Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Development of UV spectrophotometry methods for concurrent quantification of amlodipine and celecoxib by manipulation of ratio spectra in pure and pharmaceutical formulation
Contributed equally to this work with: Mahesh Attimarad, Venugopla Katarigatta Narayanswamy, Bandar Essa Aldhubaib
Roles Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Pharmaceutical Sciences, College of Clinical Pharmacy, King Faisal University, Al Ahsa, KSA
Roles Formal analysis, Funding acquisition, Methodology, Validation, Writing – original draft, Writing – review & editing
Affiliations Department of Pharmaceutical Sciences, College of Clinical Pharmacy, King Faisal University, Al Ahsa, KSA, Department of Biotechnology and Food Technology, Durban University of Technology, Durban, South Africa
Roles Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing
Roles Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing
¶ ‡ These authors also contributed equally to this work.
Roles Formal analysis, Funding acquisition, Methodology, Resources, Validation, Writing – review & editing
- Mahesh Attimarad,
- Venugopla Katarigatta Narayanswamy,
- Bandar Essa Aldhubaib,
- Nagaraja SreeHarsha,
- Anroop Balachandran Nair

- Published: September 16, 2019
- https://doi.org/10.1371/journal.pone.0222526
- Reader Comments
30 Dec 2019: Attimarad M, Venugopala KN, Aldhubaib BE, SreeHarsha N, Nair AB (2019) Correction: Development of UV spectrophotometry methods for concurrent quantification of amlodipine and celecoxib by manipulation of ratio spectra in pure and pharmaceutical formulation. PLOS ONE 14(12): e0227412. https://doi.org/10.1371/journal.pone.0227412 View correction
Recently, the United States Food and Drug Administration approved a new oral dosage preparation of amlodipine besylate (AML) and celecoxib (CEL) for the management of hypertension and osteoarthritis. However, no simultaneous estimation procedures for these two analytes have been described. Hence, two simple, accurate, and precise ultraviolet spectroscopic procedures that manipulated the ratio spectra were established for concurrent quantification of AML and CEL using ethanol as a solvent. The first method involves determining the peak-to-trough amplitude difference of the ratio spectra of AML and CEL. The second method involves determining the peak amplitude of the ratio first derivative (Δλ 4 nm) spectra of AML and CEL at 334.2 nm and 254.2 nm, correspondingly. Both methods showed linearity in the range of 1–6 μg mL-1 for AML and 5–40 μg mL-1 for CEL with an excellent correlation coefficient (<0.999). The proposed procedures were validated by following the International Conference on Harmonization guidelines for accuracy, precision, selectivity, recovery, and stability studies. It is evident from the low %RSD and %RE that both analytical procedures were found to be accurate and precise, respectively. The percent recovery of AML and CEL from the formulation was found to be 99.79% and 99.34% using the ratio-difference method and 100.13% and 99.70% using the ratio first-derivative method, with a low percent relative standard deviation. Further, the proposed techniques permit concurrent quantification of AML and CEL in different concentration ratios without interference from each other; hence, these techniques can be adopted for regular quality-control studies.
Citation: Attimarad M, Narayanswamy VK, Aldhubaib BE, SreeHarsha N, Nair AB (2019) Development of UV spectrophotometry methods for concurrent quantification of amlodipine and celecoxib by manipulation of ratio spectra in pure and pharmaceutical formulation. PLoS ONE 14(9): e0222526. https://doi.org/10.1371/journal.pone.0222526
Editor: Pablo Garcia de Frutos, Institut d'Investigacions Biomediques de Barcelona, SPAIN
Received: June 10, 2019; Accepted: September 2, 2019; Published: September 16, 2019
Copyright: © 2019 Attimarad et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: This research work was performed with the financial support provided by the Deanship of Scientific Research, King Faisal University, Al-Ahsa, Saudi Arabia (Group Project #: 1811018).
Competing interests: The authors have declared that no competing interests exist.
Introduction
Middle-aged and older adults are suffering from many health problems, such as hypertension, diabetes, and osteoarthritis due to modern lifestyles and stress. Calcium-channel blockers are the most extensively prescribed medication in the management of hypertension due to its long duration of action. Amlodipine besylate (AML; Fig 1A ), a dihydropyridine derivative, is a potent antihypertensive and antianginal drug that obstructs the migration of calcium ions in both vascular and cardiac muscles, thereby inhibiting the contraction of smooth and cardiac muscles. AML also reduces blood pressure via a peripheral vasodilation effect by reducing peripheral vascular resistance. [ 1 , 2 ]
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Chemical structures of amlodipine besylate (A) and celecoxib (B).
https://doi.org/10.1371/journal.pone.0222526.g001
Osteoarthritis is a degenerative disorder of the articular cartilage that leads to structural modifications of the bone. [ 3 ] Osteoarthritis may result from aging, obesity, and genetics. Those who are older and/or obese generally suffer from hypertension. Also, approximately 40% of those with hypertension are also diagnosed with osteoarthritis. [ 4 – 5 ] For the treatment of osteoarthritis-related pain, different nonsteroidal anti-inflammatory drugs (NSAIDs) were prescribed; however, for patients suffering from hypertension along with osteoarthritis, special attention should be paid when selecting NSAIDs, as most NSAIDs have an effect on blood pressure, and use of non-selective COX inhibitors can result in gastric ulceration. Conversely, the selective COX-2 inhibitor celecoxib (CEL; Fig 1B ) has gastroprotective effects along with little effects on blood pressure. [ 6 – 7 ] As such, the United States Food and Drug Administration (FDA) has recently approved a fixed-dose oral formulation of AML and CEL for the management of hypertension and osteoarthritis. [ 8 ].
Different analytical techniques were described in the public domain for the quantification of AML from pharmaceutical preparations and plasma. AML alone has been estimated by spectrophotometric, [ 9 , 10 ] fluorometric, [ 11 ] reverse-phase high-performance liquid chromatography (RP-HPLC), [ 12 – 14 ] and liquid chromatography–tandem mass spectrometry (LCMS/MS) methods [ 15 ]. AML, along with other antihypertensive drugs, were determined by spectrophotometric [ 16 ], high-performance thin-layer chromatography (HPTLC), [ 17 ] and electroanalytical methods [ 18 – 23 ]. Besides, few high-performance liquid chromatography (HPLC) procedures have been described for the concurrent estimation of AML with statins. [ 24 , 25 ]
Few spectroscopic procedures have been described in the estimation of CEL alone and various drug combinations. [ 26 – 28 ] Many chromatographic methods, along with stability-indicating procedures, were reported in the public domain for the concurrent estimation of CEL alone and in combination with other drug formulations and biological fluids. [ 29 – 33 ] Recently, the LCMS/MS method has been described for the quantification of CEL from human plasma. [ 34 ] In addition, the capillary zone electrophoresis (CZE) technique has been adopted for quantification of CEL in pharmaceutical formulations. [ 35 , 36 ]. However, no spectroscopic procedures have been reported for the concurrent quantification of AML and CEL in formulations. Therefore, it would be useful to establish simple, accurate, precise, and specific spectroscopic methods for the concurrent quantification of AML and CEL. The ultraviolet (UV) spectrophotometric method has been extensively used in analytical and clinical laboratories to analyze drugs in pharmaceutical formulations due to it’s simple, accurate, and reproducible results. However, many drugs have good UV absorption due to the presence of aromatic and heteroaromatic groups. Hence, multi-component formulations produce highly overlapped UV spectra, making it difficult to analyze the drugs directly in the presence of each other. Similarly, the ultraviolet (UV) spectrum of CEL completely overlaps that of AML, making it difficult to quantify both analytes by direct measurement. Hence, in the present work, we reported two ratio spectra manipulation spectroscopic procedures for the concurrent estimation of AML and CEL in a solid dosage form.
Materials and methods
Instrumentation: A Shimadzu UV-Vis spectrophotometer (1600; Shimadzu Corporation, Kyoto, Japan) associated with the workstation was used for the development of the analytical methods. Then, 10 mm quartz cuvettes were used to measure solution absorbance. The slit width and scan speed were adjusted to 1 nm and medium, respectively. The manipulation of scanned spectra was performed using Shimadzu software (UV-Probe version 2.0; Shimadzu Corporation). Analytes and formulations were weighed using a digital balance.
Chemicals: Pure samples of AML and CEL were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Analytical-grade ethanol was procured from Sigma Aldrich Co. Pure water was prepared using the Milli Q (Millipore, Billerica, MA, USA) water purification system, which was used throughout the experiments. AML (10 mg/tablet) and CEL (200 mg/capsule) were obtained from the pharmacy.
The tablets were prepared in a laboratory and included 200 mg CEL, 10 mg AML / 5 mg AML, 10 mg sodium starch glycolate type A, 12.5 mg talc, 10mg microcrystalline cellulose, 5 mg magnesium stearate and 8 mg lactose per tablet
Preparation of standard solutions
To prepare the standard stock solutions of AML and CEL, 100 mg of AML and CEL were accurately weighed into two 100 mL measuring flasks, separately. Both analytes were dissolved using 60 mL of ethanol, and the final volumetric capacity reached 100 mL with the addition of ethanol. Standard solutions were kept in the refrigerator (4°C) until use. Standard solutions were used to prepare the calibration curve and for validation purposes, and laboratory-prepared solutions were arranged by transferring the required amount of standard solutions into 10 mL volumetric flasks.
Preparing the sample solutions
AML and CEL combination tablets were not available in Saudi Arabia; hence, AML (10 mg) tablets and CEL (200 mg) capsules were used to prepare the samples. The weight of 20 AML tablets was determined; the average weight was computed, and the tablets were crushed into a smooth powder. Similarly, the powder of 20 CEL capsules was collected and the average weight was computed. AML powder corresponding to 50 mg of AML, as well as CEL capsule contents corresponding to 1,000 mg of CEL, were transferred and mixed thoroughly. Then, the powder mixture, corresponding to 10 mg of AML and 200 mg of CEL, was moved into a 100 mL measuring flask consisting of 60 mL of ethanol. The contents were dissolved with the help of ultrasonication (10 minutes). The solution was filtered using Whatman filter paper in a 100 mL measuring flask. The filter paper and remaining solution were rinsed with fresh ethanol; the final volume was adjusted up to the level with ethanol. The required amount of sample fraction was transferred into a 10 ml measuring flask and the ethanol was added to obtain the analyte quantity, which fell in the calibration range.
Preparing the sample solutions using laboratory prepared tablets
Laboratory-prepared tablets were powdered, and powder equivalent to 10 mg of AML and 200 mg of CEL, as well as 5 mg of AML and 200 mg of CEL, was transferred separately into two conical flasks. Analytes were extracted by adding ethanol (30 ml x3). Each time solutions were sonicated for 10 min, filtered into 100 ml measuring flasks and the residue was washed with fresh ethanol. The final volume of both solutions were adjusted to 100 mL with ethanol. Further, solutions were diluted with ethanol to obtain a concentration in the range of the calibration curve.
Procedure to prepare the calibration curve using ratio and first-derivative ratio spectra
The six working standard solutions comprising of 1–6 μg mL -1 (1, 2, 3, 4, 5, and 6 μg mL -1 ) of AML and 5–40 μg mL -1 (5, 10, 20, 25, 30, and 40 μg mL -1 ) of CEL were prepared by diluting the standard solutions with ethanol. AML and CEL solutions with a concentration 2 μg mL -1 and 10 μg mL -1 , respectively, were arranged independently. UV absorption spectra were recorded for all solutions in the UV range of 200–400 nm using ethanol as a blank; the spectra were stored on a computer. Then, the ratio spectra were constructed by dividing the stored spectra by the CEL spectra of the 10 μg mL -1 solution; the ratio spectra were stored to perform the ratio-difference method. Further, the ratio spectra were converted into first-derivative spectra using 4 nm as Δλ. Similarly, the ratio spectra for CEL were constructed by dividing the combined spectra of the AML and CEL solutions by the AML spectra of the 2 μg mL -1 solution. Further, these spectra were converted into first-derivative spectra using 4 nm as Δλ. For the ratio-difference method, the peak amplitude difference was calculated by subtracting the amplitude of the trough at 345.6 nm from the amplitude of the peak at 336.4 nm for AML, and 236.5 nm from 266.8 nm for CEL. Then, the calibration curves were constructed by plotting a graph between the difference in peak amplitude with the corresponding concentrations of AML and CEL. Alternatively, regression equations were developed from the calibration curve.
For the first-ratio-derivative method, peak heights were determined at 334.2 nm for AML and 254.2 nm for CEL. Then, the calibration curves were created by drawing a graph between the peak amplitude and its relevant concentration. Alternatively, regression equations were developed from the calibration curve.
Procedure to create laboratory mixed solutions
Nine mixed solutions were prepared in the laboratory by transferring the aliquot of AML and CEL stock solutions to obtain concentrations of 1:10, 2:10, 3:10, 1:20, 2:20, 3:20, 1:30, 2:30, and 3:30 μg mL -1 , respectively. Normal UV spectra were recorded for all solutions in the UV range of 200–400 nm; these spectra were converted into ratio spectra and the first-derivative of ratio spectra. Amplitudes were determined by adopting the above procedure; concentrations were determined by the regression equation.
Results and discussion
Normal absorption spectra for AML and CEL ( Fig 2 ) showed complete overlap, which hindered the direct determination of these two analytes without prior separation. In such cases, the transformation of the UV absorption spectra into ratios and/or derivative ratio graphs can be adopted for the simultaneous determination of multicomponent formulations. [ 37 , 38 ] The ratio spectra and their derived spectra permit the quantification of one component that is present alongside another component, as well as with excipients without their interference. [ 37 – 41 ]
UV spectra of pure AML(A), CEL(B) and Formulation (C).
https://doi.org/10.1371/journal.pone.0222526.g002
Theory of the ratio-absorbance-difference and ratio-derivatization techniques [ 37 – 41 ]
Hence, the component n is canceled; the peak amplitude difference represents only component m. Component m can be determined from a mixture of components by constructing the calibration curve between the difference in peak amplitudes at two different wavelengths of the ratio spectra against the concentration of m. Similarly, following the same process, another component n can be determined in the presence of component m.
In the present work, the ratio spectra of AML with different concentrations were obtained by scanning the mixture of AML and CEL in increased concentrations and dividing the normal spectra with the UV absorption spectra of CEL (10 μg mL -1 ; Fig 3A ). The difference in the peak amplitude of the ratio spectra at a peak of 336.4 nm and trough of 345.6 nm was proportional to the concentration of AML. Further, this was supported by comparing the ratio spectra of the pure AML standard solution and the ratio spectra of the mixture of CEL, as well as with AML containing the same amount of AML. The constant difference between these two spectra was due to the Cn/Cn˚ in Eq ( 2 ). This unwanted parameter can be excluded by taking the variance between peak amplitudes measured at two wavelengths (336.4 nm and 345.6 nm). Generally, the two selected wavelengths should be the peak and trough of the ratio spectra to obtain maximum sensitivity. Fig 3B shows that the difference between the peak and trough amplitudes of the mixture and standard AML ratio spectra were the same; hence, the concentration of AML can be determined even in the presence of CEL. Similarly, CEL was determined by constructing the ratio spectra, which was achieved by dividing the normal spectra of the increased concentration of AML and CEL with the UV absorption spectra of the AML (2 μg mL -1 ) solution ( Fig 4A ). The peak height (266.8 nm) and trough (236.5 nm) of the ratio spectra were selected to measure the difference, which was proportional to the concentration of CEL. Fig 4B shows that the difference between the peak and trough amplitudes of the mixture and standard CEL ratio spectra were the same, irrespective of the AML concentration in the mixture.
(A) Ratio spectra of standard AML solutions (1–6 μg mL -1 ) using 10 μg mL -1 CEL as devisor. (B) Comparison of ratio spectra of pure AML and mixture of AML and CEL using 10 μg mL -1 CEL as devisor.
https://doi.org/10.1371/journal.pone.0222526.g003
(A) Ratio spectra of standard CEL solutions (5–40 μg mL -1 ) using 2 μg mL -1 AML as devisor. (B) Comparison of ratio spectra of pure CEL and mixture of AML and CEL using 2 μg mL -1 AML as devisor.
https://doi.org/10.1371/journal.pone.0222526.g004
Ratio-first-derivative spectroscopic method
As an alternative to the aforementioned method of measuring the difference between the peaks and troughs of the ratio spectra, derivatization of the ratio spectra can be adopted to resolve the interference spectra of binary mixtures. The derivatization of ratio spectra eliminates the constant interference value (Cn/Cn˚) in Eq ( 2 ) and provides many maxima and minima. This provides an opportunity to quantify one analyte in the presence of another analyte, as well as excipients, which generally cause interference during analysis. [ 38 – 41 ] In the second method, the ratio spectra of different concentrations were converted into first-derivative spectra using 4 nm as a derivatization wavelength (Δλ). Different wavelengths (2 nm, 4 nm, 8 nm, and 10 nm) were tested; however, 4 nm showed the most accurate results. The first-derivative ratio spectra of AML ( Fig 5A ) showed 2 minima at –342.2 nm and –390.2 nm, and four maxima at 316.6 nm, 327.9 nm, 334.2 nm, and 348.8 nm. Further, the peak amplitude at 316.54 nm, 327.9 nm, 348.70 nm, and –390.15 nm was lower, whereas the peak amplitude was satisfactory at 334.2 nm and –342.2 nm. Overall, 334.2 nm provided good linearity. Hence, a wavelength of 334.2 nm was selected for further processing. The first derivative of the ratio spectra of CEL ( Fig 6A ) showed two maxima at 222.0 nm and 254.2 nm, and four minima at –230.6 nm, –260.2, –277.2, and –291.4 nm. The peak amplitude at 222.0 nm, –230.6 nm, –260.2 nm, and –277.2 nm was lower, whereas good peak amplitude, linearity, and recovery were observed at 254.2 nm and –291.4 nm.
(A) First derivative of standard AML solutions (1–6 μg mL -1 ) using 10 μg mL -1 CEL as devisor. (B) Comparison of first derivative of ratio spectra of pure AML and mixture of AML and CEL using 10 μg mL -1 CEL as devisor.
https://doi.org/10.1371/journal.pone.0222526.g005
(A) First derivative of ratio spectra of standard solutions of CEL (5–40 μg mL -1 ) using 2 μg mL -1 AML as devisor. (B) Comparison of First derivative (Δλ 4nm) of ratio spectra of pure CEL and mixture of AML and CEL using 2 μg mL -1 AML as devisor.
https://doi.org/10.1371/journal.pone.0222526.g006
Also, a comparison of the first derivative of the ratio spectra for the combined AML and CEL, as well as the pure standard AML, showed the same amplitude at 334.2 nm, ( Fig 5B ) and whereas CEL showed the same amplitude at 254.2 nm ( Fig 6B ). Hence, wavelengths 334.2 nm and 254.2 nm were used to further develop the method.
Method validation
Validation of the proposed manipulation of the UV-ratio spectroscopic methods was carried out in terms of linearity, the limit of detection, the limit of quantification, accuracy, precision, recovery studies, selectivity, and stability studies following International Conference on Harmonisation (ICH) guidelines.
Linearity: Linearity was established by converting the recorded UV absorption spectra into the ratio and first derivative of ratio spectra of both analytes. In both methods, AML and CEL showed good linearity with a concentration ranging from 1–6 μg mL -1 and 5–40 μg mL -1 , correspondingly ( S1 Fig ). The linearity equations constructed from the standardization curve were tabulated in Table 1 . In all cases, the correlation coefficient was found to be more than 0.999.
https://doi.org/10.1371/journal.pone.0222526.t001
Limit of detection (LOD) and limit of quantification (LOQ).
As per the ICH guidelines, the LOD was calculated by 3.3 x/y and LOQ by 10 x/y. In these equations, x represents the standard deviation of the intercept and y represents the slope of the standard curve. The calculated LOD and LOQ are shown in Table 1 . The small LOD and LOQ values describe the sensitivity of the projected methods.
Accuracy and precision.
Intraday accuracy and precision were evaluated by investigating the laboratory-mixed analytes at three diverse concentration points covering the entire calibration range in triplicate on the same day. The same solutions were analyzed for three successive days to determine intraday accuracy and precision ( S2 Fig ). Precision was expressed as %RSD ( Table 2 ) and it was <2%, demonstrating a high degree of precision for the procedures. Accuracy was expressed in terms of % relative error ( Table 2 ). The results showed a low % relative error, indicating the excellent accuracy of the established procedures.
https://doi.org/10.1371/journal.pone.0222526.t002
Stability studies.
The prepared stock solutions were stored in a refrigerator at 4°C. The stored solutions were analyzed daily; no difference was observed in the assay results, as determined by spectroscopic methods, which were compared to the day 1 results. Further, CEL was stable for 7 days, whereas AML began degrading after 5 days. This could be due to the instability of AML in the presence of light. [ 42 – 43 ].Hence, it is recommended that the solutions be covered with aluminum foil.
AML and CEL determination from laboratory-prepared samples
Standard solutions contacting different concentrations of both analytes were mixed and analyzed ( S3 Fig ). The recovered drug concentrations ( Table 3 ) of AML and CEL indicated the analytical power of the suggested spectroscopic procedures for the simultaneous determination of both analytes present in different ratios. Hence, these methods can be applied to analyze formulations comprising different ratios of AML and CEL (5:200, and 10:200).
https://doi.org/10.1371/journal.pone.0222526.t003
Selectivity.
The selectivity of the established procedures was confirmed by the results of the laboratory-mixed AML and CEL solutions at different concentrations. Acceptable results were achieved in the different ratios, as tabulated in Table 3 . Further, the effect of tablet excipients was investigated by analyzing the tablet excipients without the analytes. The excipients did not show any absorbance in the applied wavelength for both methods.
Determining AML and CEL in formulations
Established procedures were successfully employed for the concurrent quantification of AML and CEL from the pharmaceutical preparation. The outcomes of the investigation are presented in Table 4 . The present assays for AML were 99.38% and 98.96%, whereas, for CEL, they were 101.4% and 99.17%. Across mean recovery studies conducted using the standard addition method ( S4 Fig ), the ratio difference was found to be 100.13% and 99.79%, while the ratio-first-derivative methods yielded values of 99.70% and 99.34% for AML and CEL, respectively. The low %RSD showed the degree of precision and reproducibility of the methods. The analysis results obtained from the laboratory—prepared tablet also showed that the methods used were highly accurate ( Table 4 , S5 Fig ) The analysis and recovery results indicated that the accuracy of the methods, as well as the absence of formulation excipients interfere with the quantification of both analytes.
https://doi.org/10.1371/journal.pone.0222526.t004
Conclusions
Overall, it was found that the two proposed UV spectrophotometric procedures–ratio difference and the first derivative of the ratio spectra–could simultaneously estimate AML and CEL in pure and pharmaceutical preparations with excellent accuracy and precision. However, the absorbance difference between ratio spectra has its advantages over the ratio-derivative method in terms of simplicity, due to the involvement of only two steps (the division and measurement of absorbance differences) when compared to the three steps required for the ratio-derivative method (division, derivatization, and absorbance measurement). Further, both methods are simple, fast, sensitive, and specific; they work without applying complicated equations or separation procedures. Hence, these techniques can be utilized to perform regular quality control studies of solid dosage forms that have different ratios of AML and CEL.
Supporting information
Calibration curve for ratio difference method AML (A), CEL (B) and ratio first derivative method AML (C), CEL (D).
https://doi.org/10.1371/journal.pone.0222526.s001
S2 Fig. Ratio and first derivative ratio spectra of CEL and AML for accuracy and precision studies.
(A) Ratio spectra of CEL 20,30,40 μg ML -1 using 2 μg mL -1 solution spectra of AML. (B) First derivative of Ratio spectra of CEL 20,30,40 μg ML -1 using 2 μg mL -1 solution spectra of AML.(C) Ratio spectra of AML 1, 1.5, 2 μg mL -1 using 10 μg mL -1 solution spectra of CEL.(D) First Derivative of ratio spectra of AML 1, 1.5, 2 μg mL -1 for precision and accuracystudies using 10 μg mL -1 solution spectra of CEL for accuracy and precision studies.
https://doi.org/10.1371/journal.pone.0222526.s002
S3 Fig. UV absorption spectra of laboratory prepared solutions of AML and CEL in different ratios (AML: CEL, 1:20, 1.5:20, 2:20; 1:30, 1.5:30, 2:30; and 1:40, 1.5:40, 2:40 μg ml-1 respectively).
https://doi.org/10.1371/journal.pone.0222526.s003
S4 Fig. Ratio and first derivative ratio spectra of CEL and AML for recovery studies.
(A) Ratio spectra of CEL 20, 25, 30 35 μg ml -1 using AML 2 μg ml -1 . (B) First derivative (Δλ 4 nm) of ratio spectra of CEL 20, 25, 30 35 μg ml -1 using AML 2 μg ml -1 . (C) Ratio spectra of AML 1, 1.5, 2, 2.5 μg ml -1 using CEL 10 μg ml -1 . (D) First derivative (Δλ 4 nm) of ratio spectra of AML 1, 1.5, 2, 2.5 μg ml -1 using CEL 10 μg ml -1 for recovery studies.
https://doi.org/10.1371/journal.pone.0222526.s004
S5 Fig. Manipulated UV spectra of tablet solutions.
Ratio spectra (A) and First derivative (Δλ 4 nm) of ratio spectra (B) of AML: CEL 1:20 and 1:40 μg ml -1 tablet solution using AML 2 μg ml -1 as divisor, Ratio spectra (C) and First derivative (Δλ 4 nm) of ratio spectra (D) of AML: CEL 1:20 AND 1:40 μg ml -1 tablet solution using CEL 10 μg ml -1 as divisor.
https://doi.org/10.1371/journal.pone.0222526.s005
Acknowledgments
The authors thank Deanship of Scientific Research, King Faisal University, Al Ahsa, Saudi Arabia for the financial support (Group Project # 1811018).
- 1. Sweetman SC, Martindale, The Complete Drug Reference. 37th ed. London, UK: The Pharmaceutical Press; 2011.
- View Article
- PubMed/NCBI
- Google Scholar
- Open access
- Published: 05 April 2024
Green chemometric-assisted UV-spectrophotometric methods for the determination of favipiravir, cefixime and moxifloxacin hydrochloride as an effective therapeutic combination for COVID-19; application in pharmaceutical form and spiked human plasma
- Eman A. Madbouly 1 ,
- Abdalla A. El-Shanawani 1 ,
- Sobhy M. El-adl 1 &
- Ahmed S. Abdelkhalek 1
BMC Chemistry volume 18 , Article number: 65 ( 2024 ) Cite this article
Metrics details
As pharmaceutical analysis progresses towards environmental sustainability, there is a growing need to enhance the safety and health conditions for analysts. Consequently, the incorporation of chemometrics into environmentally friendly analytical methods represents a promising approach. Favipiravir, cefixime, and moxifloxacin hydrochloride have been currently used in COVID-19 treatment. In this study, we develop spectrophotometric methods depending on chemometric based models to measure the levels of favipiravir, cefixime, and moxifloxacin hydrochloride in pharmaceutical preparations and spiked human plasma. It is challenging to determine favipiravir, cefixime, and moxifloxacin simultaneously because of overlap in their UV absorption spectra. Two advanced chemometric models, partial least square (PLS) and genetic algorithm (GA), have been developed to provide better predictive abilities in spectrophotometric determination of the drugs under study. The described models were created using a five-level, three-factor experimental design. The outcomes of the models have been thoroughly assessed and interpreted, and a statistical comparison with recognized values has been taken into consideration. The analytical eco-scale and the green analytical procedure index (GAPI) evaluation methods were also utilized to determine how environmentally friendly the mentioned models were. The outcomes demonstrated how well the models described complied with the environmental requirements.
Peer Review reports
Introduction
The coronavirus disease 2019 (COVID-19) outbreak has become a worldwide crisis due to the devastation it has caused and its rapid spread [ 1 ]. This disease is brought on by a novel infectious positive single-stranded RNA virus called SARS-CoV2, and it frequently comes with multiple cases of atypical pneumonia. Although there has been quick progress in developing SARS-CoV-2 vaccines, drug repurposing is still a crucial part of treating various illnesses [ 2 ]. Antivirals and antibiotics are mainly used in COVID-19 treatment. Favipiravir (FPV), Fig. 1 a is a pyrazine carboxamide derivative. It is an analogue of purine nucleic acid that replaces guanine or adenine and hinders viral replication by preventing RNA-dependent RNA polymerase (RdRp). It is administered as a prodrug that, when phosphoribosylated intracellularly, can produce the active compound FPV ribofuranosyl-5B-triphosphate [ 3 ]. For the quantitative determination of FPV, various analytical approaches were reported, including liquid chromatographic [ 4 , 5 , 6 , 7 , 8 , 9 , 10 ], electrochemical [ 11 , 12 , 13 , 14 ], spectrophotometric [ 15 , 16 , 17 ], spectrofluorometric [ 6 , 18 ] and densitometric [ 19 , 20 ] methods.
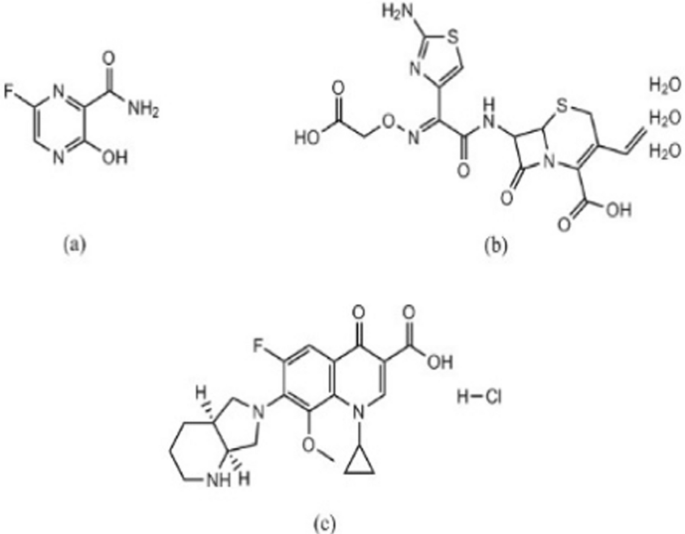
Structural formula of FPV ( a ), CEF ( b ) and MFX ( c )
Antibiotics are used to treat bacterial infections that coexist with COVID-19 infections or to exploit their possible antiviral properties. Cefixime trihydrate (CEF), Fig. 1 b, is a semi-synthetic cephalosporin antibiotic of third-generation that is taken orally. It is an antibacterial agent that is used to treat bronchitis, and pneumonia. Cefixime’s antibacterial effect is due to its ability to prevent the formation of mucopeptides in the bacterial cell wall [ 21 ].
Moxifloxacin hydrochloride (MFX), Fig. 1 c, is a fourth generation fluoroquinolone antibiotic. Its mechanism of action relies on inhibition DNA gyrase, also known as topoisomerase II, an enzyme that is necessary for the replication of bacterial DNA [ 22 ]. The combination of MFX and CEF has been approved by the FDA [ 23 ]. So, this combination can be used as adjuncts therapy in treating patients who have COVID-19. A survey of the literature indicates that the most popular analytical method for CEF/MFX analysis is high-performance liquid chromatography (HPLC) [ 24 , 25 , 26 ]. Nevertheless, the documented HPLC techniques have certain drawbacks, such as the unusual use of potentially harmful organic solvents in the mobile phase as acetonitrile as well as laborious separation processes. Additionally, choosing the right stationary and mobile phases is one of the crucial factors that needs to be precisely adjusted for the best peak resolution. On the other hand, spectrophotometric methods for drug analysis can eliminate the aforementioned issues with increased ease, effectiveness, and precision. Available spectrophotometric techniques used for CEF/MFX determination include mathematical manipulation techniques like first derivative, and first derivative of the ratio spectra [ 23 , 27 ]. These techniques have drawbacks as well, such as inefficient data collection that could lower the throughput of analytical methodology. These approaches also have drawbacks since they are ineffective at gathering unnecessary data, which could lower the throughput of analytical methodologies due to wasteful data collection. Furthermore, when a data spectrum is analyzed using only one or two points, these methods are extremely sensitive to interfering factors because it is challenging to discern the analyte signal from an interferent. Moreover, every drug needs a calibration curve, and a number of tests are needed to choose the appropriate divisor for the next derivative of the ratio spectra [ 28 , 29 ]. As a result, chemometrics has garnered a lot of interest lately as a successful post-processing method that can address the aforementioned drawbacks [ 30 ]. Partial least squares (PLS) and genetic algorithm partial least squares (GA-PLS) were popular two assisted chemometric spectrophotometric methods for the quantitative analysis of complex mixtures without any recommended need for a prior separation [ 31 , 32 ].
In addition, no method for simultaneously evaluating FPV, CEF and MFX in co-formulation as co-administered drugs has been reported. This means that hospitalized inpatients require a method to determine those medications simultaneously in order to evaluate their therapeutic drug monitoring [ 33 ]. The aim of this study is to develop and validate two new multivariate chemometric methods (PLS and GA-PLS) for the simultaneous analysis of the cited drugs in bulk powder, pharmaceutical dosage forms and spiked human plasma. Also, this study aims to develop the first analytical method capable of estimating those co-administered drugs in the co-formulation while taking into consideration green analytical chemistry concepts. Several tools, including the analytical eco-scale [ 34 ] and the green analytical procedure index [ 35 ] were used to assess the models' level of greenness. Also, the models given showed superiority with the greenness characteristics in terms of the conventional green metric values. Through the integration of chemometric tools and their application with green assessment metrics, the authors aim to offer a promising challenge for accomplishing green goals.
Experimental
FPV (99.65%) pure powder was kindly supplied by Biophore India Pharmaceuticals Private Limited (Telangana, India). (CEF) (99.50%) pure powder was graciously donated by Kahira Pharmaceutical and Chemical Industrial Company-Cairo-Egypt. MFX (99.45%) pure powder was graciously donated by EVA Pharmaceutical Industrial Company (Cairo, Egypt). All of the chemicals were of analytical grade, the solvents were HPLC grade, and the water was freshly distilled throughout the entire process.
Favipiravir® Tablet (400 mg FPV/Tablet), manufactured by ZHEJIANG HISUN Pharmaceutical Company (batch number 23006020), was purchased from the Chinese market. Moxinow® Tablet (400 mg CEF & 400 mg MFX/Tablet) manufactured by Lupin Ltd (batch number 005G23OS), was purchased from the Indian market.
Apparatus and software
A UV-1800 PC double-beam Shimadzu UV–Vis spectrophotometer, with UV probe software, was utilized. PLS and GA were implemented in MATLAB R2015a (8.5.0.197613) employing the PLS toolbox software version 2.1.
Standard solutions
By dissolving 10 mg of each standard in 70 mL of distilled water in separate 100 mL volumetric flasks, and then bringing the volume to 100 mL with distilled water, three distinct stock solutions (100 μg/mL) of FPV, CEF and MFX have been obtained.
PLS and GAPLS models design
Arguably, one of the most important steps to improve the likelihood of obtaining representative and instructive data is to plan your experiments well. A partial five-level/three-factor factorial design would have been ideal for creating calibration and validation sets. In the beginning, twenty-five FPV, CEF and MFX mixtures were created and split into calibration and validation sets.
The calibration set was prepared using five concentration levels for each component to produce 13 laboratory-prepared mixtures with various concentrations ranges: 3–7 μg/mL for each of FPV, CEF and MFX.
The design’s central level is 5 μg/mL for each drug. To prevent any overfitting of the created models, a total of twelve combinations of the three medications under study were selected as the validation set. The calibration and validation sets’ concentrations were established using the partial factorial experimental design approach. The results are shown in Table 1 .
Application to pharmaceutical preparation
Ten Favipiravir® tablets (400 mg/tablet) were finely ground and weighted. A precise weight measurement was used to determine the appropriate amount of powder, equivalent to 10 mg of FPV. The powder was then transferred to a 100-mL volumetric flask, and the volume was increased to approximately 70 mL using distilled water. After 15 min of vigorous shaking and filtration, the volume was filled with distilled water until a volumetric concentration of 100 μg/mL was achieved.
CEF and MFX
Ten Moxinow® Tablet (400 mg CEF & 400 mg MFX/Tablet) were finely ground and weighted. A precise weight measurement was used to determine the appropriate amount of powder, equivalent to 10 mg of FPV. The powder was then transferred to a 100-mL volumetric flask, and the volume was increased to approximately 70 mL using distilled water. After 15 min of vigorous shaking and filtration, the volume was filled with distilled water until a volumetric concentration of 100 μg/mL was achieved.
Favipiravir, cefixime and moxifloxacin hydrochloride (co-formulated)
The fixed-dose combination was formulated because FPV, CEF, and MFX fixed-dose tablets were not readily available. Ten Tablets of each pharmaceutical preparation including Favipiravir® tablets (400 mg/tablet) and Moxinow® tablets (400 & 400 mg/tablet) were weighted, finely powdered and mixed well and calculating the average weight has been done. We weighed amount of powder containing (10 mg for FPV, 10 mg for CEF and 10 mg for MFX) and transferred it to a 100-mL volumetric flask, after which the volume was diluted with distilled water to approximately 70 mL. After 15 min of vigorous shaking, the volume was completed to 100 mL with distilled water and then filtered to obtain a concentration of (100 μg for FPV, 100 μg for CEF and 100 μg for MFX per mL). Using the proposed methods, the FPV, CEF, and MFX contents were determined.

Procedure for determination of FPV, CEF and MFX in spiked human plasma
Various aliquots (0.3, 0.4, 0.5, 0.6, 0.7 mL) of FPV, CEF and MFX standard solutions (100 μg/mL) were pipetted and transferred to 10 mL centrifuge tubes that already contained 1 mL of drug-free plasma. Then, add 3 mL of methanol to denaturate the protein. After mixing the contents of centrifuge tubes with a vortex shaker, the tubes were centrifuged for 30 min at 4000 rpm. The resulting protein-free supernatants were evaporated to dryness using a rotary evaporator under vacuum, then reconstituted in distilled water, placed in 10-mL volumetric flasks, and then the volume was diluted to 10 mL with distilled water. For each drug, the overall method was repeated with aliquots encompassing the working concentration range. Using the proposed methods, the FPV, CEF, and MFX contents were determined.
Results and discussion
Spectral characteristics.
The FPV, CEF and MFX UV spectral characteristics were measured between 200 and 400 nm in wavelength. After taking a quick look at these spectra, Fig. 2 illustrates a severe overlap that explains the difficulty in directly determining that drugs simultaneously. Thus, we utilized two chemometric assisted calibration methods, namely PLS and GA-PLS, to address such overlap and determine FPV, CEF and MFX concurrently in their pharmaceutical dosage form and spiked human plasma.
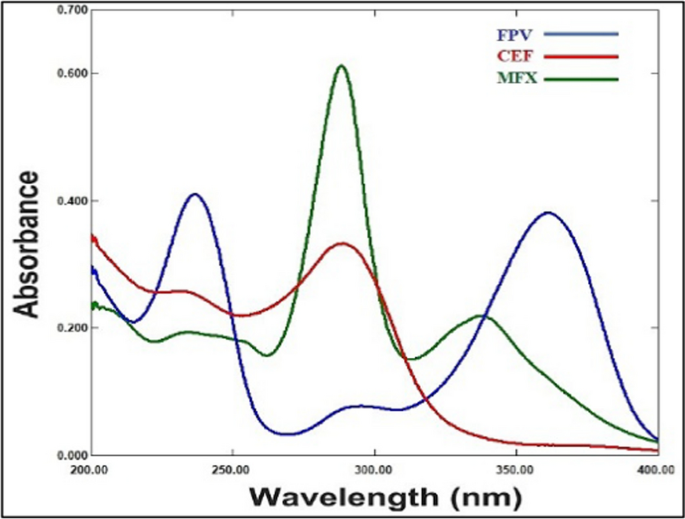
Absorption spectra of 5 μg/mL FPV, 5 μg/mL CEF and 5 μg/mL MFX
PLS and GA-PLS
The spectral matrix of the calibration data was fitted with the PLS model, a popular regression model, to infer it into new dimensions known as latent variables (LVs). PLS model was used to design a calibration model between the concentration of the studied drugs and the latent variables of the data matrix. Its ability to use all of the information in the recorded spectral data ensures greater accuracy for the spectral analysis. Additionally, PLS model has the advantage of choosing the most informative variables and excluding the uninformative ones which improves the quality of the applied model. A calibration set of 13 calibration spectra was used in conjunction with the cross validation approach, which involves removing samples one at a time, to determine the number of factors in the PLS algorithm. Consequently, the root-mean-square error cross-validation (RMSECV) was calculated after a series of LVs were gradually added to the model. Using Haaland and Thomas’s criteria [ 36 ], the best number of latent variables was chosen. The model with the best latent variable shows no statistically significant difference the corresponding root mean squares error of cross-validation and the minimum root mean squares error of cross-validation.
As shown in Fig. 3 , it was discovered that two latent variables were optimal for FPV and three latent variables for CEF and MFX with RMSECV values of 0.110, 0.160 and 0.111, respectively.
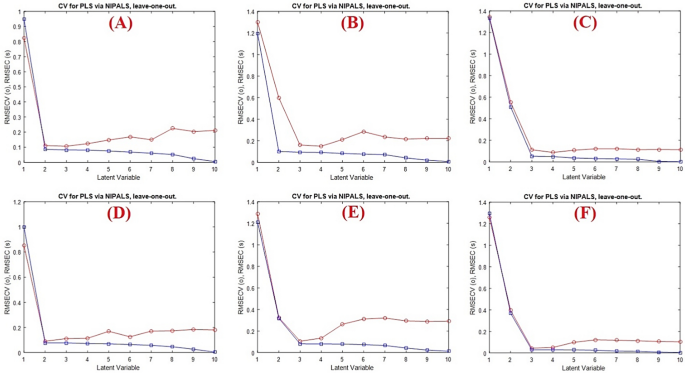
Cross validation results of the full PLS models for A FPV, B CEF, C MFX and the GA-PLS models for D FPV, E CEF, F MFX. The optimum number of latent variables shows significant decrease in their RMSECV values
Fascinatingly, the GA procedure was employed as an informative variable’s selection technique in order to increase the PLS model’s predictive ability. To eliminate irrelevant variables while retaining informative ones, the GA model was applied to 201 variables for FPV, CEF and MFX (200–400 nm). A key factor in achieving successful GA performance is the modification of GA parameters, as indicated in Table 2 . When using GAs, one of the most important factors is the population size. Choosing the right population size is an intricate issue. Larger population sizes are able to search larger spaces, which leads to an early convergence to the solution, while smaller populations perform poorly due to their limited ability to search the solution space [ 37 ]. Another crucial feature of GA was its rate of mutation, which changed one or more GA chromosomes’ genes to maintain the diversity of genetic populations and impede rapid convergence. It was discovered that the appropriate mutation rate for every medication was 0.005. Other parameters are the maximum number of LVs using the full PLS model, the number of subsets, and the number of cross-validation iterations at each generation were also estimated. Fascinatingly, it was discovered that GA reduces the absorbance matrix to roughly 33% for FVP, 25% for CEF and 23% for MFX (66 variables for FVP, 50 variables for CEF and 46 for MFX). As indicated in Tables 3 and 4 , it is interesting to note that the GA-PLS models for the three drugs have lower values in terms of standard deviation (SD) of the % recoveries when compared to the full model.
Models validation
The models described was validated regarding to linearity range, accuracy, precision, limits of detection (LOD), limits of quantitation (LOQ) and selectivity parameters.
Range of linearity
Regarding the developed PLS experimental design, which took into account the concentration range of 1–15 μg/mL for FPV, 2–15 μg/mL for CEF and 1–10 μg/mL for MFX, acceptable results were obtained over this range. While the concentration range of 1–15 μg/mL for FPV, 1–15 μg/mL for CEF and 0.5–10 μg/mL for MFX shows acceptable results for the developed GA-PLS experimental design, as indicated in Table 5 .
Limits of detection and quantitation
LOD and LOQ were calculated, and the results were listed in Table 5 . The results demonstrated the sensitivity of the proposed model for drug analysis.
Accuracy and precision
The proposed procedure was used to determine three concentration levels in triplicate that covered the linearity ranges of the three drugs (4, 5, and 6 μg/mL for each drug). The method's precision, calculated as %RSD, was evaluated by using the proposed procedure for triplicate determination of three concentration levels that covered the linearity range of each drug (4, 5, and 6 μg/mL for each drug) within one day for repeatability and on three consecutive days for intermediate precision. Excellent %R, as displayed in Table 5 , proves the proposed method’s accuracy. Additionally, small RSD values, as displayed in Table 5 , prove the high method’s precision. Also, a variety of validation parameters, such as root mean square error of calibration (RMSEC), root mean square error of prediction (RMSEP), and relative root mean square error of prediction (RRMSEP), had been computed and displayed in Table 5 in order to interpret the accuracy and predictive ability of the models. Additionally, the precision or variance of the prediction was measured using the bias corrected mean square error of prediction (BCMSEP) parameter (Table 5 ), and the best outcomes were obtained.
Selectivity
The standard addition technique on the already analyzed pharmaceutical samples, Table 6 , was also used to assess the effect of excipients on estimation of the drugs. According to Table 7 , the results obtained by application of the standard addition technique demonstrate the selectivity of the method in avoiding interference from excipients.
Application of the proposed models for determination of FPV, CEF and MFX pharmaceutical dosage forms
To compare the outcomes with those of the reported methods, statistics were used [ 23 ]. As shown in Table 8 , the proposed approach for analyzing the drug under investigation in its pharmaceutical dosage form did not produce any statistically significant differences when the student’s t-test and the F-test were conducted at a 95% confidence level. This suggests that the suggested method is accurate and precise.
Determination of FPV, CEF and MFX in spiked human plasma
The new method was successful in monitoring FPV, CEF and MFX at therapeutic levels in spiked human plasma samples because the proposed models’ linearity and detection limits, along with the mean plasma C max values for FPV (12.69–41.39 μg/mL), CEF (4.7263 ± 1.2069 μg/mL) and MFX (3.56 mg/L) [ 38 , 39 , 40 ], allowed for this degree of determination. As shown in Table 9 , the models discussed were appropriate for determining the drugs under study in human plasma without interfering with endogenous plasma matrix components.
Green assessment of the described models
Two new approaches to assessing the greenness of the suggested method were presented: the analytical eco-scale [ 34 , 41 ] and the green analytical procedure index. The eco-scale relies on penalization points calculated from reagents, instruments, and waste to facilitate their development as semi-quantitative methods. The method relies on subtracting the total number of penalty points from 100. The higher the value of the result, the more environmentally friendly the newly developed approach [ 35 ]. In Table 10 , the sum of the penalty points for the suggested technique were 3 and 9 points for application of Pharmaceutical dosage forms and Spiked human plasma, respectively that resulted a total scoring of 91 and 97. This shows that the suggested approach is just as environmentally friendly as the reported spectrophotometric method for CEF and MFX [ 23 ], but it is more environmentally friendly than the reported techniques for favipiravir [ 10 , 42 ]. Using five pictograms and a unique symbol, the GAPI metrics rate how environmentally friendly each stage of the analytical process is. Every pictogram is made up of different fields and denotes a specific stage in an analytical procedure. The environmental effects of each field are classified as low, medium, and high (green, yellow, and red), and their corresponding quantities are computed. Furthermore, a specific circle indicates whether or not the approach being studied includes quantification [ 35 ].
The described models for the proposed method had nine and seven green zones for application in pharmaceutical dosage forms and spiked human plasma, respectively. This indicates the proposed model has the same greenness as the previously reported spectrophotometric method for CEF and MFX [ 23 ]. Comparing with other reported methods of favipiravir, the proposed method has more green zones with the same number of red zones when applied in the same matrix. In conclusion, the green metrics' findings provided a thorough environmental friendliness profile and, for the most part, verified compliance with green practices.
In the proposed study, two novel multivariate chemometric methods were used to validate a new analytical tool for the simultaneous determination of FPV, CEF and MFX in pharmaceutical preparations and spiked human plasma. Without requiring a separation step, the chemometric techniques under study demonstrated excellent sensitivity and resolving power. This in turn provides more economical alternatives, higher levels of simplicity, and faster analysis times all of which are necessary for the numerous regular daily analyses that pharmaceutical research and quality control laboratories perform. To enable integrated green spectrophotometric determination of the drugs under study, chemometric models were built and refined. The Green Analytical Procedure Index, and the analytical eco-scale were used to assess the greenness of the models. In terms of the official green metric values, the results demonstrated that the models described complied and met the environmental friendliness requirements.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Favipiravir
Cefixime trihydrate
Moxifloxacin hydrochloride
Partial least squares
Genetic Algorithm
International Conference on Harmonization
Green Analytical Procedure Index
Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B, Chen Y, Zhang Y. COVID-19: immunopathogenesis and immunotherapeutics. Signal Transduct Target Ther. 2020;5:128. https://doi.org/10.1149/10701.17797ecst .
Article CAS PubMed PubMed Central Google Scholar
Chakraborty C, Sharma AR, Bhattacharya M, Agoramoorthy G, Lee S-S. The drug repurposing for COVID-19 clinical trials provide very effective therapeutic combinations: lessons learned from major clinical studies. Front Pharmacol. 2021;12: 704205. https://doi.org/10.3389/fphar.2021.704205 .
Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11:11022. https://doi.org/10.1038/s41598-021-90551-6 .
Bulduk İ. Comparison of HPLC and UV spectrophotometric methods for quantification of favipiravir in pharmaceutical formulations. Iran J Pharm Res IJPR. 2021;20:57. https://doi.org/10.22037/ijpr.2020.114199.14725 .
Article CAS PubMed Google Scholar
Harahap Y, Noer RF, Simorangkir TPH. Development and validation of method for analysis of favipiravir and remdesivir in volumetric absorptive microsampling with ultra high-performance liquid chromatography–tandem mass spectrophotometry. Front Med. 2023;10:1022605. https://doi.org/10.3389/fmed.2023.1022605 .
Article Google Scholar
Mikhail IE, Elmansi H, Belal F, Ibrahim AE. Green micellar solvent-free HPLC and spectrofluorimetric determination of favipiravir as one of COVID-19 antiviral regimens. Microchem J. 2021;165: 106189. https://doi.org/10.1016/j.microc.2021.106189 .
Nadendla R, Abhinandana P. A validated high performance liquid chromatographic method for the quantification of favipiravir by PDA detector. Int J Life Sci Pharma Res. 2021;11:181–8. https://doi.org/10.3390/molecules26133789 .
Article CAS Google Scholar
Nishanth VG, Spandana T, Sri CD, Nataraj V, Vikram PH, Gurupadayya B. Multivariate optimization for determination of favipiravir, a SARS-CoV-2 molecule, by the reverse-phase liquid chromatographic method using a QbD approach. J Chromatogr Sci. 2022. https://doi.org/10.1093/chromsci/bmac041 .
Tiris G, Gazioglu I, Furton KG, Kabir A, Locatelli M. Fabric phase sorptive extraction combined with high performance liquid chromatography for the determination of favipiravir in human plasma and breast milk. J Pharm Biomed Anal. 2023;223: 115131. https://doi.org/10.1016/j.jpba.2022.115131 .
Marzouk HM, Rezk MR, Gouda AS, Abdel-Megied AM. A novel stability-indicating HPLC-DAD method for determination of favipiravir, a potential antiviral drug for COVID-19 treatment; application to degradation kinetic studies and in-vitro dissolution profiling. Microchem J. 2022;172: 106917. https://doi.org/10.1016/j.microc.2021.106917 .
Ali MF, Saraya RE, El Deeb S, Ibrahim AE, Salman BI. An innovative polymer-based electrochemical sensor encrusted with Tb nanoparticles for the detection of favipiravir: a potential antiviral drug for the treatment of COVID-19. Biosensors. 2023;13:243. https://doi.org/10.3390/bios13020243 .
Allahverdiyeva S, Yunusoğlu O, Yardım Y, Şentürk Z. First electrochemical evaluation of favipiravir used as an antiviral option in the treatment of COVID-19: A study of its enhanced voltammetric determination in cationic surfactant media using a boron-doped diamond electrode. Anal Chim Acta. 2021;1159: 338418. https://doi.org/10.1016/j.aca.2021.338418 .
El-Wekil MM, Hayallah AM, Abdelgawad MA, Abourehab MA, Shahin RY. Nanocomposite of gold nanoparticles@ nickel disulfide-plant derived carbon for molecularly imprinted electrochemical determination of favipiravir. J Electroanal Chem. 2022;922: 116745. https://doi.org/10.1016/j.jelechem.2022.116745 .
Mehmandoust M, Khoshnavaz Y, Tuzen M, Erk N. Voltammetric sensor based on bimetallic nanocomposite for determination of favipiravir as an antiviral drug. Microchim Acta. 2021;188:1–15. https://doi.org/10.1007/s00604-021-05107-2 .
Chakraborty S, Mondal S. Green eco-friendly analytical method development, validation, and stress degradation studies of favipiravir in bulk and different tablet dosages form by UV-spectro-photometric and RP-HPLC methods with their comparison by using ANOVA and in-vitro dissolution studies. Int J Pharm Investig. 2023. https://doi.org/10.5530/ijpi.13.2.039 .
Panchale WA, Bisen SB, Manwar JV, Bakal RL, Tidke TV, Paithankar MN, Vakhare AG. Development of visible spectrophotometric methods for the analysis of favipiravir in pure drug and tablet formulation. GSC Biol Pharm Sci. 2022;20:184–95. https://doi.org/10.30574/gscbps.2022.20.2.0320 .
Rele RV, Tiwatane PP. Simple extractive spectrophotometric method for determination of favipiravir from pharmaceutical formulation. Asian J Res Chem. 2022. https://doi.org/10.52711/0974-4150.2022.00053 .
Megahed SM, Habib AA, Hammad SF, Kamal AH. Experimental design approach for development of spectrofluorimetric method for determination of favipiravir; a potential therapeutic agent against COVID-19 virus: application to spiked human plasma. Spectrochim Acta Part A Mol Biomol Spectrosc. 2021;249: 119241. https://doi.org/10.1016/j.saa.2020.119241 .
Balap AR, Dudhe AP. Stability indicating HPTLC method development and validation for quantification of favipiravir in bulk and commercial film coated tablet. Int J Pharm Investig. 2023. https://doi.org/10.5530/ijpi.13.3.071 .
Jain B, Jain R, Jaiswal PK, Zughaibi T, Sharma T, Kabir A, Singh R, Sharma S. A non-instrumental green analytical method based on surfactant-assisted dispersive liquid–liquid microextraction–thin-layer chromatography–smartphone-based digital image colorimetry (SA-DLLME-TLC-SDIC) for determining favipiravir in biological samples. Molecules. 2023;28:529. https://doi.org/10.3390/molecules28020529 .
Hooper DC, Wolfson JS. Mechanisms of quinolone action and bacterial killings, quinolone antimicrobial agents, vol 1, 2nd edn. Washington, DC: American Society for Microbiology 1993; pp. 53–57
Google Scholar
Hooper DC, Jacoby GA. Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance. Cold Spring Harb Perspect Med. 2016;6:a025320.
Article PubMed PubMed Central Google Scholar
Attimarad M, Al-Dhubiab BE, Alhaider IA, Nair AB, Sree HN, Mueen AK. Simultaneous determination of moxifloxacin and cefixime by first and ratio first derivative ultraviolet spectrophotometry. Chem Central J. 2012;6:1–7. https://doi.org/10.1186/1752-153X-6-105 .
Devika G, Sudhakar M, Rao JV. Simultaneous estimation of cefixime and moxifloxacin in bulk and its pharmaceutical dosage form by RP-HPLC. Orient J Chem. 2012;28:1743–50.
Chauhan RS, Chabhadiya MB, Patel AK, Shah SA. Simultaneous estimation of cefixime trihydrate and moxifloxacin hydrochloride in their combined tablet dosage form by RPHPLC. J Der Pharma Chemica. 2013;5:197–201.
CAS Google Scholar
Rao A. Development and validation of novel HPLC method for simultaneous estimation of cefixime and moxifloxacin in combined tablet dosage form. Int J Pharm. 2013;3:621–7.
Patel M, Kakadiya J, Shah N. Development and validation of first order derivative spectrophotometric method for simultaneous estimation of cefixime trihydrate and moxifloxacin hydrochloride in combined tablet dosage form. Asian J Pharm Sci Thechnol. 2013;3:19–24.
Fayez YM, Tawakkol SM, Fahmy NM, Lotfy HM, Shehata MA-A. Comparative study of the efficiency of computed univariate and multivariate methods for the estimation of the binary mixture of clotrimazole and dexamethasone using two different spectral regions. Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;194:126–35. https://doi.org/10.1016/j.saa.2018.01.00 .
Obaydo RH, Al Zakri DJ, Sakur AA, Lotfy HM. Ultraviolet spectrophotometric methods for the determination of the minor component presented in fixed-dose pharmaceutical combinations through the last two decades (2000–2020). Future J Pharm Sci. 2021;7:1–9. https://doi.org/10.1186/s43094-021-00192-9 .
Abdelazim AH, Shahin M. Different chemometric assisted approaches for spectrophotometric quantitative analysis of lesinurad and allopurinol. Spectrochim Acta Part A Mol Biomol Spectrosc. 2021;251: 119421. https://doi.org/10.1016/j.saa.2020.119421 .
Brereton RG. Multilevel multifactor designs for multivariatecalibration. Analyst. 1997;122:1521–9.
Yehia AM, Mohamed HM. Chemometrics resolution and quantification power evaluation: application on pharmaceutical quaternary mixture of Paracetamol, Guaifenesin, Phenylephrine and p-aminophenol. Spectrochim Acta Part A Mol Biomol Spectrosc. 2016;152:491–500.
Mahdavi R, Talebpour Z. Analytical approaches for determination of COVID-19 candidate drugs in human biological matrices. TrAC Trends Anal Chem. 2023. https://doi.org/10.1016/j.trac.2023.116964 .
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC, Trends Anal Chem. 2012;37:61–72. https://doi.org/10.1016/j.trac.2012.03.013 .
Płotka-Wasylka J. A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta. 2018;181:204–9. https://doi.org/10.1016/j.talanta.2018.01.013 .
Haaland DM, Thomas EV. Partial least-squares methods for spectral analyses. 1. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal Chem. 1988;60:1193–202.
Serag A, Hasan MA, Tolba EH, Abdelzaher AM, Elmaaty AA. Analysis of the ternary antiretroviral therapy dolutegravir, lamivudine and abacavir using UV spectrophotometry and chemometric tools. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022;264:120334. https://doi.org/10.1016/j.saa.2021.120334 .
Moise P, Birmingham M, Schentag J. Pharmacokinetics and metabolism of moxifloxacin. Drugs Today (Barcelona, Spain: 1998). 2000;36:229–44. https://doi.org/10.1358/dot.2000.36.4.570201 .
Zakeri-Milani P, Valizadeh H, Islambulchilar Z. Comparative bioavailability study of two cefixime formulations administered orally in healthy male volunteers. Arzneimittelforschung. 2008;58:97–100. https://doi.org/10.1055/s-0031-1296475 .
Gülhan R, Eryüksel E, Gülçebi İdriz Oğlu M, Çulpan Y, Toplu A, Kocakaya D, Tigen E, Ertürk Şengel B, Sili U, Olgun Yıldızeli Ş. Pharmacokinetic characterization of favipiravir in patients with COVID-19. Br J Clin Pharmacol. 2022;88:3516–22. https://doi.org/10.1111/bcp.15227 .
Sajid M, Płotka-Wasylka J. Green analytical chemistry metrics: a review. Talanta. 2022;238: 123046. https://doi.org/10.1016/j.talanta.2021.123046 .
Elama HS, Zeid AM, Shalan SM, El-Shabrawy Y, Eid MI. Eco-friendly spectrophotometric methods for determination of remdesivir and favipiravir; the recently approved antivirals for COVID-19 treatment. Spectrochim Acta Part A Mol Biomol Spectrosc. 2023;287: 122070. https://doi.org/10.1016/j.saa.2022.122070 .
Download references
Acknowledgements
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). There is no funding to declare.
Author information
Authors and affiliations.
Department of Medicinal Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
Eman A. Madbouly, Abdalla A. El-Shanawani, Sobhy M. El-adl & Ahmed S. Abdelkhalek
You can also search for this author in PubMed Google Scholar
Contributions
EAM: conceptualization, methodology, investigation, writing—original draft. AAE: conceptualization, writing—review & editing, supervision. SME: conceptualization, writing—review & editing, supervision. ASA: conceptualization, writing—review & editing, supervision. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Eman A. Madbouly .
Ethics declarations
Ethics approval and consent to participate.
The study was carried out according to the Declaration of Helsinki and approved by Zagazig University Institutional Review Board (ZU-IRB) under the number (ZU-IRB #11330). The need for informed consent was waived by Zagazig University Institutional Review Board (ZU-IRB) as the human plasma was provided kindly by Zagazig University Hospitals. All described procedures were performed in accordance with relevant guidelines and regulations.
Consent for publication
Competing interests.
All authors confirm that there are no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Madbouly, E.A., El-Shanawani, A.A., El-adl, S.M. et al. Green chemometric-assisted UV-spectrophotometric methods for the determination of favipiravir, cefixime and moxifloxacin hydrochloride as an effective therapeutic combination for COVID-19; application in pharmaceutical form and spiked human plasma. BMC Chemistry 18 , 65 (2024). https://doi.org/10.1186/s13065-024-01168-5
Download citation
Received : 26 November 2023
Accepted : 20 March 2024
Published : 05 April 2024
DOI : https://doi.org/10.1186/s13065-024-01168-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Spectrophotometry
- Chemometrics
- Partial least square
- Genetic algorithm
BMC Chemistry
ISSN: 2661-801X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Adv Pharm Technol Res
- v.3(4); Oct-Dec 2012
A simple Ultraviolet spectrophotometric method for the determination of etoricoxib in dosage formulations
Shipra singh.
Department of Pharmaceutical Chemistry, Central Facility of Instrumentation, College of Pharmacy, IFTM Lodipur-Rajput, Moradabad, India
Amrita Mishra
Anurag verma, ashoke k. ghosh, arun k. mishra.
The present study was undertaken to develop a validated, rapid, simple, and low-cost ultraviolet (UV) spectrophotometric method for estimating Etoricoxib (ETX) in pharmaceutical formulations. The analysis was performed on λ max 233 nm using 0.1 M HCl as blank/diluent. The proposed method was validated on International Conference on Harmonization (ICH) guidelines including parameters as linearity, accuracy, precision, reproducibility, and specificity. The proposed method was also used to access the content of the ETX in two commercial brands of Indian market. Beer's law was obeyed in concentration range of 0.1–0.5 μ g/ml, and the regression equation was Y = 0.418x + 0.018. The mean accuracy values for 0.1 μ g/ml and 0.2 μ g/ml concentration of ETX were found to be 99.76 ± 0.52% and 99.12 ± 0.84, respectively, and relative standard deviation (RSD) of interday and intraday was less than 2%. The developed method was suitable and specific to the analysis of ETX even in the presence of common excipients. The method was applied on two different marketed brands and ETX contents were 98.5 ± 0.56 and 99.33 ± 0.44, respectively, of labeled claim. The proposed method was validated as per ICH guidelines and statistically good results were obtained. This method can be employed for routine analysis of ETX in bulk and commercial formulations.
INTRODUCTION
Chemically Etoricoxib (ETX) is 5-chloro-3-(4-methanesulfonylphenyl)-2-(6-methylpyridin-3-yl) pyridine [ Figure 1 ].[ 1 ] It is a selective COX-2 inhibitor, which belongs to a family of pain killers called non-steroidal anti-inflammatory drugs (NSAIDs). It is mainly used to treat patients suffering from joint pain and swelling caused by osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, and gout. It is also used to reduce swelling and joint stiffness.[ 2 ]

Chemical structure of Etoricoxib
Analysis is an essential step of formulation development, and it must include a simple, reliable, and cost-effective method. Keeping in view this objective, the present work was undertaken to develop and validate simple UV spectrophotometric method for estimation of ETX in dosage formulations.
Earlier methods including high performance liquid chromatography (HPLC),[ 3 , 4 ] high performance thin layer chromatography (HPTLC),[ 5 , 6 ] liquid chromatography-mass spectrometry (LC–MS),[ 7 ] capillary zone electrophoresis,[ 8 ] and ultra performance liquid chromatography (UPLC)[ 9 ] for quantification of ETX in pharmaceutical dosage forms are reported. These reported methods involve a tedious sample preparation process, costly equipments, as well as time-consuming steps. The long run time of these processes limits their applicability for large number of samples.
Upon literature survey and as per our present knowledge, there is no simple and reliable method for estimation of ETX from pharmaceutical dosage forms as well as in bulk formulation. In this study, a simple UV spectrophotometric method was developed and validated as per International Conference on Harmonization (ICH) guidelines.[ 10 ] This method involves simple instrument and cost-effective solvents, and consumes less time. The reproducibility of the proposed method was high, which accounts this method novel and reliable. The method was also used in the determination of the content of ETX in two marketed ETX products in India.
MATERIALS AND METHODS
Instruments and materials.
Schimazdu 1800 double beam UV/Vis spectrophotometer, digital balance (Citizen Co. Mumbai, India), and micropipette (The Modern scientific industries, Meerut, India) were used in this study. ETX was obtained as a gift sample from the Torrent Research Centre, Hyderabad, India. The other chemicals and reagents used were of analytical grade.
Standard Stock Solution
Standard drug solution of ETX was prepared by dissolving 10 mg of ETX in 5 ml 0.1 N HCl in a 10-ml volumetric flask, shaken well, and finally the volume was adjusted to get a solution of concentration of 1 mg/ml. This 1 mg/ml solution was used as a stock solution.
Calibration Curve
Five milliliters of 1 mg/ml aliquot solution was further diluted up to 50 ml by 0.1 N HCl in a 100-ml volumetric flask and the final volume was adjusted up to 100 ml. This was scanned spectrophotometrically in the wavelength region 190–800 nm to determine the wavelength of maximum (λmax) absorption. The λmax was found to be 233 nm against blank [ Figure 2 ]. From 1 mg/ml stock solution, the serial dilution pattern was followed to obtain aliquots of 0.1–0.5 μg/ml concentration. The calibration curve was plotted between concentration and absorbance. The optical characteristics of different aliquots are presented in Table 1 .

Scanned spectra of Etoricoxib
Calibration curve data of etoricoxib in 0.1 N HCl

Sample Solution Preparations
The proposed method was applied on two different commercial brands of ETX. Twenty tablets of each brand were weighed and powdered. Ten milligrams of ETX powder equivalents was weighed and transferred in a 10-ml volumetric flask by diluting it with 10 ml 0.1 N HCl. After a continuous shaking for 10 min, the solutions were filtered through Whatman filter paper separately. The filtrate was further suitably diluted in order to get 0.2 μg/ml concentration. Against a blank solution, the absorbance was measured at 233 nm wavelength. Finally, employing the calibration curve and linear equation, the concentration was calculated. Determination of accuracy was done by % addition method and is presented in Table 2 . Amount of drug estimated through this method is presented in Table 2 .
Determination of accuracy (by percentage recovery method) and precision

RESULTS AND DISCUSSION
The linearity of the drug was obtained for 0.1–0.5 μg/ml concentration range of ETX. The calibration curve was obtained by plotting absorbance versus concentration and linear regression analysis was performed to get linear equation.[ 11 ] The linear equation found was y = 0.418x + 0.018 and r 2 was 0.997. The calibration curve was found to be linear in stated concentration.
Accuracy of the method was estimated by standard addition recovery method. In this, known amount of standard ETX was added to pre-analyzed sample.[ 12 ] This was done for 0.1 μg/ml, 0.2 μg/ml, and performed in triplicate. The accuracy values for 0.1 μg/ml and 0.2 μg/ml concentration of ETX were found to be 99.76 ± 0.52 and 99.12 ± 0.84%, respectively [ Table 2 ].
The precision of the assay was determined by repeatability (intraday) and intermediate precision (interday) and reported as % relative standard deviation (RSD).[ 13 ] For this, 0.1 μg/ml and 0.2 μg/ml concentration solution was measured three times in day and the same was measured in the next 3 days. The %RSD was calculated [ Table 2 ].
Robustness of the method was determined by carrying out the analysis under different temperature conditions, i.e. at room temperature and at 18°C.[ 14 ] The respective absorbance of 0.3 μg/ml was noted and the result was indicated as %RSD [ Table 3 ].
Robustness analysis

The ruggedness of the method was determined by carrying out the analysis by different analysts and the respective absorbance of 0.3 μg/ml was noted. The result was indicated as %RSD [ Table 4 ].
Ruggedness analysis

Limit of Detection and Limit of Quantification
The limit of detection (LOD) and limit of quantification (LOQ) for ETX were determined by using standard deviation of response and slope.[ 15 ] The LOD and LOQ values are presented in Table 5 .
Validation parameters

The stability of ETX in 0.1 N HCl solution was studied by the developed method. Sample solutions (0.3 μg/ml) were prepared in triplicate and heated to maintain 50°C and 60°C for 60 min.[ 16 ] The absorbance data of these samples reveled information about the stability of ETX [ Table 6 ].
Stability study

Determination of Active Ingredients in Different Brands of Tablets
The proposed and validated method was applied to estimate the amount of active ingredient, ETX, in two different brands of tablets using 20 tablets in each batch. Results of quantitative analysis are presented in Table 7 . The findings of analysis suggest that both the marketed formulations fulfilled the % amount requirement (98–102%) with respect to labeled claim.
Determination of active ingredients (%)

The developed method was found to be simple, rapid, cost-effective, and reproducible, with high accuracy and precision value. The parameters were validated as per ICH guidelines. The satisfactory findings of the work suggest that the method may be applied for quantitative estimation of ETX from bulk and pharmaceutical dosage formulations. This method may also be used in routine quality-control aspects.
ACKNOWLEDGMENT
Authors would like thanks to Prof. R.M.Dubey (Honourable Vice-Chancellor of IFTM University) for his kind support.
Source of Support: Nil
Conflict of Interest: Nil.

IMAGES
VIDEO
COMMENTS
Based on the non-availability of methods, the authors developed two UV spectrophotometric methods using water as a solvent. The drug shows a maximum absorption of 263 nm in water.
Ultraviolet spectroscopy is one important and advanced analytical instrument in the Pharmaceutical industry and used for the last 35 years to obtain the absorbance spectra of a compound in solution or as a solid. Ultraviolet spectroscopy is one important and advanced analytical instrument in the Pharmaceutical industry and used for the last 35 years. The method of analysis is based on ...
This UV-spectrophotometric technique is quite simple, accurate, precise, reproducible, and sensitive. The UV method has been developed for quantification of terbinafine hydrochloride in tablet formulation. The validation procedure confirms that this is an appropriate method for their quantification in the formulation.
Ultraviolet and Visible absorption spectrophotometry is the technique based on attenuation of electromagnetic radiation measurement by an absorbing substance [9].This radiation, has a spectral range approximately around 190-800 nm, which also differ in terms of energy ranges, and type of excitation from other related regions (Table 1).This attenuation results from the reflection, scattering ...
The ultraviolet (UV) spectrophotometric method has been extensively used in analytical and clinical laboratories to analyze drugs in pharmaceutical formulations due to it's simple, accurate, and reproducible results. However, many drugs have good UV absorption due to the presence of aromatic and heteroaromatic groups.
1. Introduction. The spectrophotometer is sometimes popularly known as "The Workhorse of the Modern Laboratory." Precisely, ultraviolet and visible (UV-vis) spectrophotometric study is considered a preferable choice in many research and academic laboratories involving the analysis of inorganic and/or organic materials extensively for different processes and products, which include ...
n-wavelengths method. The spectrophotometric resolution of mixtures of two components on the basis of the well-known extended Beer's law relies on absorbance measurements at two different wavelengths and the fulfillment of the law of the additivity of absorbances [2]. This methodology has several shortcomings like the use of only two ...
The literature review shows that only LCMS methods are introduced for the determination of RDV in biological samples, which significantly increases the cost of analysis. So far, all studies reported in the literature have been performed on biological fluids, particularly plasma samples. ... developing HPLC and UV-Spectrophotometric methods that ...
A few UV spectrophotometric[4 ... Review of the literature revealed that there is no spectrophotometric method available for determination of this combination. The first-order derivative, ratio derivative, corrected absorbance spectrophotometric methods for the combinations were developed in the same laboratory. Therefore, the same combination ...
Literature survey reveals that no method has been reported for estimation of EPE and PAR in combination. ... Shah R, Kadikar H, Patani P, Shukla M. Method development and statistical validation of UV spectrophotometric method for estimation of Tolperisone hydrochloride and Paracetamol in synthetic mixture and combined dosage form. Int J Pharm ...
ranges including Ultraviolet and visible (UV-Vis), near-infrared (NIR), mid- infrared (MIR), far infrared (FIR), Raman, Microwaves, radio waves, and nuclear magneticresonance. UV-Vis spectroscopy is sensitive method in molecular spectroscopy that uses ultraviolet and visible light in wavelength range between 200 and 780 nm.
Ultraviolet spectrophotometry is among the most convenient and useful quantitative and qualitative methods, especially in multicomponent analysis [1] by minimizing the cumbersome task of ...
The analytical methods were developed by scanning analytical solutions in the UV region. The proposed methods were validated in accordance with the ICH guideline and the United States Pharmacopoeia.
A survey of the literature indicates that the most popular ... F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. ... Obaydo RH, Al Zakri DJ, Sakur AA, Lotfy HM. Ultraviolet spectrophotometric methods for the determination of the minor ...
The reported method. Simultaneous eq. UV spectrophotometric method using 243 and 255 nm was used for the simultaneous determination of AML and CXB, respectively [20]. First derivative UV spectrophotometric method for the determination of AML at 254.34 nm (zero-crossing point of RMP) and RMP at 211.87 nm (zero-crossing point of AML) [24]. 3.
This communication describe simple, sensitive, rapid, accurate, precise and cost effective absorption ratio method(Q-Analysis)zero crossing method for the simultaneous determination of Atenolol and Losartan Potassium in combined dosage form. The utility of absorption ratio method data processing program is its ability to calculate unknown concentration of components of interest in a mixture ...
Two simpl e, accurate and precise UV spectroscopic methods were developed for the simultaneous estimation of atenolol and indapamide in their combined dosage form, one of which being dual wavelength method, in which two wavelengths were selected for each drug in a way so that the difference in absorbance is zero for another drug.
An accurate, precise and reproducible UV-spectrophotometric methods and liquid chromatographic assay method were developed and validated for the determination of Dapagliflozin propanediol and Glimepiride in synthetic mixture. Spectrophotometric estimation was done by derivative spectroscopic method and methanol as solvent. In
Conclusion: A cheap and rapid UV spectrophotometric method was developed and validated for the quantitative estimation of Indinavir sulphate in capsules as per ICH guidelines and hence it can be ...
Ultraviolet-visible (UV-visible) spectrophotometry is primarily a quantitative analytical technique concerned with the absorption of near-UV (180-390 nm) or visible (390-780 nm) radiation by chemical species in solution. These regions of the electromagnetic spectrum (see Table 1) provide energy that gives rise to electronic transitions.
UV-Method water: gly cerol (1% v/v) is used as solvent as well as Methanol water used a s solvent. Solution of drugs were scanned in spectrophotometer in the range 200-400nm.
Upon literature survey and as per our present knowledge, there is no simple and reliable method for estimation of ETX from pharmaceutical dosage forms as well as in bulk formulation. In this study, a simple UV spectrophotometric method was developed and validated as per International Conference on Harmonization (ICH) guidelines. This method ...
From the literature survey, it was found that topiroxostat was estimated by analytical methods such as Spectrophotometry, HPLC and HPTLC in single form or combination with other drugs. The aim of the present study is to develop a simple, precise and accurate spectrophotometric method for the estimation
Zhou and Qian proposed a spectrophotometric method for the assay of vitamin A in feeds and fish liver oil pills. The UV-absorption wavelength of vitamin A has been found at 326.5 nm. p-Toluene sulfonic acid has been used to dehydrate vitamin A for its determination in feeds and fish liver oil pills.