrhea, myopathy and skin reactions with colchicine treatment; gastrointestinal bleeding, cardiovascular events, renal impairment and skin reactions with NSAIDs; hyperglycemia, hypertension and cardiovascular events with steroids; and local skin reaction, infection and neutropenia with anakinra.
Contraindications to conventional therapies and comorbidities were as described [ 4 ], except for osteoporosis and hyperlipidemia, which we did not consider a comorbidity limiting prescription of conventional therapies.
Statistical analysis
Data are reported as mean ± SD or median (interquartile range (IQR)) or number (%). Non-parametric or Fisher's exact test was used to compare quantitative or categorical data, respectively. A two-tailed P <0.05 was considered statistically significant.
Baseline characteristics
We investigated data for 40 patients (32 men) who received anakinra for gouty arthritis. Their baseline characteristics are shown in Table 1 . In all, 79% and 92% of patients showed clinical tophi and gouty arthropathy, respectively.
At baseline, the median (IQR) pain level was 73.5 (70.0 to 80.0) mm. The median TJC and SJC was 5.0 (3.5 to 8.0) and 4.0 (3.0 to 5.5), respectively. The median CRP level was 130.5 (55.8 to 238.8) mg/L. The mean SUA was 534 ± 172 μM. In all, 17 (43%) patients had received urate-lowering therapy (allopurinol ( n = 11), febuxostat ( n = 6), benzbromarone ( n = 1)). Diuretic drugs were prescribed for 14 patients (hydrochlorothiazides ( n = 3), loop diuretics ( n = 11)). All patients had a contraindication to or past history of adverse events with conventional treatments for acute gouty arthritis (Table 1 ).
The number of patients with gouty arthritis that was acute (<6 weeks), subacute (6 to 12 weeks) and chronic (>12 weeks) was 34, 2 and 4, respectively.
Among the 40 patients, 23 received anakinra following the protocol used by So et al .[ 14 ]: 100 mg daily for three days subcutaneously. Seven patients received anakinra for <15 days (100 mg/day: n = 6, 100 mg/2 days: n = 1). The 10 remaining patients received anakinra for the long term (>15 days), followed by a spacing of the dose regimen (median total duration: 5.0 (2.3 to 11.8) months).
Anakinra response for gouty arthritis
Whole population.
Of the 40 patients, good, partial and non-response to anakinra were noted in 36 (90%), 2 (5%) and 2 patients (5%), respectively. Pain score decreased from 73.5 (70.0 to 80.0) to 25.0 (20.0 to 32.5) mm, P <0.0001), as did CRP level (130.5 (55.8 to 238.8) to 16.0 (5.0 to 29.5) mg/L, P <0.0001) (Figure 1 ). In all, 30 patients received treatment to prevent relapse (Table 2 ). After a median follow-up of 7.0 (2.0 to 13.0) months, relapse occurred in 13 (32.5%) patients with a median delay of 15.0 (10.0 to 70.0) days. Relapse occurred particularly in patients not receiving therapy to prevent acute flare (7/10 versus 6/30, P = 0.006). No relapse occurred with long-term use of anakinra (>15 days).
Pain on a visual analog scale (VAS) and C-reactive protein level (CRP) on days 1 and 4 of anakinra treatment for gouty arthritis . VAS, visual analog scale (mm); CRP, C-reactive protein (mg/l).
Anakinra response according to the dose regimen
A total of 23 patients received anakinra, 100 mg/day, for up to three days, with good response in 20 (87%); 17 (74%) showed relapse prevention after resolution of the flare. After a median follow-up of 6.0 (1.5 to 14.0) months, relapse occurred in six (26%) patients at a median delay of 15.0 (6.0 to 26.3) days.
In all, 17 patients received anakinra for more than three days, with good response in 16 (94%); 13 (76%) showed relapse prevention after flare resolution. After a median follow-up of 8.0 (3.0 to 13.0) months, relapse occurred in seven (41%) patients at a median delay of 60.0 (12.5 to 125.0) days.
No patient reported anakinra-related skin hypersensitivity. A total of seven infectious complications, mainly staphylococcal infections, were reported in six patients (Table 2 ). One H1N1 viral infection occurred one day after anakinra was started (previously reported in [ 17 ]). All other infectious complications occurred in patients with long-term use of anakinra and were successfully treated with antibiotics. Of the six patients, five restarted anakinra after the resolution of infection. No patient has shown tuberculosis or pneumococcal infection.
Recently, the emerging role of IL-1β in the pathogenesis of inflammation in crystal-induced arthritis [ 8 , 18 , 19 ] led to considering anti-IL-1 therapies as a relevant alternative to conventional therapies for gouty arthritis. Here, we report on our experience with anakinra therapy, a recombinant receptor antagonist against IL-R blocking both IL-1β and β, for gouty arthritis in a large series of patients. Our results are in good agreement with those of the first open-label trial of anakinra showing improved patient-reported symptoms by at least 50% of all 10 patients enrolled [ 14 ]. Chen et al . suggested an efficiency in 6 of 10 patients with good response [ 13 ]. Similar to So et al ., we observed a rapid decrease of both VAS pain and CRP levels. Recently, randomized controlled trials found that two anti-IL-1β biologic agents (rilonacept and canakinumab) prevented gouty arthritis during the initiation of urate-lowering therapy with allopurinol [ 10 , 11 ], but only canakinumab demonstrated efficacy for acute gouty arthritis [ 9 , 12 ]. In these cases, rilonacept failed to induce a rapid relief of symptoms [ 20 ] but could decrease pain in chronic gouty arthritis [ 15 ]. These data strengthen the argument to target IL-1 blockade for acute gouty arthritis. Of interest, canakinumab recently obtained European authorization [ 21 ].
In our study, relapse occurred frequently among patients not receiving therapy to prevent acute flare, and conversely, no patient with long-term use of anakinra experienced relapse. The daily subcutaneous injection could limit the use of the drug in preventing flare, although skin tolerance was excellent in our study. The short-term regimen was well tolerated: only one viral infection was observed. This short-term good tolerance agrees with previous studies of anakinra for gouty arthritis [ 13 , 14 ] and pseudogout [ 22 , 23 ]. However, long-term use was poorly tolerated, with six infectious events, notably one septic arthritis and one patient with pulmonary abscesses. These data suggest a risk of infection with prolonged administration. To date, the role IL-1 antagonists could play in clinical practice is unclear. Nonetheless, their cost (38€ per injection in France, 2013) may not be excessive in managing acute attacks, particularly in patients with contraindications to, or who cannot tolerate, conventional therapies. Recently, American College of Rheumatology recommendations allow for use of anti-IL1 agents when conventional therapies have failed or are contraindicated [ 2 ]. Of note, anakinra, with the shortest half-life of the IL-1 blockers, could be a relevant option to manage acute gouty arthritis.
Our study had some limitations. First, data were retrospectively collected, with varied use of anakinra, and we had no control group. However, this real-life observational study is the largest reported series for this treatment. Randomized controlled studies are necessary to clarify the place of anakinra in the management of gouty arthritis flare.
Our results provide evidence that anakinra is effective, relatively well tolerated with short-term use, and could be a good alternative for treating gouty arthritis in patients for whom conventional therapies are ineffective or contraindicated. Although these findings are promising, this was a retrospective study, and future randomized controlled trials are required definitely to determine the place of anakinra in managing gouty arthritis.
Abbreviations
C-reactive protein
interleukin-1
interquartile range
monosodium urate
nonsteroidal anti-inflammatory drugs
standard deviation
swollen joint count
serum uric acid
tender joint count
visual analog scale.
Richette P, Bardin T: Gout. Lancet. 2009, 375: 318-328.
Article PubMed Google Scholar
Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, Kaldas M, Gogia M, Perez-Ruiz F, Taylor W, Liote F, Choi H, Singh JA, Dalbeth N, Kaplan S, Niyyar V, Jones D, Yarows SA, Roessler B, Kerr G, King C, Levy G, Furst DE, Edwards NL, Mandell B, Schumacher HR: 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012, 64: 1447-1461.
Article CAS Google Scholar
Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Liote F, McCarthy G, Netter P, Nuki G, Perez-Ruiz F, Pignone A, Pimentao J, Punzi L, Roddy E, Uhlig T, Zimmermann-Gorska I: EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006, 65: 1312-1324. 10.1136/ard.2006.055269.
Article PubMed Central CAS PubMed Google Scholar
Keenan RT, O'Brien WR, Lee KH, Crittenden DB, Fisher MC, Goldfarb DS, Krasnokutsky S, Oh C, Pillinger MH: Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med. 2011, 124: 155-163. 10.1016/j.amjmed.2010.09.012.
Terkeltaub RA: Colchicine update: 2008. Semin Arthritis Rheum. 2009, 38: 411-419. 10.1016/j.semarthrit.2008.08.006.
Article CAS PubMed Google Scholar
Park SC, Chun HJ, Kang CD, Sul D: Prevention and management of non-steroidal anti-inflammatory drugs-induced small intestinal injury. World J Gastroenterol. 2011, 17: 4647-4653. 10.3748/wjg.v17.i42.4647.
Brater DC: Anti-inflammatory agents and renal function. Semin Arthritis Rheum. 2002, 32: 33-42. 10.1053/sarh.2002.37216.
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006, 440: 237-241. 10.1038/nature04516.
So A, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, Arulmani U, Sallstig P, Schlesinger N: Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010, 62: 3064-3076. 10.1002/art.27600.
Schumacher HR, Evans RR, Saag KG, Clower J, Jennings W, Weinstein SP, Yancopoulos GD, Wang J, Terkeltaub R: Rilonacept (interleukin-1 trap) for prevention of gout flares during initiation of uric acid-lowering therapy: results from a phase III randomized, double-blind, placebo-controlled, confirmatory efficacy study. Arthritis Care Res (Hoboken). 2012, 64: 1462-1470. 10.1002/acr.21690.
Schlesinger N, Mysler E, Lin HY, De Meulemeester M, Rovensky J, Arulmani U, Balfour A, Krammer G, Sallstig P, So A: Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann Rheum Dis. 2011, 70: 1264-1271. 10.1136/ard.2010.144063.
Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, Krammer G, Murphy V, Richard D, So AK: Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis. 2012, 71: 1839-1848. 10.1136/annrheumdis-2011-200908.
Chen K, Fields T, Mancuso CA, Bass AR, Vasanth L: Anakinra's efficacy is variable in refractory gout: report of ten cases. Semin Arthritis Rheum. 2010, 40: 210-214. 10.1016/j.semarthrit.2010.03.001.
So A, De Smedt T, Revaz S, Tschopp J: A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007, 9: R28-10.1186/ar2143.
Article PubMed Central PubMed Google Scholar
Terkeltaub R, Sundy JS, Schumacher HR, Murphy F, Bookbinder S, Biedermann S, Wu R, Mellis S, Radin A: The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009, 68: 1613-1617. 10.1136/ard.2009.108936.
Zhang W, Doherty M, Pascual E, Bardin T, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Liote F, McCarthy G, Netter P, Nuki G, Perez-Ruiz F, Pignone A, Pimentao J, Punzi L, Roddy E, Uhlig T, Zimmermann-Gorska I: EULAR evidence based recommendations for gout. Part I: diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006, 65: 1301-1311. 10.1136/ard.2006.055251.
Nocturne G, Ora J, Ea HK, Liote F: Influenza A H1N1 and anakinra exposure in a patient with gout. Joint Bone Spine. 2010, 77: 369-370. 10.1016/j.jbspin.2010.04.005.
Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J: Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012, 36: 388-400. 10.1016/j.immuni.2012.01.018.
Church LD, Cook GP, McDermott MF: Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol. 2008, 4: 34-42.
Terkeltaub RA, Schumacher HR, Carter JD, Baraf HS, Evans RR, Wang J, King-Davis S, Weinstein SP: Rilonacept in the treatment of acute gouty arthritis: a randomized, controlled clinical trial using indomethacin as the active comparator. Arthritis Res Ther. 2013, 15: R25-10.1186/ar4159.
Euopean Medicines Agency: Ilaris. 2013, [ http://www.ema.europa.eu/docs/fr_FR/document_library/EPAR_-_Summary_for_the_public/human/001109/WC500031677.pdf ]
Google Scholar
Molto A, Ea HK, Richette P, Bardin T, Liote F: Efficacy of anakinra for refractory acute calcium pyrophosphate crystal arthritis. Joint Bone Spine. 2012, 79: 621-623. 10.1016/j.jbspin.2012.01.010.
Ottaviani S, Brunier L, Sibilia J, Maurier F, Ardizzone M, Wendling D, Gill G, Palazzo E, Meyer O, Dieude P: Efficacy of anakinra in calcium pyrophosphate crystal-induced arthritis: a report of 16 cases and review of the literature. Joint Bone Spine. 2013, 80: 178-182. 10.1016/j.jbspin.2012.07.018.
Download references
Acknowledgements
We thank Laura Smales for copyediting.
Author information
Authors and affiliations.
Université Paris Diderot, Sorbonne Paris Cité, UFR de Médecine, F-75205 Paris, France; AP-HP, Service de Rhumatologie, Hôpital Bichat, 75018, Paris, France
Sébastien Ottaviani, Ghislaine Gill, Lauren Brunier, Elisabeth Palazzo, Olivier Meyer & Philippe Dieudé
Sorbonne Paris Cité, UFR de Médecine, F-75205 Paris, France; AP-HP, Service de Rhumatologie, Pôle appareil Locomoteur, Hôpital Lariboisière, Université Paris Diderot, F-75475, Paris, France
Anna Moltó, Hang-Korng Ea, Pascal Richette, Thomas Bardin & Frédéric Lioté
Université René Descartes, Service de Rhumatologie B, Hôpital Cochin, APHP, Paris, France
Séverine Neveu & Maxime Dougados
Université René Descartes, Service de Rhumatologie A, Hôpital Cochin, APHP, Paris, France
Yannick Allanore
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Philippe Dieudé .
Additional information
Competing interests.
Frédéric Lioté received unrestricted grants from SOBI France and Novartis since 2010 for helping set up an annual European Workshop on crystal-induced inflammation and human diseases. The other authors declare they have no competing interests.
Authors' contribution
SO and PD conceived of the study, made substantial contributions to the acquisition of data, participated in its design, and wrote the manuscript. AM, HKE, SN, GG, LB, EP, PR, TB, YA, FL, MD and OM made substantial contributions to the acquisition of data and helped to draft the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
Ottaviani, S., Moltó, A., Ea, HK. et al. Efficacy of anakinra in gouty arthritis: a retrospective study of 40 cases. Arthritis Res Ther 15 , R123 (2013). https://doi.org/10.1186/ar4303
Download citation
Received : 19 March 2013
Revised : 24 June 2013
Accepted : 17 September 2013
Published : 17 September 2013
DOI : https://doi.org/10.1186/ar4303
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Arthritis Research & Therapy
ISSN: 1478-6362
- General enquiries: [email protected]
- Case report
- Open access
- Published: 09 October 2007
An unusual case of gout in the wrist: the importance of monitoring medication dosage and interaction. A case report
- Craig L Jacobs 1 &
- Paula J Stern 1
Chiropractic & Osteopathy volume 15 , Article number: 16 ( 2007 ) Cite this article
15k Accesses
7 Citations
Metrics details
Gouty arthritis of the wrist is uncommon although gout itself is the most common inflammatory arthritis in older patients. Some known risk factors for the development of gout include trauma, alcohol use, obesity, hyperuricaemia, hypertension and diabetes mellitus. As well, certain medications have been shown to promote the development of gout. These include thiazide diuretics, low dose salicylates and cyclosporine. We present a case of gouty wrist pain possibly precipitated by a medication dosage increase as well as medication interactions.
Case presentation
A 77 year old male presented with right wrist pain. Redness and swelling was present at the dorsal aspect of his wrist and range of motion was full with pain at end range upon examination. One week prior, his anti-hypertensive medication dosage had been increased. The patient's situation continued to worsen. Radiographic examination revealed changes consistent with gouty arthritis.
It is important for clinicians treating joint conditions to be aware of patients' comorbidities, medication usage and changes in dosages. Education of patients with gout is of prime importance. Clinicians should educate patients that gout may occur at any joint in the body not only the lower limb. Patients should be aware of the signs and symptoms of an acute gouty attack and be made aware that changes in certain medication dosages may precipitate an attack. Awareness of radiographic changes associated with gout is still of importance although these changes are not seen as frequently as they have been in the past due to better control of the disease.
Joint pain accompanied with swelling is a common complaint seen in clinical practice. The challenge is to determine the underlying etiology and to provide the appropriate treatment. Many joint diseases present as acute monoarthritis with the most common causes due to gout or calcium pyrophosphate dihydrate crystal deposition disease (CPPD) [ 1 ]. The peak incidence of gout is between the ages of 30–50 with the prevalence increasing with age [ 2 ]. Both the incidence and prevalence of gout has been on the rise in recent years [ 3 ]. The increased prevalence is believed to be related to several factors which include increased age and obesity in the population and widespread diuretic use for hypertension treatment [ 3 , 4 ].
Gout is five times more common in men. Most acute gouty attacks occur in a single joint in the lower limb with the first metatarsal joint most commonly affected [ 2 , 5 ]. On clinical presentation, the joint often appears red, swollen and very tender. Some differentials to keep in mind include septic arthritis, rheumatoid arthritis, osteoarthritis and erosive arthritis [ 1 ]. Some known risk factors for the development of gout include trauma, alcohol use, obesity, hyperuricaemia, hypertension and diabetes mellitus [ 2 , 5 ]. As well, certain medications have been shown to promote the development of gout. These include thiazide diuretics, low dose salicylates and cyclosporine [ 2 , 5 ].
We present an unusual case of gouty wrist pain possibly precipitated by a medication dosage increase as well as medication interactions.
A 77 year old male was treated at a chiropractic clinic for low back pain resulting from lumbar facet arthrosis and lateral canal stenosis. On a subsequent visit he reported right wrist pain which began while lifting a heavy box. On examination, redness and swelling was noted on the dorsal aspect of his right wrist. Range of motion was full with pain at end range of flexion and extension. His health history included two hip replacements, two previous episodes of gout in both first metatarsophalangeal joints (2 and 5 years prior), and hypertension. Medications for hypertension included perindopril (4 mg), hydrochlorothiazide (25 mg), and Norvasc (10 mg). In addition, he was prescribed 80 mg of aspirin/day and took a daily multivitamin. One week prior, the patient's general practitioner had increased his Norvasc dosage and also prescribed Tylenol 3 to be taken as needed for his back pain.
Two days later the swelling had increased in the dorsal aspect of his right wrist and hand. Wrist flexion was limited by 80% with severe pain. Pain was present on palpation of the scaphoid bone. Due to the suspicion of fracture, the patient was referred to his general practitioner for radiographs. The radiologist who read the films described tiny cysts at the distal radius and concluded that these were most likely due to old trauma. Mild osteoarthritic changes were noted at the carpal-metacarpal joint at the base of the thumb. The report stated that the radiographs were otherwise normal.
The patient's symptoms worsened with increased pain and swelling over the next few days. Due to the worsening symptoms, repeat radiographs were performed five days later and were read by a radiologist. The radiographs revealed well-marginated juxta-articular bony erosions at the radial styloid process and the dorsal rim of the distal radius with soft tissue swelling. These findings were deemed to be consistent with gouty arthritis. (See figure 1 ).
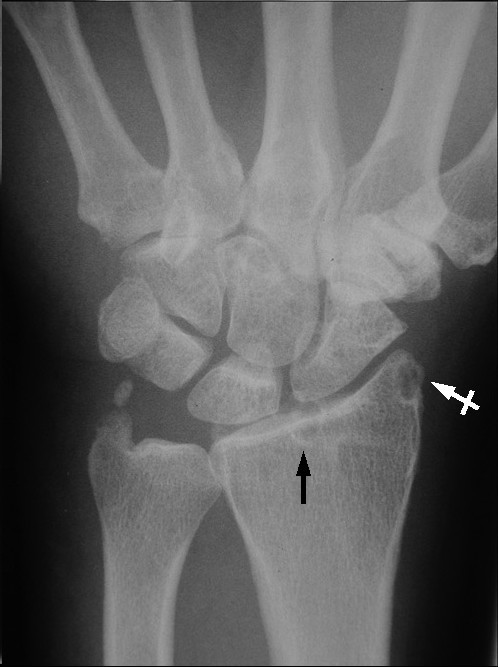
PA view of right wrist reveals a subchondral cyst in the distal radius (black arrow) and a well-marginated juxta-articular bony erosion at the radial styloid process (white crossed arrow).
The patient was referred back to his general practitioner with the radiologist's report and the patient was immediately put on colchicine. At follow up two days later, the wrist swelling had decreased significantly. One week after the initiation of colchicine, no swelling was present and only mild pain was noted with flexion/extension of his wrist and fingers. The patient was taken off colchicine due to diarrhea. A recurrence of wrist pain occurred several weeks later. The patient was referred to a rheumatologist who prescribed colchicine for one month duration. When the pain resolved completely, the patient was prescribed allopurinol. Anti-hypertensive medications were not altered. At one year follow up, no further gouty attacks had been reported.
A review of the literature reveals that gouty arthritis of the wrist is rare in isolation although gout itself is the most common inflammatory arthritis in older patients [ 4 , 6 – 9 ]. Gout at the wrist as the initial appearance of the condition occurs between 0.8 to 2% of all gout cases [ 9 ]. Gout patients who are not treated have a 19–30% chance of developing gout in the wrist during their lifetime [ 9 ]. Reported cases of carpal tunnel syndrome, tendon entrapment or rupture, and scapholunate dissociation have been reported in the literature due to tophaceous deposits in the wrist due to gout [ 6 , 8 , 9 ]. The prevalence of gout in the USA ranges between 0.5–2.8% in men and 0.1–0.6% in women [ 2 ]. The prevalence rises to 4.4% of men and 1.8% of women over the age of 65 [ 4 ]. A two-fold increase in incidence of gout has been reported in the USA and New Zealand over the past 30 years, while the prevalence of gout has been reported to have risen three-fold in the UK over 20 years of follow-up [ 10 ]. This rise may be attributed in part to the continuous aging of the population as well as the widespread use of diuretics for treatment of hypertension [ 4 ].
Gout is a clinical syndrome caused by the deposition of monosodium urate monohydrate crystals into synovial, bursal, and cartilaginous tissue. The underlying metabolic disorder is hyperuricemia. The exact trigger of an acute attack of gout is poorly understood however predictors for the development of gout in hyperuricemic individuals have been identified. These include: increasing uric acid level, alcohol consumption, hypertension, use of diuretic drugs (thiazides and loop diuretics), increased body mass index, and family history of gout [ 4 , 5 , 11 ]. These predictors appear to have an additive effect on the risk of developing gout [ 11 ].
Hyperuricemia results from either decreased renal excretion (which occurs in 90% of gout patients) or hyperproduction of uric acid [ 4 ]. Drugs that may cause hyperuricemia and gout include: diuretics, cyclosporine, low-dose aspirin, ethambuthol, pyrzinamide, and nicotinic acid [ 4 ]. As some are commonly prescribed medications, it is imperative that health care practitioners dealing with joint and musculoskeletal conditions be aware of the medications that their patients are taking. In addition, they should be prudent to be made aware of any changes in prescribed dosages. In this case, the patient was taking diuretics as well as other anti-hypertensive medications, aspirin, and vitamin B3 (nicotinic acid).
A retrospective cohort study found a substantially increased risk of receiving treatment for gout among elderly hypertensive patients who were prescribed thiazide diuretics when compared to those subjects who were receiving non-thiazide antihypertensive medications [ 12 ]. The thiazide diuretic therapy subjects were almost twice as likely to have undergone anti-gout therapy. This risk increased even more so when thiazide diuretics were combined with any other non-thiazide antihypertensive medication. In this case, the patient's non-thiazide antihypertensive medication was increased. This change in blood chemistry may have contributed to the precipitation of the acute gouty attack however the exact trigger of this acute gouty attack cannot be determined and is most likely multifactorial. The mechanisms by which diuretics contribute to elevated serum uric acid levels are: decreased filtration of uric acid, increased reabsorption, as well as decreased secretion [ 12 ]. Ene-Stroescu and Gorbien state that due to these mechanisms, diuretics are the most common cause of secondary gout with diuretic use being reported in over 75% of patients with late-onset gout and even approaching 100% in women [ 4 ]. The amount of risk is also related to dosage. Thiazide diuretic dosages less than 25 mg/day did not have a significant increase in risk, whereas dosages ≥25 mg/day had a relative risk of between 2.10–2.16 [ 12 ]. In this case, the patient was taking a dosage of 25 mg/day and thus had an increased risk of gouty attack. Gurwitz et al state that low doses of thiazide diuretics can be just as efficacious as larger doses with a reduced risk of metabolic disturbances. Doses as low as 6.25 mg can be effective when combined with another low dose anti-hypertensive medication [ 12 ].
Aspirin is prescribed widely in the elderly. Given that aspirin is attainable without prescription, this leads to problems with self-prescription and dosage issues. Low-dose aspirin, up to 2 g/day has the potential to increase uric acid retention [ 11 , 13 ]. The combination of low-dose aspirin and diuretics compounds this effect [ 13 ]. Clinicians should inquire regarding aspirin usage in patients due to this widespread and often unmonitored use.
The three clinical stages of gout are: acute gouty arthritis, intercritical gout, and chronic tophaceous gout. Acute gouty arthritis refers to acute inflammation due to the precipitation of urate crystals within a joint. Gouty attacks may be precipitated by trauma, starvation, surgery, ingestion of high purine content food, excessive alcohol intake, and drugs that affect urate concentration [ 4 ]. It should be noted that drugs that reduce urate concentration may also precipitate a gouty attack [ 4 ]. Initial attacks most commonly occur in the lower limbs and are usually monoarticular with up to 50–60% occurring at the first metatarsophalangeal joint [ 2 , 4 , 14 ]. Gout may occur in any joint including the ankle, knee, hand, wrist, elbow, sacroiliac joint and other joints of the spine, however most commonly occurs in the lower extremity. In this case, although the patient had suffered two previous gouty attacks in the first metatarsophalangeal joint he was unaware that gout could occur in his wrist.
Typical presentation includes sudden onset of intense pain, redness and swelling of the joint. Examination will reveal a red, swollen, and extremely tender joint. Natural history of an acute attack ranges from a few days to a few weeks. Radiographs during early attacks may only reveal soft-tissue swelling. Serum uric acid levels may be normal during an attack due to pro-inflammatory cytokines [ 5 ].
The majority of untreated patients will experience another acute attack within 2 years [ 4 ]. Prophylactic treatment is usually recommended in patients who have more than 2–3 gouty attacks per year [ 5 ]. Recent studies have advocated the avoidance of diuretics, weight gain and alcohol consumption. A low carbohydrate, high protein and unsaturated fat diet has also been recommended as it enhances insulin sensitivity and may reduce serum uric acid levels [ 13 ].
Patients who experience multiple attacks of acute gouty arthritis are predisposed to the development of polyarticular gouty arthritis [ 14 ]. Attacks can then occur in more than one joint simultaneously, especially in the lower extremity. This emphasizes the unusual presentation in this case of an isolated attack of gout in the wrist. Acute onset of polyarticular gouty arthritis is more frequently seen in older patients most of whom are receiving diuretics for the management of hypertension [ 4 ]. Radiographic findings also tend to lag behind the clinical manifestations of gout by 5–10 years [ 14 ]. This is an especially unusual aspect of this case in that the patient had no previous gouty attacks in the wrist and radiographic changes were present during this first acute episode in his wrist.
The success of prophylactic measures has led to a significant decrease in the numbers of patients developing chronic tophaceous gout [ 14 ]. Chronic tophaceous gout occurs after years of recurrent acute gouty attacks and is characterized by persistent pain and swelling in the affected joints. Classic radiographic features include soft tissue densities (tophi) and para-articular bony erosions [ 14 ]. Joint space is generally well maintained. Subchondral cysts may be present as they were in this case. (See Figure 1 ) Due to the increasing rarity of these x-ray changes because of better management, it is possible that clinicians may not be as familiar with these changes, especially in the early stages of bone and joint destruction. Radiographs still remain the imaging examination of choice for gouty arthritis although advanced imaging techniques may be used. The appearance of gout in MR imaging is variable. Joint effusion and para-articular edema may be present in an inflamed joint. Tophaceous deposits will appear low to intermediate signal intensity on T1-weighted images and range from low to high signal intensity on T2-weighted images depending on the degree of hydration of the tophi [ 2 ].
Differential diagnoses to consider include rheumatoid arthritis, osteoarthritis, septic arthritis, calcium pyrophosphate dihydrate crystal deposition disease, erosive arthritis, psoriatic arthritis, xanthomatosis, and amyloidosis. The definitive diagnosis of gout is made by examination of synovial fluid aspirated from the joint. Joint aspiration is of prime importance in order to rule out infection.
This is an uncommon and unusual case of gout in the wrist which occurred in isolation and which may have been induced by a change in anti-hypertensive medication dosage. This case demonstrates several issues that clinicians should keep in mind when assessing patients with a history of gout. Patient education is very important and patients who have had a previous attack of gout should be made aware of common signs and symptoms, treatment protocols during an acute attack, and that gout may occur in any joint of the body, not only in the lower limb. Clinicians should be aware of the various comorbidities associated with gout which include hypertension, cardiovascular disease, and diabetes. Awareness of prescribed medications and any dosage changes is important due to the effects they may have on serum urate levels. Patients should be made aware that dosage changes of certain drugs may precipitate a gouty attack as well as bringing to their attention the effect of aspirin on serum urate levels. Awareness of radiographic changes associated with gout is still of importance although these changes are not seen as frequently as they have been in the past due to better control of the disease.
Siva C, Velazquez C, Mody A, Brasington R: Diagnosing acute monoarthritis in adults: a practical approach for the family physician. American Family Physician. 2003, 68: 83-90.
PubMed Google Scholar
Monu JUV, Pope TL: Gout: a clinical and radiologic review. Radiologic Clinics of North America. 2004, 42: 169-184. 10.1016/S0033-8389(03)00158-1.
Article PubMed Google Scholar
Hunter DJ, York M, Chaisson CE, Woods R, Niu J, Zhang Y: Recent diuretic use and the risk of recurrent gout attacks: the online case-crossover gout study. Journal of Rheumatology. 2006, 33: 1341-5.
Ene-Stroescu D, Gorbien MJ: Gouty arthritis: a primer on late-onset gout. Geriatrics. 2005, 60: 24-31.
Li EK: Gout: a review of its aetiology and treatment. Hong Kong Medical Journal. 2004, 10: 261-70.
CAS PubMed Google Scholar
Ohishi T, Koide Y, Takahashi M, Miyata R, Kushida K: Scapholunate dissociation caused by gouty arthritis of the wrist. Case report. Scand J Plast Reconstr Surg Hand Surg. 2000, 34 (2): 189-191. 10.1080/02844310050160079.
Article CAS PubMed Google Scholar
Kamimura T, Hatakeyama M, Okazaki H, Minota S: Acute gout attack in the wrist joint. Internal Medicine. 2004, 43: 641-2. 10.2169/internalmedicine.43.641.
Schuind FA, van Geertruyden J, Stallenberg B, Remmelink M, Pasteels JL: A rare manifestation of gout at the wrist--a case report. Acta Orthop Scand. 2002, 73 (5): 594-596. 10.1080/000164702321022910.
Raimbeau G, Fouque PA, Cesari B, Le Bourg M, Saint-Cast Y: Arthropathie goutteuse du poignet a propos de cinq cas. Chirurgie de la Main. 2001, 20: 325-31. 10.1016/S1297-3203(01)00054-3.
Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR, Saag KG: Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Annals of the Rheumatic Diseases. 2005, 64: 267-272. 10.1136/ard.2004.024091.
Article PubMed Central CAS PubMed Google Scholar
Terkeltaub RA: Gout. The New England Journal of Medicine. 2003, 349: 1647-55. 10.1056/NEJMcp030733.
Gurwitz JH, Kalish SC, Bohn RL, Glynn RJ, Monane M, Mogun H, Avorn J: Thiazide diuretics and the initiation of anti-gout therapy. Journal of Clinical Epidemiology. 1997, 50: 953-959. 10.1016/S0895-4356(97)00101-7.
Schlesinger N, Schumacher HR: Gout: can management be improved?. Current Opinion in Rheumatology. 2001, 13: 240-244. 10.1097/00002281-200105000-00016.
Yochum TR, Rowe LJ: Essentials of Skeletal Radiology. 1996, Williams and Wilkins, 2: 929-936. 2
Google Scholar
Download references
Acknowledgements
Written consent for publication was obtained from the patient.
The authors wish to thank Dr. William Hsu and Dr. Tania Pringle for their contribution to the interpretation of the radiographs and providing key information relevant to this case.
No funding was received for the publication of this manuscript.
Author information
Authors and affiliations.
Graduate Education and Research Programs, Canadian Memorial Chiropractic College, 6100 Leslie St., Toronto, ON, M2H 3J1, Canada
Craig L Jacobs & Paula J Stern
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Craig L Jacobs .
Additional information
Competing interests.
The author(s) declare that they have no competing interests.
Authors' contributions
CLJ and PJS both contributed substantially to the conception, writing and editing of the manuscript. Both authors read and approved the final manuscript.
Craig L Jacobs and Paula J Stern contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Jacobs, C.L., Stern, P.J. An unusual case of gout in the wrist: the importance of monitoring medication dosage and interaction. A case report . Chiropr Man Therap 15 , 16 (2007). https://doi.org/10.1186/1746-1340-15-16
Download citation
Received : 08 May 2007
Accepted : 09 October 2007
Published : 09 October 2007
DOI : https://doi.org/10.1186/1746-1340-15-16
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Carpal Tunnel Syndrome
- Hyperuricemia
- Thiazide Diuretic
- Serum Uric Acid Level
Chiropractic & Manual Therapies
ISSN: 2045-709X
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Advanced Search
- Journal List
- Medicine (Baltimore)
- v.100(16); 2021 Apr 23
Establishment of a clinical diagnostic model for gouty arthritis based on the serum biochemical profile
a National Pharmaceutical Engineering Center for Solid Preparation in Chinese Herbal Medicine, Jiangxi University of Traditional Chinese Medicine
Ruowen Ding
Shilin yang, wanyuan chen.
b Department of Pathology, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou
c State Key Laboratory of Innovative Drug and Efficient Energy-Saving Pharmaceutical Equipment, Nanchang, China.
Associated Data
The disease progression of gouty arthritis (GA) is relatively clear, with the 4 stages of hyperuricemia (HUA), acute gouty arthritis (AGA), gouty arthritis during the intermittent period (GIP), and chronic gouty arthritis (CGA). This paper attempts to construct a clinical diagnostic model based on blood routine test data, in order to avoid the need for bursa fluid examination and other tedious steps, and at the same time to predict the development direction of GA.
Serum samples from 579 subjects were collected within 3 years in this study and were divided into a training set (n = 379) and validation set (n = 200). After a series of multivariate statistical analyses, the serum biochemical profile was obtained, which could effectively distinguish different stages of GA. A clinical diagnosis model based on the biochemical index of the training set was established to maximize the probability of the stage as a diagnosis, and the serum biochemical data from 200 patients were used for validation.
The total area under the curve (AUC) of the clinical diagnostic model was 0.9534, and the AUCs of the 5 models were 0.9814 (Control), 0.9288 (HUA), 0.9752 (AGA), 0.9056 (GIP), and 0.9759 (CGA). The kappa coefficient of the clinical diagnostic model was 0.80.
This clinical diagnostic model could be applied clinically and in research to improve the accuracy of the identification of the different stages of GA. Meanwhile, the serum biochemical profile revealed by this study could be used to assist the clinical diagnosis and prediction of GA.
1. Introduction
Gouty arthritis (GA) is a kind of chronic disease caused by purine metabolic disorders, and its progression is relatively clear, which can be roughly divided into the following 4 stages: hyperuricemia (HUA), acute gouty arthritis (AGA), gouty arthritis during the intermittent period (GIP), and chronic gouty arthritis (CGA). When the uric acid (UA) level in blood is above 7 mg/L or 420 μmol/L (HUA), monosodium urate (MSU) crystals might accumulate in the joint capsule, bursa, cartilage, bone, or other periarticular tissues, which can stimulate the synovial membrane of the joint and produce pathological reactions, such as synovial vasodilation, as well as increased permeability and leukocyte exudation (AGA). However, some patients with HUA do not progress to AGA and only show excessive serum UA levels. [ 1 ] Statistics show that up to 30% of AGA patients had normal UA values. [ 2 , 3 ] Therefore, a high UA level in blood is a necessary and insufficient condition for AGA. Clinically, AGA is most frequently encountered in the major joints, especially in the first metatarsophalangeal joint, ankle, and foot joints. Other complications include chronic renal injury, ureteral calculi, and arthritis malformation. [ 4 , 5 ] Long-term intermittent repeated episodes of AGA would lead to the deposition of uratoma and eventually evolve into CGA, and there would be an asymptomatic interval (GIP) from the evolution of AGA to CGA (see Figure S1, Supplemental Content, which demonstrates the abridged general view of the evolution of GA).
According to statistics, the incidence rate of GA is increasing every year globally (about 1%–2%, 2018), especially in developing countries. [ 6 , 7 ] With the deepening of research in recent years, GA has been gradually defined as an autoimmune inflammatory disease. [ 8 ] Some preliminary research conclusions on the pathogenesis of AGA and CGA have been made by researchers at present. The pathogenesis of AGA might be related to the activation of Toll-like receptors, the NLRP3 inflammasome, or the P2X7 receptor, [ 9 ] while CGA might be related to the stimulation of the generation of extracellular neutrophil traps [ 10 ] or endoplasmic reticulum stress responses. [ 11 ] Some scholars also analyzed the metabolites of gout in HUA patients and healthy volunteers by nuclear magnetic resonance (NMR) [ 12 ] and ion chromatography-mass spectrometry (IC-MS), [ 13 ] and thus obtained the relevant biomarkers of gout and HUA including oxalic acid, l-homocysteic acid, lipids, and amino acids, which could help in developing lab tests for GA.
The clinical diagnosis of GA has been generally based on joint swelling, [ 14 ] CT findings, [ 15 ] smear test results, [ 16 ] and patient descriptions, which involve uncertainties (MSU crystal smear of HUA has positive results). [ 17 ] At present, there are different treatment methods for GA patients at different stages, such as diet control, drug treatment, and surgical treatment. However, in the absence of clear diagnostic markers, there is still a lack of methods to prevent the occurrence of GA and predict the evolution trend of GA. Therefore, the white blood cell (WBC) count, C-reactive protein (CRP) level, UA level, blood urea nitrogen (BUN) level, creatinine (Cre) level, hemoglobin (Hem) level, erythrocyte sedimentation rate (ESR), high/low-density lipoprotein (HDL/LDL) level, total cholesterol (TC) level, triglyceride (TG) level, demographics (age, body mass index [BMI], and sex), and living habit (smoking and alcohol drinking habit) have been considered as candidate risk factors of the progression of GA in this research. For most patients, the symptoms of GA and rheumatoid arthritis (RA) are similar, so it is easy to ignore the condition and delay the optimal treatment time. As a consequence, we also distinguished the 2 by the difference in biochemical indicators. Furthermore, principal component analysis (PCA), orthogonal partial least squares discrimination analysis (OPLS-DA), non-repetitive one-way analysis of variance (ANOVA), [ 18 ] correlation analysis, [ 19 ] and multiple logistic regression analysis [ 20 ] were used to screen the important indicators affecting GA in each stage, and to distinguish GA and RA. Finally, multiple logistic regression analysis was used to establish a clinical diagnosis model, [ 21 ] so as to improve the success rate of clinical diagnosis and prediction (see Figure S2, Supplemental Content, which demonstrates the overview of the study design).
2.1. Study design and group forming criterion
This study was implemented from November 30, 2017 to November 10, 2019, at Zhejiang Provincial People's Hospital (ZPPH), the largest comprehensive first-class hospital in Zhejiang province of China. All participants were categorized as healthy controls, HUA patients, AGA patients, GIP patients, and CGA patients based on the level of serum UA and the definition of GA as follows (based on the 2015 ACR-EULAR Gout Classification Criteria) [ 22 ] :
- (1) healthy controls were considered as those with a serum UA level of 150 to 416 μmol/L in men or 80 to 357 μmol/L in women and without GA or other types of arthritis, such as RA, osteoarthritis, and psoriatic arthritis.
- (2) HUA patients were considered as those with a serum UA level of >416 μmol/L in men or >357 μmol/L in women.
- (3) AGA patients were considered as those with persistent swelling and intense pain in the peripheral joints or bursae, with symptom peak within 24 hours and resolution within 14 days.
- (4) GIP patients were considered as those in whom it had been over 4 weeks since the last AGA attack and who had not received UA lowering therapy (ULT).
- (5) CGA patients were considered as those with joint swelling, pressing pain, deformity, dysfunction, and hypodermic tophus.
- (6) RA patients were considered as those with multi-joint swelling, disease duration over 6 weeks and rheumatoid factor (RF) >20. In the HUA and AGA groups, all synovial fluid samples were examined using polarized-light microscopy to ensure the presence of MSU crystals.
2.2. Measurement method and instruments
In addition to HUA, the onset of GA was usually associated with age, BMI, sex, smoking, and alcohol drinking habit. [ 3 ] Therefore, uric acid level, WBC, CRP, urea nitrogen, creatinine, hemoglobin, erythrocyte sedimentation rate (ESR), LDL, HDL, total cholesterol, triglyceride, demographic data, living habits, comorbidities (tumors and cardiovascular diseases), disease duration, and medical situations were collected through questionnaires and case history. Serum UA levels and other blood biochemical indexes of participants in each group were determined by the CHEMIX-180 automatic biochemistry analyzer (Sysmex Corp., Kobe, Japan). Each participant was visited, and serum was collected only once during the study period.
2.3. Study population
A total of 579 serum specimens were collected from ZPPH, including 379 in the training set (80 healthy volunteers [21.1%], 62 patients with HUA [16.4%], 69 patients with AGA [18.2%], 74 patients with GIP [19.5%], 62 patients with CGA [16.4%], and 32 patients with RA [8.4%]). As well as 200 in the validation set (80 healthy volunteers [40.0%], 30 patients with HUA [15.0%], 30 patients with AGA [15.0%], 30 patients with GIP [15.0%], and 30 patients with CGA [15.0%]).
About 70% of the patients were inpatients and another 30% were outpatients. The acute episodes of AGA lasted no <3 days, and the course of CGA was >11 years. Among the 207 patients with GA (mean age 49.23 ± 17.90 years), 188 were men (90.8%) and 19 (9.2%) were women. Compared with controls, GA patients had a higher average BMI (25.88 ± 3.01 vs 22.9 ± 2.7 kg/m 2 ; P = .01) and UA level (420.81 ± 116.86 vs 158.2 ± 89.0 μmol/L; P = .001). Meanwhile, the proportions of individuals with smoking and alcohol drinking habits were higher among GA patients than among healthy volunteers (smoking: 34.4% vs 55.8%; drinking: 25.0% vs 46.3%).
2.4. Statistical analysis methods
Statistical descriptions of other biochemical indicators in all serum specimens are provided in Table Table1, 1 , and the trends of biochemical indicators in the 5 disease progressions are indicated by box plots (Fig. (Fig.1). 1 ). The difference in measurement with a 2-sided alpha level of 0.05 could be ensured by this sample size using Fisher exact test. Simca 16.1 (Umetrics Inc., Sweden) was used for PCA and partial least square analysis in the groups (control group, HUA group, AGA group, GIP group, and CGA group). SPSS 23.0 (SPSS Inc., Chicago, IL) was used to perform repetitive single-factor ANOVA for the serum samples from the different groups of patients. Later, Pearson correlation coefficients were determined for indexes that may affect the progression of GA by using R language packages (corrplot). By applying the code written by our research team and combining 7 R language packages (quality plan, pROC package, ggplot2 package, mlogit package, RMS package, corrplot package, and survival package), we carried out multiple logistic regression analysis, established a GA diagnosis model, and conducted verification and visualization.
Baseline characteristics (demographic, living habit, medication, blood biochemical values) of the HUA, AGA, GIP, CGA, RA, and control groups in training set (n = 379).

The 8 selected blood biochemical indexes for the identification of the 5 GA stages and RA. A–L Box plots show the blood concentrations of the 8 indexes (left y -axis), including the WBC count, CRP level, uric acid level, urea nitrogen level, HDL/LDL level, cholesterol level, triglyceride level, creatinine level, hemoglobin level, BMI, and ESR across all 5 GA stages and RA. In the box plot, the median is expressed by the center line, 75% is represented by the upper bound of the box, 25% is expressed by the lower bound of the box, minimum is expressed by the lower whisker, and maximum is expressed by the upper whisker. Dot plots and lines show the trend of indexes across all 5 GA stages and RA. BMI = body mass index, CRP = C reactive protein, ESR = erythrocyte sedimentation rate; GA = gouty arthritis, HDL/LDL = high/low-density lipoprotein, RA = rheumatoid arthritis, WBC = white blood cell.
3.1. Statistical analysis of patient information and serum biochemical indicators
Data, including demographic data, living habits, comorbidities, disease durations, and medical situations, were collected through questionnaires and case histories. The UA level, WBC count, CRP level, BUN level, creatinine level, hemoglobin level, ESR, HDL/LDL level, TC level, and TG level of the participators in each group were determined by the automatic biochemistry analyzer (see Table S1 and Table S2, Supplemental Content, which respectively contain the detailed biochemical index data of patients in the training set and the validation set). The outcome of statistical analysis is provided in Table Table1 1 (training set), and the trends of biochemical indicators in the 5 stages are indicated by box plots (Fig. (Fig.1). 1 ). The RA group (n = 32) was added in this study in order to find a method that could be used to distinguish CGA from RA.
3.2. Principal component analysis and orthogonal partial least squares discrimination analysis
Multivariate analysis was performed on SIMCA-P 16.1 with the raw data of training set (n = 379). In order to determine the intrinsic differences among the 6 groups (control, HUA, AGA, GIP, CGA, and RA), PCA, and OPLS-DA were utilized. The PCA score plots (Fig. (Fig.2A) 2 A) showed a satisfactory separating effect of data among the 6 groups, while R 2 X was 1.00 and Q 2 was 0.978. Moreover, OPLS-DA score plots (Fig. (Fig.2B) 2 B) showed a better consequence, while R 2 X was 0.707, R 2 Y was 0.483, and Q 2 was 0.453. As can be seen from Fig. Fig.2, 2 , the biochemical index profile of each group was significantly different from that of the control group, with the greatest difference between the CGA group and the control group. Furthermore, the serum biochemical profiles of CGA and RA were relatively different, indicating that the biochemical indicators selected in this study could adequately distinguish CGA from RA.

PCA and OPLS-DA results of the serum biochemical profiles of the 6 groups. A represents the PCA score plots, B represents the OPLS-DA score plots, C is the variable important in projection (VIP) value of each biochemical index in the OPLS-DA model, D represents the 3D OPLS-DA score plots. The confidence interval is 95%. The creatinine level, age, ESR, BMI, hemoglobin level, WBC count, CRP level, and UA level contributed significantly to the difference between groups (VIP >1). BMI = body mass index, CRP = C reactive protein, ESR = erythrocyte sedimentation rate; GA = gouty arthritis, OPLS-DA = orthogonal partial least squares discrimination analysis, PCA = principal component analysis, UA = uric acid, VIP = variable important in projection, WBC = white blood cell.
3.3. Correlation analysis of factors influencing the course of GA
The corrplot package written by our research group in R language was applied for correlation analysis of biochemical indicators associating with progression of GA. The matrix heat map of correlation is shown in Fig. Fig.3A 3 A (see Table S3, Supplemental Content, which contains Pearson correlation and Significance). Here, we assume that the GA process is gradually evolved from the control group, HUA group, AGA group, GIP group, and CGA group from light to heavy. As can be seen from Fig. Fig.3A, 3 A, LDL and TC levels were significantly correlated, indicating that the correlation was not affected by the disease and had little significance for the evolution of GA. In addition, the UA level, creatinine level, WBC count, CRP level, BUN level, ESR, BMI, and HDL level were significantly correlated with the progression of GA, among which the UA level, creatinine level, WBC count, CRP level, BUN level, and ESR were remarkably positively correlated with the progression of GA, while the HDL level and BMI were remarkably negatively correlated with it.

The matrix heat map of the correlation of biochemical indicators (A) and ROC curves (B) associated with the progression of GA. A: Each dot represents a correlation between 2 indicators. The dot size is proportional to the P value, and the color gradation of the dots represents the magnitude of the correlation. The number at the bottom left represents the corresponding correlation coefficient with the dot. Blue indicates positive correlation, and red indicates negative correlation. As can be seen, the creatinine level (0.62), ESR (0.36), BUN level (0.32), WBC count (0.31), and UA level (0.23) had the strongest correlations with the progression of GA; B: The areas under the curve (AUCs) of the 5 models are 0.9814 (Control), 0.9288 (HUA), 0.9752 (AGA), 0.9056 (GIP), and 0.9759 (CGA). AGA = acute gouty arthritis, BUN = blood urea nitrogen, CGA = chronic gouty arthritis, ESR = erythrocyte sedimentation rate, GA = gouty arthritis, GIP = during the interictal period, ROC = receiver operating characteristic, UA = uric acid, WBC = white blood cell.
3.4. Clinical diagnostic model associating with progression of GA based on serum biochemical indicators
The MASS, PROC, ggplot2, mlogit, RMS, and survival packages in R language were used to carry out ordinal multiple logistic regression analyses for a clinical diagnostic model associated with the progression of GA. The independent variables were without influence upon the regression coefficient and segmentation point of the dependent variables, and there was no multicollinearity between the independent variables. The dependent variable was transformed into the corresponding dummy variable before modeling, and then, the continuous variables (among the independent variables, “sex” was the classified variable and the rest were continuous variables) were standardized, followed by factor analysis. The training set data (n = 379) were adopted to build the model. The 5 stages of GA (control, HUA, AGA, GIP, and CGA) were regarded as dependent variables, and logistic stepwise regression was performed for each stage. Meanwhile, combining the results of “3.1” to “3.4,” risk factors were screened to form the best logistic regression equation for each stage. The prediction probability of the sample under each regression equation was calculated, and the stage of GA in which the maximum probability was located was considered as the diagnosis. The verification set data (n = 200) were adopted to evaluate and verify the model. Details of the clinical diagnostic model of GA are provided in Table Table2 2 .
Details of clinical diagnostic model of GA.
3.5. Evaluation and verification of a clinical diagnostic model associated with the progression of GA
The validation set data were substituted into the above models, and the predicted results were outputted and compared with the actual results. The receiver operating characteristic (ROC) curves of 5 models are shown in Fig. Fig.3B. 3 B. The total area under the curve (AUC) of the clinical diagnostic model associated with the progression of GA was 0.9534, and the kappa coefficient was applied to evaluate the consistency between the predicted results of the model and the actual results. Encouragingly, the kappa coefficient of the clinical diagnostic model was 0.80, which indicated a substantial consistency of this model (the magnitude of the kappa coefficient could be divided into 5 degrees as follows: 0.0–0.20, slight consistency; 0.21–0.40, fair consistency; 0.41–0.60, moderate consistency; 0.61–0.80, substantial consistency; 0.81–1, almost perfect consistency). It shows that the model has high accuracy and reliability.
3.6. Visualizing the clinical diagnostic model associated with the progression of GA
In order to achieve greater application of the GA diagnostic model in clinical practice, a nomogram of the clinical diagnostic model associated with the progression of GA was made by adopting the R language rms package (see Figure S3, Supplemental Content, which demonstrates the nomogram of 5 models. A: Control group, B: HUA group, C: AGA group, D: GIP group, E: CGA group). According to the regression coefficient of each influencing factor in the multiple regression model, each influencing factor was scored. The summation function of the score was converted into the probability of the occurrence of the outcome event, and the stage of the maximum probability was the diagnosis result. This method can transform the complex regression equation into a simple and visual chart, making the results of the prediction model more readable and useful.
4. Discussion
Statistical studies showed that the prevalence of GA is increasing every year, especially in developing countries, with a 2% increase in the prevalence of GA from 2017 to 2019. [ 23 , 24 ] The incidence of GA in European and American countries is 0.13% to 0.37%, and the annual incidence is 0.20% to 0.35%. [ 25 ] The prevalence of HUA in the general population in China is about 10%. [ 26 ] So far, >80% of the published articles on disease prediction models for arthritis were associated with RA, [ 27 – 30 ] according to our literature review and document retrieval. Thus, it could be seen that GA is not a research hotspot in the field of arthritis, which is not commensurate with its high incidence. In order to reverse this abnormal phenomenon, the present study assessed a clinical diagnostic model associated with the progression of GA based on serum biochemical indicators.
We found a strong correlation between LDL and TC levels in all groups (LDL/TC in the control, HUA, AGA, GIP, and CGA groups: 0.54, 0.51, 0.59, 0.58, and 0.55), which is consistent with literature reports. [ 31 ] The correlation was not affected by GA progression, which indicated that LDL and TC were not independent risk factors (IRFs) of the progression of GA. Moreover, according to line charts in Fig. Fig.1, 1 , hemoglobin tended to flatten out in the 5 stages of GA. Therefore, LDL, TC, and hemoglobin levels were excluded in the establishment of a diagnostic model.
Box plots C and I in Fig. Fig.1 1 clearly indicated that BUN and creatinine levels showed significant elevation in the CGA group compared with the other 5 groups. The kidney is the main organ for excreting BUN, and as with serum creatinine, BUN could be in the normal range in the early stages of renal function impairment. However, BUN and creatinine levels would rise rapidly when the glomerular filtration rate (GFR) drops below 50% of the normal rate. Studies have shown that chronically high UA levels in the blood could significantly increase the risk of kidney disease, [ 32 ] and 5.6% of 13,338 participants (mean serum UA level = 5.9 ± 1.5 mg/dL) had incident kidney disease defined by a GFR decrease of >30% over 8.5 years. [ 33 ] Another population-based cohort study showed that patients with CGA who were treated with ULT had a greater risk of incident chronic kidney disease. [ 34 ] Therefore, BUN and creatinine could be regarded as IRFs for the progression of GA, and significant increases in BUN and creatinine levels indicate that the course of GA has entered the CGA stage. However, there were no significant differences in BUN and creatinine levels among the control, HUA, AGA, and GIP groups, so the establishment of a clinical diagnostic model for the progression of GA requires the contribution of other indicators. Besides, BUN and creatinine could be considered as diagnostic indicators of CGA and are serum biochemical indicators that could distinguish CGA from RA in addition to RF.
Moreover, as can be seen in Fig. Fig.2, 2 , the biochemical profile of each group was significantly different from that of the control group, with the greatest difference between the CGA group and the control group, and the creatinine level, age, ESR, BMI, hemoglobin level, WBC count, CRP level, and UA level contributed significantly to the difference between groups (VIP >1). Furthermore, the serum biochemical profiles of CGA and RA were relatively different, indicating that the biochemical indicators selected in this study could adequately distinguish the 6 groups including the 5 GA stages and RA. On this account, we suppose that the bias of the overall profile of the patient's serum biochemical indicators to a certain stage may lead to the appearance of symptoms. Therefore, we believe that the previous single indicator diagnosis method should be improved, and the approach should adopt multiple indicators for comprehensive clinical diagnosis and prediction. Meanwhile, according to the results of correlation analysis, the UA level, creatinine level, WBC count, CRP level, BUN level, TG level, ESR, and HDL level were significantly correlated with the progression of GA, among which the creatinine level (0.62), ESR (0.36), BUN level (0.32), WBC count (0.31), and UA level (0.23) had the strongest correlations with it. The findings are consistent with the results of multivariate statistics.
The training set data (n = 379) were adopted to build the clinical diagnostic model associated with the progression of GA (consists of 5 phases), and the verification set data (n = 200) were adopted to evaluate and verify it. The AUCs of the 5 models were 0.9814 (Control), 0.9288 (HUA), 0.9752 (AGA), 0.9056 (GIP), and 0.9759 (CGA). The kappa coefficient applied to evaluate the consistency was 0.80, indicating substantial consistency of this model. Furthermore, the visualization of the model is realized by using nomograms, which facilitates more clinical application of the model. Both doctors and patients can judge the development and changes of GA according to the model, which provides an effective reference for distinguishing the development stages of GA.
After this study, non-targeted metabolomics analysis will be performed on the serum samples of GA patients collected above to obtain potential diagnostic biomarkers related to the progression of GA, including absolutely qualitative and quantitative verification. The changes in biomarkers and serum biochemical indexes in different progressions of GA were found to optimize the clinical diagnostic model of GA based on the serum biochemical indexes proposed in this paper. There is an expectation to develop a new clinical diagnosis method for GA similar to the “GA diagnostic kit” which can help clinicians predict the progress of GA faster and more accurately.
5. Conclusion
In this study, serum biochemical indicators from clinical assessment were adopted to establish a clinical diagnostic model and serum biochemical profile associated with the progression of GA. It is the first study to investigate the serum biochemical profile at different stages of GA. An obvious difference in the serum biochemical profile among GA stages was found in this paper, which could effectively distinguish them. The hypothetical algorithm of GA diagnostic model was P = 1/1 + e – Z , in which P stands for prediction probability, Z stands for independent risk factor variables at each stage of GA (health: 5.38–0.0251 × UA; HUA: 4.12 + 2.11 × HDL + 0.0114 × UA − 0.0560 × CRP; AGA: 14.8 + 0.0464 × CRP + 0.0132 × UA + 0.287 × ESR; GIP: 4.92 + 0.184 × WBC − 3.52 × HDL + 0.00608 × UA − 0.310 × ESR; CGA: 6.58 + 0.602 × BUN + 0.0436 × ESR). The prediction probability of the sample under each regression equation was calculated, and the stage of GA in which the maximum probability was located was considered as the diagnosis. We suppose that the bias of the overall profile of a patient's serum biochemical indicators to a certain stage may lead to the appearance of symptoms. A simple evaluation tool was developed, and we have reason to believe it will be useful for clinicians and researchers wishing to consolidate their clinical diagnosis in regard to the stage and tendency of GA patients. This clinical diagnostic model could be applied clinically and in research to improve the accuracy of the identification and prediction of these stages of GA patients.
Author contributions
Conceptualization: Shang Lyu, Shilin Yang, Peng Liu.
Data curation: Ruowen Ding, Hui Ouyang.
Funding acquisition: Shilin Yang, Yulin Feng.
Investigation: Shang Lyu, Ruowen Ding, Wanyuan Chen, Peng Liu.
Software: Yi Rao.
Validation: Shilin Yang.
Visualization: Ruowen Ding.
Writing – original draft: Shang Lyu.
Supplementary Material
Abbreviations: AGA = acute gouty arthritis, ANOVA = analysis of variance, AUC = area under curve, BMI = body mass index, CGA = chronic gouty arthritis, CRP = C reactive protein, CV = coefficient of variation, CVD = cardiovascular disease, GA = gouty arthritis, GIP = gouty arthritis during the intermittent period, HUA = hyperuricemia, JUTCM = Jiangxi University of Traditional Chinese Medicine, MSU = monosodium urate, MVA = multivariate analysis, OPLS-DA = orthogonal projections to latent structures-discriminant analysis, PCA = principal component analysis, RA = rheumatoid arthritis, RF = rheumatoid factors, ROC = receiver operating characteristic curve, ULT = urate-lowering therapy, WBC = white blood cell, ZPPH = Zhejiang Provincial People's Hospital.
How to cite this article: Lyu S, Ding R, Yang S, Chen W, Rao Y, OuYang H, Liu P, Feng Y. Establishment of a clinical diagnostic model for gouty arthritis based on the serum biochemical profile: a case-control study. Medicine . 2021;100:16(e25542).
This work was supported by grants from Key R&D project of Jiangxi Province (2020YBBGWL002), Jiangxi Science and Technology Innovation Platform Project (20194AFD45001), Special Project for Central Guidance of Local Science and Technology Development (20192ZDD02002), Natural Science Foundation of China (No.81560636, 81760702) and National Key R&D Program of China (No.2019YFC1712300), Science and Technology Research Project of Education Department of Jiangxi Province (GJJ201257), Science and Technology Project of Health Commission of Jiangxi Province (202110124), Doctoral Research Foundation of Jiangxi University of Traditional Chinese Medicine (2020BSZR016).
Ethics approval and consent to participate: The medical ethics committee of ZPPH approved this study (acceptance number: KY2018048, approval number: 2018KY046), the ethics committee was constituted and operated in accordance with the principles of SFDA/GCP and the Declaration of Helsinki. Health screening of participants in the study were enrolled at ZPPH from 2018 to 2019. Demographic data, living habits, comorbidities, disease durations and medical situations were recorded in a normative questionnaire.
Consent for publication: The publication of all case reports has been consent, and the personal data contained in the manuscript have been consent to publish by the patient.
Competing interests: The authors declare that they have no competing interests.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
Data were presented as mean ± SD except where stated otherwise.
AGA = acute gouty arthritis, BMI = body mass index, CGA = chronic gouty arthritis, CRP = C-reactive protein, CVD = cardiovascular disease, GIP = gouty arthritis during the intermittent period, HUA = hyperuricemia, RA = rheumatoid arthritis, RF = rheumatoid factors, UTL = uric acid lowering therapy, WBC = white blood cell.
∗ P < .05.
∗∗ P < .01, compared with control.
# P < .05.
## P < .01, compared with the RA group.
The calculation results of P value were shown in Table S3.
AGA = acute gouty arthritis, CI = confidence interval, CGA = chronic gouty arthritis, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, GIP = gouty arthritis during the intermittent period, HDL = high-density lipoprotein, HUA = hyperuricemia, UA = uric acid, WBC = white blood cell.
OR = odds ratio, indicates the strength of the association between factors and models; P in regression equations: prediction probability.
Z1–Z5 in regression equations: Z1 = 5.38–0.0251 × UA, Z2 = 4.12 + 2.11 × HDL + 0.0114 × UA − 0.0560 × CRP, Z3 = 14.8 + 0.0464 × CRP + 0.0132 × UA + 0.287 × ESR, Z4 = 4.92 + 0.184 × WBC − 3.52 × HDL + 0.00608 × UA − 0.310 × ESR, Z5 = 6.58 + 0.602 × BUN + 0.0436 × ESR.

IMAGES
VIDEO
COMMENTS
Abstract. Gout is the most common inflammatory arthritis which is caused by the buildup of uric acid crystals in the joints, that leads to severe pain, swelling, and stiffness. The condition typically affects the first metatarsophalangeal joint but it can impact other joints in the body. We present a case in which a 43-year-old male with a past ...
A 24-year-old white male with no known medical history presented to the hospital with chief complaints of 2 weeks of progressively worsening pain and swelling of his right wrist and left ankle joints. He did not report any fevers, chills, night sweats, weight loss or rash. He was employed as a fireman. He denied alcohol use disorder, smoking cigarettes, or any recreational drugs. Family ...
Background Gout is a metabolic disease that can manifest as acute or chronic arthritis, and deposition of urate crystals in connective tissue and kidneys. It can either manifest as acute arthritis or chronic tophaceous gout. Case presentation We present a 39-year-old male patient who developed acute arthritis during his hospital course. Later on, after a careful physical examination, patient ...
The current epidemiologic studies show that the prevalence of gout in patients between the ages of 18 and 44 is <0.3%. As of 2019, only 159 cases of spinal gout have been reported; gout is typically diagnosed in the fourth decade of life, the average age in men 30 years old. Around 50% of patients present with neurological sequela requiring ...
Background: Gout is an inflammatory arthritis that results from faulty purine metabolism, affecting approximately 4% of adults in the US, and predominately affects people in the fourth decade of life. Further, spinal gout is rarely the first presentation of gout, especially in younger individuals. Case description: A 26-year-old male came to the emergency room with acute lower extremity ...
Gouty arthritis is one of the most common rheumatic diseases. The clinical burden of gouty arthritis has historically been well recognized; however, gout is often misdiagnosed and mismanaged. The prevalence of gout is rising and is likely attributed to several factors including increased incidence of comorbidities, lifestyle factors, and ...
Gout is the most common inflammatory arthritis in the United States. Gouty arthritis is associated with significant morbidity and mortality and is the result of chronic hyperuricemia. Gout is effectively managed and potentially cured by decreasing the overall urate burden with serum urate-lowering therapy. When serum urate is maintained at less than 6.0 mg/dL, urate deposition is resolved ...
Joint pain is a common complaint in pediatrics and is most often attributed to overuse or injury. In the face of persistent, severe, or recurrent symptoms, the differential typically expands to include bony or structural causes versus rheumatologic conditions. Rarely, a child has two distinct causes for joint pain. In this case, an obese 15-year-old male was diagnosed with gout, a disease ...
Because these results suggested gout, the patient was ad-vised to start colchicine 0.6 mg twice daily and allopurinol 100 mg daily, titrated every 2 weeks to a target dose of 300 mg daily. He was advised to reduce his daily dosage of aspirin to 81 mg, to discontinue indomethacin, and to take acetaminophen as needed, up to 4 g/day. In addition, the
Case study: a case of debilitating gout in the 1st metatarsophalangeal joint. 2015 Mar;25 (1):45-50. doi: 10.1016/j.foot.2014.11.004. Epub 2014 Dec 6. Gout is a painful arthritic condition that affects many people worldwide. The disease has been associated with hyperuricaemia and life style risk factors such as obesity, alcohol intake, meat and ...
Studies in patients with gout are restricted to small case series in ten or fewer patients [18, 19]. In one case series, patients with a history of recurrent gouty arthritis or tophaceous gout received open-label anakinra at a dose of 100 mg/day administered as a subcutaneous injection for 3 days [ 19 ].
Atypical Presentation of Gout: A Case Report Ameen Abdel-Khalek , Ammarah Tariq , Joseph A. White , Amara R. Cheema , Sirjana Dhillon , ... arthritis, gout and hypertension. His chronic arthritis ... The Global Burden of Disease Study 2017 published that there are 41 million living with gout worldwide [3]. The prevalence of gout in 1990 was 20.2
Gout is a metabolic disease characterized by recurrent episodes of acute arthritis. Gout has been reported in many locations but is rarely localized in the shoulder joint. We describe a rare case of gouty arthritis involving bilateral shoulder joints and leading to severe destructive changes in the right shoulder glenoid. A 62-year-old male was referred for pain and weakness in the right ...
Gouty tophi typically arise in the intercondylar notch, suprapatellar bursa, infrapatellar fat pad, lateral gutter, and tibial plateau [1].In the present case, the gouty tophi arose in almost the same location as in the previous report [5, 17].Regarding treatment of gouty knee arthritis, Wang et al. [8] performed a randomized controlled trial and reported the efficacy of arthroscopic ...
Rheumatoid arthritis (RA) is one of the most common symmetric, peripheral polyarthritis, chronic inflammatory rheumatic diseases of an unknown etiology. The general prevalence of RA is about 0.5% to 1% in the adult population with a male and female prevalence ratio of 1:3. [1,2] Meanwhile, gout is a deposition of monosodium urate crystals which ...
Case Studies in Gouty Arthritis 1-3 May 2005 • New York, New York • TAP Pharmaceuticals, Inc. Edited by Kenneth G. Saag MD - The University of Alabama at Birmingham, Birmingham, Alabama
Abstract. Gout is a picturesque presentation of uric acid disturbance. It is the most well understood and described type of arthritis. Its epidemiology is studied. New insights into the pathophysiology of hyperuricemia and gouty arthritis; acute and chronic allow for an even better understanding of the disease.
The disease progression of gouty arthritis (GA) is relatively clear, with the 4 stages of hyperuricemia (HUA), acute gouty arthritis (AGA), gouty arthritis during the intermittent period (GIP), and chronic gouty arthritis (CGA). ... A case-control study Medicine (Baltimore). 2021 Apr 23;100(16):e25542. doi: 10.1097/MD.0000000000025542. ...
Introduction Gout is a common arthritis that occurs particularly in patients who frequently have associated comorbidities that limit the use of conventional therapies. The main mechanism of crystal-induced inflammation is interleukin-1 production by activation of the inflammasome. We aimed to evaluate the efficacy and tolerance of anakinra in gouty patients. Methods We conducted a multicenter ...
A review of the literature reveals that gouty arthritis of the wrist is rare in isolation although gout itself is the most common inflammatory arthritis in older patients [4, 6-9].Gout at the wrist as the initial appearance of the condition occurs between 0.8 to 2% of all gout cases [].Gout patients who are not treated have a 19-30% chance of developing gout in the wrist during their ...
Similar to our case, all patients responded remarkably well once started on treatment for acute crystal arthropathy. It is known that both, gout and calcium pyrophosphate deposition disease (CPPD) arthritis, elicit their inflammatory response by engaging the recruitment of leucocytes and release of cytokines as discussed before.
Arthritis and Gout Case Study. This case study looks at the effects of being diagnosed with a long-term condition and provides an insight and appreciation of how living with the condition effects a person's life and their family. I interviewed a 38-year-old male of Indian descent, who lives in Auckland in a flatting situation with two other ...
A case-control study. Shang Lyu, PhD, a Ruowen Ding, PhD, a Shilin Yang, ... Gouty arthritis (GA) is a kind of chronic disease caused by purine metabolic disorders, and its progression is relatively clear, which can be roughly divided into the following 4 stages: hyperuricemia (HUA), acute gouty arthritis (AGA), gouty arthritis during the ...