Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 April 2024

Integrative common and rare variant analyses provide insights into the genetic architecture of liver cirrhosis
- Jonas Ghouse ORCID: orcid.org/0000-0002-9634-5964 1 , 2 ,
- Gardar Sveinbjörnsson ORCID: orcid.org/0000-0003-2429-9468 3 ,
- Marijana Vujkovic ORCID: orcid.org/0000-0003-4924-5714 4 , 5 , 6 ,
- Anne-Sofie Seidelin 7 ,
- Helene Gellert-Kristensen ORCID: orcid.org/0000-0002-9771-9876 7 ,
- Gustav Ahlberg ORCID: orcid.org/0000-0003-0066-2779 2 ,
- Vinicius Tragante ORCID: orcid.org/0000-0002-8223-8957 3 ,
- Søren A. Rand ORCID: orcid.org/0000-0002-1892-1911 1 , 2 ,
- Joseph Brancale ORCID: orcid.org/0000-0002-1948-5103 8 ,
- Silvia Vilarinho ORCID: orcid.org/0000-0002-2099-4212 8 ,
- Pia Rengtved Lundegaard ORCID: orcid.org/0000-0002-8284-1844 2 ,
- Erik Sørensen 9 ,
- Christian Erikstrup ORCID: orcid.org/0000-0001-6551-6647 10 ,
- Mie Topholm Bruun ORCID: orcid.org/0000-0002-8819-5388 11 ,
- Bitten Aagaard Jensen 12 ,
- Søren Brunak ORCID: orcid.org/0000-0003-0316-5866 13 ,
- Karina Banasik ORCID: orcid.org/0000-0003-2489-2499 14 ,
- Henrik Ullum 15 ,
- DBDS Genomic Consortium ,
- Niek Verweij 16 ,
- Luca Lotta ORCID: orcid.org/0000-0002-2619-5956 16 ,
- Aris Baras ORCID: orcid.org/0000-0002-6830-3396 16 ,
- Regeneron Genetics Center ,
- Tooraj Mirshahi ORCID: orcid.org/0000-0002-0754-3820 17 ,
- David J. Carey 17 ,
- Geisinger-Regeneron DiscovEHR Collaboration ,
- VA Million Veteran Program ,
- David E. Kaplan ORCID: orcid.org/0000-0002-3839-336X 4 , 5 ,
- Julie Lynch ORCID: orcid.org/0000-0003-0108-2127 18 , 19 ,
- Timothy Morgan 20 , 21 ,
- Tae-Hwi Schwantes-An 20 , 22 ,
- Daniel R. Dochtermann 23 ,
- Saiju Pyarajan 23 , 24 ,
- Philip S. Tsao 25 , 26 ,
- Estonian Biobank Research Team ,
- Triin Laisk ORCID: orcid.org/0000-0003-1501-9030 27 ,
- Reedik Mägi 27 ,
- Julia Kozlitina ORCID: orcid.org/0000-0001-7720-2290 28 ,
- Anne Tybjærg-Hansen 7 ,
- David Jones 29 ,
- Kirk U. Knowlton 30 , 31 ,
- Lincoln Nadauld 29 , 32 ,
- Egil Ferkingstad ORCID: orcid.org/0000-0001-8090-7988 3 ,
- Einar S. Björnsson 33 , 34 ,
- Magnus O. Ulfarsson 3 , 35 ,
- Árni Sturluson 3 ,
- Patrick Sulem ORCID: orcid.org/0000-0001-7123-6123 3 ,
- Ole B. Pedersen ORCID: orcid.org/0000-0003-2312-5976 36 , 37 ,
- Sisse R. Ostrowski ORCID: orcid.org/0000-0001-5288-3851 9 , 36 ,
- Daniel F. Gudbjartsson ORCID: orcid.org/0000-0002-5222-9857 3 , 38 ,
- Kari Stefansson ORCID: orcid.org/0000-0003-1676-864X 3 ,
- Morten Salling Olesen ORCID: orcid.org/0000-0001-9477-5322 1 , 2 ,
- Kyong-Mi Chang 4 , 5 ,
- Hilma Holm ORCID: orcid.org/0000-0002-9517-6636 3 ,
- Henning Bundgaard 1 , 36 na1 &
- Stefan Stender ORCID: orcid.org/0000-0003-0281-5900 7 , 36 na1
Nature Genetics ( 2024 ) Cite this article
1516 Accesses
2 Altmetric
Metrics details
- Genome-wide association studies
- Liver cirrhosis
We report a multi-ancestry genome-wide association study on liver cirrhosis and its associated endophenotypes, alanine aminotransferase (ALT) and γ-glutamyl transferase. Using data from 12 cohorts, including 18,265 cases with cirrhosis, 1,782,047 controls, up to 1 million individuals with liver function tests and a validation cohort of 21,689 cases and 617,729 controls, we identify and validate 14 risk associations for cirrhosis. Many variants are located near genes involved in hepatic lipid metabolism. One of these, PNPLA3 p.Ile148Met, interacts with alcohol intake, obesity and diabetes on the risk of cirrhosis and hepatocellular carcinoma (HCC). We develop a polygenic risk score that associates with the progression from cirrhosis to HCC. By focusing on prioritized genes from common variant analyses, we find that rare coding variants in GPAM associate with lower ALT, supporting GPAM as a potential target for therapeutic inhibition. In conclusion, this study provides insights into the genetic underpinnings of cirrhosis.
Similar content being viewed by others
Genome-wide association study of serum liver enzymes implicates diverse metabolic and liver pathology
Vincent L. Chen, Xiaomeng Du, … Elizabeth K. Speliotes

Multiomics study of nonalcoholic fatty liver disease
Gardar Sveinbjornsson, Magnus O. Ulfarsson, … Kari Stefansson
A multiancestry genome-wide association study of unexplained chronic ALT elevation as a proxy for nonalcoholic fatty liver disease with histological and radiological validation
Marijana Vujkovic, Shweta Ramdas, … Kyong-Mi Chang
Cirrhosis of the liver results from prolonged hepatic inflammation and replacement of healthy liver tissue with scar tissue. It is an irreversible and progressive disease that is associated with high morbidity and mortality due to liver failure, cardiovascular and renal complications and a high rate of hepatic malignancies 1 . Major lifestyle and environmental risk factors for cirrhosis include chronic viral hepatitis, alcohol abuse and fatty liver disease. Mirroring the obesity pandemic, obesity-associated fatty liver disease is projected to soon become the most common cause of cirrhosis globally 1 , 2 .
Estimates from twin studies have shown that approximately half of the variation in cirrhosis risk is attributed to genetic factors 3 . Identifying the implicated risk loci has progressed steadily over the last decade, mainly due to ever-larger genome-wide association studies (GWAS). The largest of these included 4,829 cases and 72,705 controls in the discovery cohort and identified 12 loci to be associated with cirrhosis in multitrait GWAS with plasma alanine aminotransferase (ALT), a biochemical marker of liver cell injury 4 . However, the number of sequence variants linked to cirrhosis is low when compared to the hundreds of risk loci identified for other complex traits and diseases.
A better understanding of the genetic factors that predispose to cirrhosis may improve our ability to predict, prevent and ultimately treat the disease. Using polygenic risk scores (PRSs) that account for the influence of multiple risk loci may assist in identifying individuals who are at an increased risk of developing cirrhosis 5 . Furthermore, the discovery of genetic variants linked to a reduced risk of cirrhosis may provide potential molecular targets for pharmacological intervention 6 , 7 , 8 , 9 .
The aims of this study were fourfold. First, we conducted meta-analyses of 12 cohorts comprising 18,265 cirrhosis cases and nearly 1.8 million controls, aiming to discover new risk loci for cirrhosis. Second, we conducted a range of analyses to elucidate the potential of PRSs to predict the onset and progression of cirrhosis. Third, we examined interactions of the genetic risk variants with alcohol consumption, adiposity and type 2 diabetes mellitus (T2D). Fourth, we used whole-exome sequencing data to gauge the expected effects of therapeutic inhibition of both known and new risk genes.
Genome-wide association results
An overview of the study design is shown in Fig. 1 . In stage 1, we performed a GWAS meta-analysis of nine studies, comprising 15,225 cases with cirrhosis and 1,564,786 controls of European ancestry (Supplementary Table 1 ). The genomic inflation factor ( λ GC ) for the European-specific meta-analysis was 1.11 with linkage disequilibrium (LD) score regression (LDSC) intercept of 1.02 (s.e. = 0.007), indicating that the observed inflation is due to polygenicity. In stage 1, we identified 12 genome-wide significant variants (Fig. 1 and Supplementary Table 2 ), 5 of which have not been previously reported in a cirrhosis GWAS. In stage 2, we conducted a cross-ancestry fixed-effects meta-analysis with individuals of East Asian (9.9%), African American (1.2%), Hispanic (1.0%) and European (87.9%) ancestries (Supplementary Table 1 ), totaling 18,265 cases and 1,782,047 controls. In the cross-ancestry meta-analysis, we identified 15 variants, including 8 previously unreported variants (Fig. 1 , Table 1 and Supplementary Table 3 ). Of the 15 unique variants identified in stage 2, 3 were specific to the cross-ancestry analysis, whereas 12 reached genome-wide significance in both stages (Supplementary Table 3 ). The ALDH2 locus was driven by a missense variant (rs671, p.Glu504Lys), which is common in East Asian populations, but is rare or absent in other ancestries (Supplementary Table 3 ). Similarly, the missense variant in SERPINA1 ( rs28929474 , p.Glu366Lys) was only present in Europeans. The following two variants showed heterogeneous effects across ancestries ( P < 0.003): PNPLA3 rs738408 and TM6SF2 rs739846 . We estimated the heritability (the proportion of variation that is attributed to common genetic variants) using LDSC. We found that the SNP-based heritability estimates were consistent between Europeans ( h 2 = 5.1%, 95% confidence interval (CI): 3.5–6.8) and East Asians ( h 2 = 2.7%, 95% CI: −2.7 to 8.1; Supplementary Table 4 ). We could not estimate heritability in African American and Hispanic samples because of limited statistical power (Supplementary Table 4 ). We also reappraised variants that have been previously linked to cirrhosis in GWAS or candidate gene studies but were not detected in stage 1 or 2 of our study (Supplementary Table 5 ). Of the nine variants, we found evidence to support association ( P < 0.05) with cirrhosis for four (in CENPW , TOR1B , MBOAT7 and MAFB ).
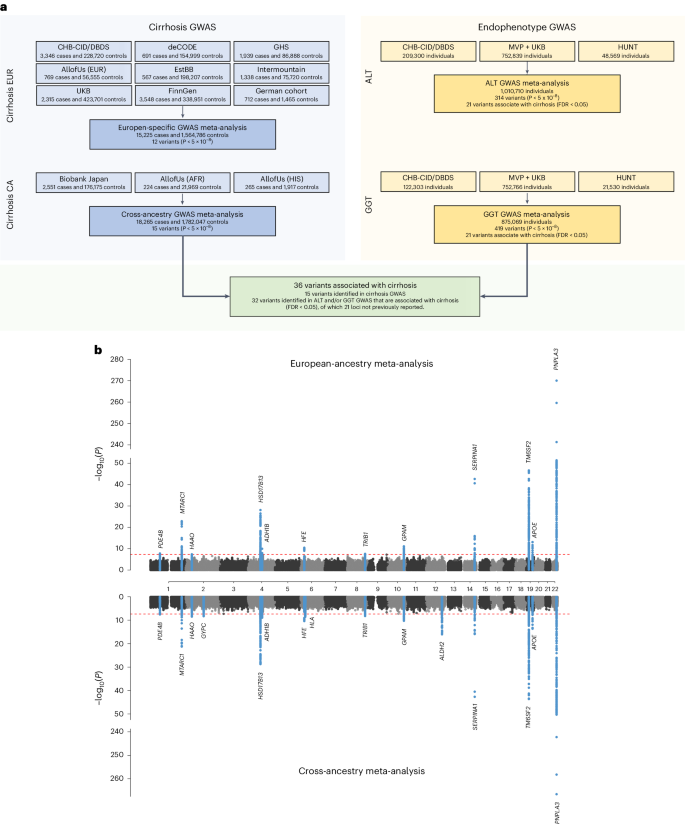
a , Overall study design, with stage 1 representing the European-specific GWAS meta-analysis and stage 2 representing the cross-ancestry GWAS meta-analysis. b , Miami plot of cirrhosis GWAS. The x axis is the chromosomal location of SNPs and the y axis is the strength of association −log 10 ( P ). Note that the y axis includes a break at 50. The lead SNPs and SNPs located within ±1 Mb are highlighted, and the nearest genes are annotated. The top plot shows results from the European-specific GWAS meta-analysis, whereas the bottom plot displays results from the cross-ancestry GWAS meta-analysis. The dashed red line represents the threshold for genome-wide significance ( P < 5 × 10 −8 ). P values were two-sided and based on an IVW fixed-effects meta-analysis, and not adjusted for multiple testing.
Endophenotype-driven analyses
We used liver enzyme GWAS associations as priors to enhance genomic discovery for liver cirrhosis. Analysis of up to 1 million individuals of European ancestry yielded 314 independent genome-wide signals for ALT, including 114 previously unreported (Fig. 1a and Supplementary Table 6 ), and 419 independent genome-wide signals for γ-glutamyl transferase (GGT), with 106 previously unreported (Fig. 1a and Supplementary Table 7 ). Of the 307 ALT and 403 GGT lead variants that were available in the cirrhosis datasets, 21 ALT and 20 GGT variants were associated with cirrhosis (false discovery rate (FDR) < 0.05; Table 1 and Supplementary Table 8 ). Nine variants were identified through both ALT- and GGT-informed analyses. Of 32 unique variants, 11 were genome-wide significant in the cirrhosis meta-analyses, 2 had been implicated in cirrhosis in prior GWAS ( TOR1B rs7029757 and MBOAT7 rs4806498 ) 4 , 10 , whereas the remaining 19 have not been associated with cirrhosis before (Table 1 and Supplementary Table 8 ). Of the 21 ALT variants, 2 were directionally discordant with cirrhosis risk, specifically rs9663238 in HKDC1 and rs79287178 in TNFSF10 (Supplementary Table 8 ), whereas all 20 GGT variants had concordant direction of effects with cirrhosis. We found that a PRS using these 21 variants identified via endophenotype-driven analysis associated significantly with cirrhosis in the UK Biobank (UKB; odds ratio (OR): 1.15 per s.d., 95% CI: 1.11–1.20, P = 1.8 × 10 −11 ) and Million Veteran Program (MVP, OR: 1.09 per s.d., 95% CI: 1.07–1.11, 1.20, P = 3.6 × 10 −19 ), but contributed only little to the variance explained ( r 2 UKB = 0.2% and r 2 MVP = 0.1%; Supplementary Table 9 ).
A total of 36 risk variants were identified through cirrhosis GWAS and/or the endophenotype-informed analysis, of which 35 were available for replication in the MVP cohort (21,689 cases and 617,729 controls). Replication of ALDH2 rs671 was not possible due to its absence in non-East Asian populations. Of the 35 variants, 14 (40%) reached Bonferroni significance ( P < 1.4 × 10 −3 (0.05/35 variants)) and 6 were nominally significant ( P < 0.05) with concordant directions of effect in MVP (Supplementary Table 10 ). Of the 20 variants associated with a P < 0.05 in MVP, 10 were initially identified in stage 1 or 2 of the GWAS, whereas 10 were identified solely via endophenotype-informed analyses. For the 15 variants that did not replicate at P < 0.05, we found a high level of concordance in the magnitudes and directions of effects between the two datasets (Pearson’s r 2 = 0.73, P = 1.8 × 10 −3 ; Supplementary Table 10 ).
Phenome-wide association study (PheWAS)
To identify the mechanism by which a variant or gene is linked to a disease, it is important to comprehend the range of phenotypic consequences resulting from carrying a specific sequence variant. Here we tested each of the 36 risk variants identified in GWAS and/or endophenotype-driven analyses for association with 41 binary and quantitative traits. As expected, many of the variants were associated with risk factors for liver disease (for example, alcohol dependence and lipids) and/or with fatty liver disease (Fig. 2 ). For example, more than half of the variants ( n = 21/36) were associated with non-high density lipoprotein cholesterol (non-HDL-C; FDR < 0.05), 16/36 variants were associated with liver fat and 18/36 variants were associated with a registry-based diagnosis of nonalcoholic fatty liver disease (NAFLD; Fig. 2 and Supplementary Table 11 ). Variants associated with either liver fat or NAFLD had directionally concordant effects on cirrhosis. Three variants ( rs739846 in TM6SF2 , rs80215559 in HFE and rs738408 in PNPLA3 ) associated with higher liver fat, but lower levels of non-HDL-C (Supplementary Table 11 ). Other variants were associated with proteins and metabolites produced by the liver, such as uric acid ( n = 23/36), sex hormone-binding globulin ( n = 20/36) and albumin ( n = 19/36). Of the newly identified variants, rs1229984 in ADH1B associated with a lower risk of alcohol dependence, alcoholic liver disease (ALD), cardiovascular risk factors (for example, hypertension, body mass index (BMI)) and cardiovascular disease (for example, coronary artery disease). Similarly, rs1937455 in PDE4B was associated with a lower risk of alcohol dependence, lower GGT levels and lower BMI. rs9663238 in HKDC1 associated with lower HbA1c levels and T2D risk. Other previously unreported variants associated with lipid traits and fatty liver disease, including rs2792735 in GPAM , rs2980888 in TRIB1 , rs13389219 in COBLL1 , rs8178824 in APOH and rs339969 in ICE2 (Fig. 2 and Supplementary Table 11 ).
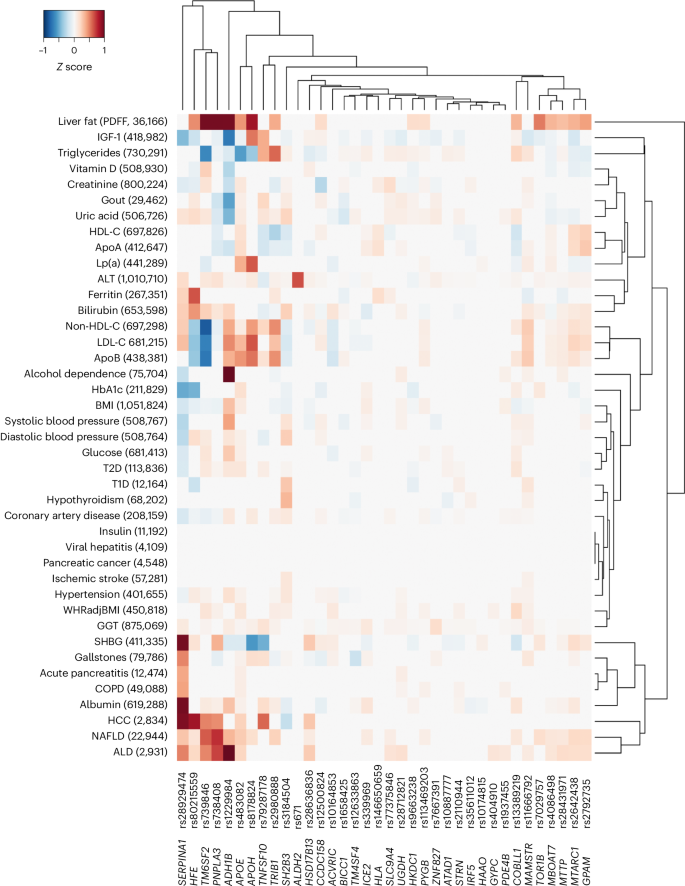
The heatmap shows associations of variants identified through cirrhosis GWAS meta-analysis or endophenotype-driven analyses with 41 binary and quantitative traits sampled from meta-analysis of data from CHB-CID/DBDS, deCODE, Intermountain Healthcare, FinnGen, UKB and external sources, where available. The number of cases for binary traits and sample size for quantitative traits are shown in parenthesis following each trait. Shown are variants and phenotypes with significant associations after correcting for multiple testing using an FDR of <0.05. P values (two-sided) were derived from linear and logistic regression models. Hierarchical clustering was performed on a variant level using the complete linkage method based on Euclidian distance. Coloring represents z scores for each respective trait or disease, oriented toward the cirrhosis risk-increasing allele. Red indicates an increase in the trait or disease risk, while blue indicates a decrease in the trait or disease risk. SHBG, sex hormone-binding globulin; IGF-1, insulin growth factor 1; ApoA, apolipoprotein A; ApoB, apolipoprotein B; COPD, chronic obstructive pulmonary disease; WHRadjBMI, waist-to-hip-ratio adjusted for BMI; LDL-C, low-density lipoprotein cholesterol; T1D, type 1 diabetes.
Comparison of genetic effects on NAFLD, ALD and cirrhosis
Next, we compared the effect sizes on cirrhosis and NAFLD ( n cases = 22,944) of 18 previously reported NAFLD variants along with the 36 cirrhosis variants identified here (totaling 38 distinct variants). Eight variants had significantly higher effects on cirrhosis compared with NAFLD ( P value for heterogeneity ( P Het ) < 0.05/38; Fig. 3a and Supplementary Table 12 ). Of those, we found that rs72613567 in HSD17B13 and known risk variants in SERPINA1 (p.Glu366Lys) and HFE (p.Cys282Tyr) exhibited stronger effects on cirrhosis than on NAFLD. Moreover, we found that the variants near HKDC1, HLA-DQB1 and MAMSTR likely influence cirrhosis via pathways distinct from those related to fatty liver disease. We also found that variants in TRIB1, TM6SF2 and APOE had stronger effects on NAFLD compared with cirrhosis, indicating that they may primarily exert their effect on cirrhosis via fatty liver disease. Variation in GCKR was strongly associated with NAFLD but had no effect on cirrhosis. The previously reported NAFLD variants p.Thr165Ala in MTARC1 and p.Ile148Met in PNPLA3 had proportional effects on cirrhosis. We then compared the effects of the 36 cirrhosis variants with their respective effects on ALD ( n cases = 2,931) and NAFLD ( n cases = 22,944; Fig. 3b ). We found proportional effects between NAFLD and ALD, except for three variants (p.His48Arg in ADH1B, P Het = 4.4 × 10 −14 ; rs28636836 in HSD17B13, P Het = 1.8 × 10 −4 ; and rs28712821 in KLB, P Het = 7.9 × 10 −4 ), which had significantly larger effects on ALD than on NAFLD ( P Het < 0.05/36).
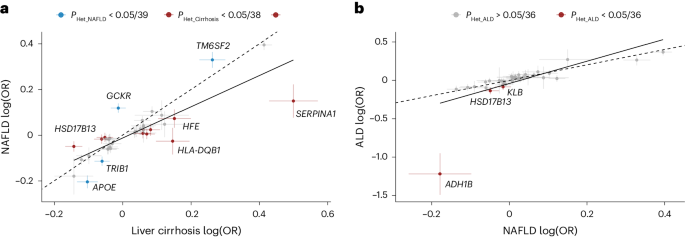
a , Shown are the effects of 18 previously reported NAFLD variants and 36 cirrhosis variants identified in this study, totaling 38 distinct signals. The NAFLD effects were derived from meta-analysis of data from deCODE, UKB, CHB, Intermountain Healthcare and FinnGen ( n = 22,944). Cirrhosis effects were derived from the cross-ancestry meta-analysis. Variants that had stronger effects ( P Het < 0.05/38, that is corrected for 38 tests) on NAFLD compared with cirrhosis are colored blue, while variants with stronger effects on cirrhosis compared with NAFLD are colored red. b , Shown are the effects of the 36 cirrhosis variants on NAFLD ( n = 22,944) and ALD ( n = 2,931). Variants with stronger effects ( P Het < 0.05/36, that is corrected for 36 tests) on ALD compared with NAFLD are colored red. For both a and b , points refer to effect estimates (log(OR), measure of center), error bars represent 95% CI and the solid line represents the line of best fit. The dashed identity line ( y = x ) is shown for reference. P Het were two-sided and obtained using a likelihood ratio test (Cochran’s Q ).
Mendelian randomization (MR)
To explore potential causal relationships between significant PheWAS findings and cirrhosis risk, we performed MR analyses. Consistent with observational data, we found evidence to support a causal role of higher BMI ( P IVW = 3.1 × 10 −16 ) and higher alcohol intake ( P IVW = 3.3 × 10 −7 ) with increased risk of cirrhosis (see Supplementary Table 13 for summary of results). The results were not driven by the effect of individual variants (see Supplementary Figs. 1 and 2 for effect and leave-one-out plots). To evaluate the potential mediating effect of NAFLD on the association between higher BMI, alcohol intake and cirrhosis, we conducted multivariable Mendelian randomization (MVMR) analyses while accounting for the influence of NAFLD. Despite observing a slight attenuation in the effect estimates, we observed significant independent associations between higher BMI ( β = 0.252 s.d. units, s.e. = 0.048, P IVW = 2.7 × 10 −7 ) and alcohol intake ( β = 0.971 s.d. units, s.e. = 0.186, P IVW = 1.3 × 10 −6 ) and risk of cirrhosis.
Interactions with alcohol, obesity and T2D
Environmental risk factors and comorbid metabolic disorders, such as alcohol consumption, obesity and T2D exacerbate the impact of known genetic risk factors on cirrhosis 4 , 11 . To determine whether similar interactions exist between environmental factors and newly identified risk variants, we examined the effects of 35 genetic variants (excluding HLA ) on cirrhosis risk in combination with environmental factors in the UKB. We found that rs738408 in PNPLA3 interacted significantly with T2D ( P = 7.9 × 10 −6 ), BMI ( P = 3.0 × 10 −6 ) and weekly alcohol intake ( P = 1.2 × 10 −5 ) on the risk of cirrhosis (Supplementary Table 14 ). PNPLA3 rs738408 was only weakly associated with T2D (OR: 1.03, P = 0.007), BMI ( β = −0.04 kg m − 2 , P = 0.001) and not associated with weekly alcohol intake ( β = −0.04 units per week, P = 0.160). We then examined the common missense variant p.Ile148Met in PNPLA3 ( rs738409 , r 2 = 1 with rs738408 ) and its interaction with the same environmental risk factors on a broader range of liver-related outcomes. We found that high alcohol intake (>14 units per week), obesity (BMI > 30 kg m − 2 ) and T2D also amplified the effect of PNPLA3 p.Ile148Met on hepatocellular carcinoma (HCC) and all-cause liver disease (Fig. 4 and Supplementary Table 15 ). For instance, among obese individuals, homozygous carriers of the G-allele had a sevenfold increased risk of HCC compared to noncarriers. Among nonobese individuals (BMI < 30 kg m − 2 ), the corresponding risk was only 2.6-fold higher ( P for interaction = 0.003; Fig. 4 and Supplementary Table 15 ).
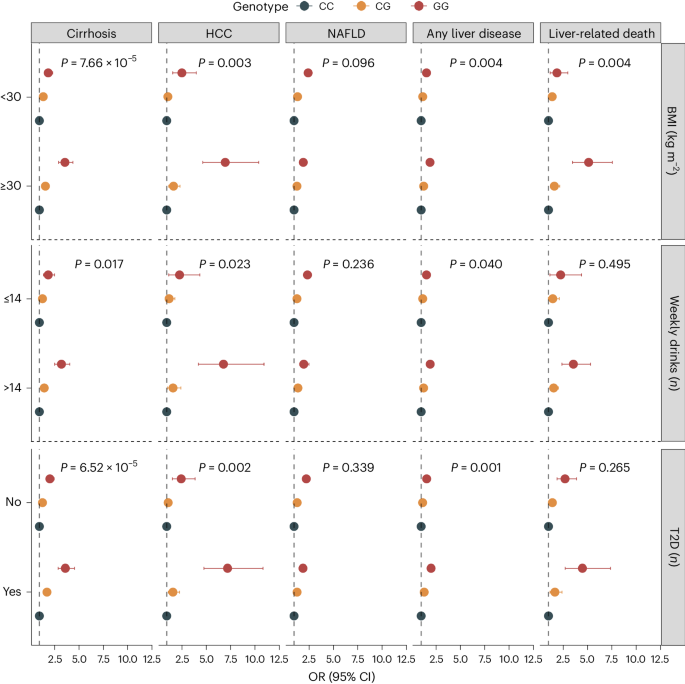
Shown are the association between rs738409 carrier status and risk of five liver outcomes according to BMI categories (<30 versus ≥30 kg m − 2 ), weekly alcohol intake (≤14 versus >14 weekly drinks) and T2D (no versus yes). Points refer to OR (measure of center), error bars represent 95% CIs and P represents P value for interaction. We used logistic regression models, adjusted for age, sex and ten principal components (PCs). Interactions between the variant and environmental factors were evaluated using likelihood ratio tests, comparing a main-effect model (variant + environmental factor) with a model including an interaction term (variant × environmental factor). The number of exposed individuals and the number of outcomes within each subcategory are listed in Supplementary Table 15 .
Gene prioritization
To prioritize potential causal genes at the identified risk loci, we used the following six approaches: (1) identification of coding variants, (2) estimation of effects on gene expression using expression quantitative trait locus (eQTL) data from two datasets (GTEx v.8 and deCODE 12 , 13 ), (3) associations with quantified splicing using splicing quantitative trait locus (sQTL) data from whole blood (deCODE), (4) effects on plasma protein levels using protein quantitative trait locus (pQTL) data from deCODE 14 and UKB 15 , (5) a similarity gene-based method (polygenic priority score (PoPS)) and (6) Open Targets Variant-to-Gene (V2G) algorithm. Of the 36 cirrhosis variants, we identified protein-altering variants in LD ( r 2 > 0.8) with the lead variant at 16 loci (Supplementary Table 16 ), including 3 splice variants in HSD17B13 ( rs72613567 , c.812+2dupT), MAMSTR ( rs11666792 , c.219+3G>A) and PYGB ( rs2261790 , c.1518+6T>C). Using gene-expression data, we found significant colocalization (posterior probability (PPa) >0.70) at six loci (Supplementary Tables 17 and 18 ), proposing 12 potentially causal genes, and a single gene at two loci ( HSD17B13 and TOR1B ). Only MBOAT7 and HKDC1 showed evidence of colocalization in liver tissue. Additionally, we found six variants that associated with splicing QTLs (Supplementary Table 19 ) and two variants (in ADH1B and APOH ) with significant cis associations with protein levels (Supplementary Table 20 ). Using the similarity-based approach, PoPS, we identified at least one gene at 23 loci that had a score among the top 10% of the PoPS distribution (Supplementary Table 21 ). Using the Open Targets Genetics V2G score, all variants were successfully mapped to a nearby gene. By considering the number of lines of evidence supporting a given gene, we found that 18 of 36 loci had at least two lines of evidence and 9 loci had at least three lines of evidence (Supplementary Table 22 ).
Convergence between common and rare variant associations
We examined exome sequencing data in the UKB to assess convergence in disease risk between common and rare protein-truncating variants. We selected 18 genes based on gene-prioritization analyses that had at least two lines of evidence and then evaluated rare variants (allele frequency <0.1%) that were predicted to cause loss-of-function (pLoF) and/or missense variants (with a Combined Annotation Dependent Depletion (CADD) score of at least 20) for their association with ALT and cirrhosis. We found three genes ( ADH1B, GPAM and TM6SF2 ) that were significantly associated with ALT ( P < 6.9 × 10 −4 ; Supplementary Table 23 ). Notably, rare pLoF variants in GPAM were associated with lower ALT levels (−0.29 s.d. units per allele, 95% CI: −0.40 to −0.16, P = 5.8 × 10 −6 ) and numerically lower odds of cirrhosis, although the latter association did not reach statistical significance (OR: 0.36, 95% CI: 0.05–2.42, P = 0.296; Supplementary Table 23 ). In contrast, the missense variant p.Ile42Val ( rs2792751 ) in GPAM had a positive effect on both ALT levels (0.006 s.d. units per allele, 95% CI: 0.005–0.007, P = 7.0 × 10 −45 ) and cirrhosis (OR: 1.09, 95% CI: 1.06–1.12, P = 6.4 × 10 −11 ). Similar to the missense variant p.Glu167Lys ( rs58542926 ), rare coding variants in TM6SF2 were also associated with higher ALT levels (0.10 s.d. units per allele, 95% CI: 0.07–0.13, P = 2.0 × 10 −10 ) and increased risk of cirrhosis (OR: 2.07, 95% CI: 1.43–3.00, P = 1.0 × 10 −4 ). The underlying mechanism by which the common missense variant p.Ile148Met ( rs738409 ) in PNPLA3 leads to hepatic steatosis and progressive liver injury has been a topic of discussion. We observed that rare coding variants (pLoF + missense) and pLoF variants (excluding missense) in PNPLA3 were both nominally associated with increased cirrhosis risk (pLoF + missense—OR: 1.86; 95% CI: 1.19–2.90; P = 6.0 × 10 −3 ; pLoF—OR: 2.97; 95% CI: 1.09–8.15; P = 0.034; Supplementary Table 23 ). We also found that rare coding variants in PNPLA3 associated nominally with liver enzymes (pLoF + missense: 0.04 s.d. units per allele, 95% CI: 0.00–0.07, P = 0.034), but not when restricting to pLoF only (0.05 s.d. units per allele, 95% CI: −0.01 to 0.12, P = 0.121). This finding is similar to the direction of effect observed for p.Ile148Met (OR: 1.58, 95% CI: 1.54–1.62, P = 3.1 × 10 −260 ). After adjusting for p.Ile148Met, associations were slightly attenuated (pLoF + missense—OR: 1.56; 95% CI: 1.04–2.33; P = 0.032; pLoF—OR: 2.01; 95% CI: 1.00–4.04; P = 0.051, respectively).
PRS and hepatobiliary outcomes
We created the following six distinct PRSs: a European-specific (PRS EUR ), a cross-ancestry PRS (PRS CA ), a PRS based on ALT (PRS ALT ) and three different weighted scores, each incorporating varying numbers of risk variants identified in this study. We then compared the predictive ability of each of these PRSs. We found that the PRS 15-SNP explained the highest proportion of phenotypic variation ( r 2 = 1.7%; Supplementary Table 9 ), change in area under the curve (AUC) (+0.031, 95% CI: 0.023–0.039; Supplementary Table 24 ) and yielded an OR for cirrhosis of 1.42 per s.d. increase in PRS (Supplementary Table 9 and Fig. 5a ). In comparison, the PRS ALT accounted for 1.3% of cirrhosis phenotypic variance, had a change in AUC of 0.021 and an OR of 1.38 per s.d. increase in PRS (Fig. 5a ). The difference in predictive ability between PRS 15-SNP and PRS ALT was statistically significant (change in AUC + 0.005, 95% CI: 0.003–0.017, P = 0.005). Next, we evaluated the reclassification of individuals after the addition of the PRS 15-SNP to a baseline model containing age, sex and ten PCs. Adding PRS 15-SNP resulted in a net percentage of individuals with cirrhosis correctly classified upward (event net reclassification index (NRI)) of 8.4% (95% CI: 3.1–13.7), and of individuals without cirrhosis correctly classified downward (nonevent NRI) of 21.3% (95% CI: 20.1–22.7). These changes resulted in an overall continuous NRI of 29.7% (95% CI: 23.4–36.1). Following this, we investigated how the various PRSs associated with a broader range of hepatobiliary outcomes. We found that the PRS EUR had the highest OR for HCC, for which a 1 s.d. higher PRS conferred an OR of 1.67 (95% CI: 1.52–1.82), followed by liver-related death (OR: 1.56 (95% CI: 1.44–1.69)) and alcoholic cirrhosis (OR: 1.47 (95% CI: 1.39–1.57)). Across the range of outcomes, the PRS 15-SNP tended to have slightly larger per s.d. effect sizes than PRS CA , PRS ALT and PRS 5-SNP , but comparable to the PRS EUR . Notably, PRS 5-SNP performed similarly to PRS ALT and PRS CA , despite being based on only five SNPs. To test the generalizability of the PRS 5-SNP , we investigated its association with cirrhosis in a general population cohort from Copenhagen, Denmark (428 cases and 95,321 controls, all Danish ancestry), and in a multi-ancestry case–control study from Dallas, Texas (825 cases and 3119 controls; 21% Hispanic, 46% Black and 31% White). The per s.d. ORs for cirrhosis in the two cohorts were 1.35 (95% CI: 1.24–1.48) and 2.35 (95% CI: 2.10–2.63), respectively.
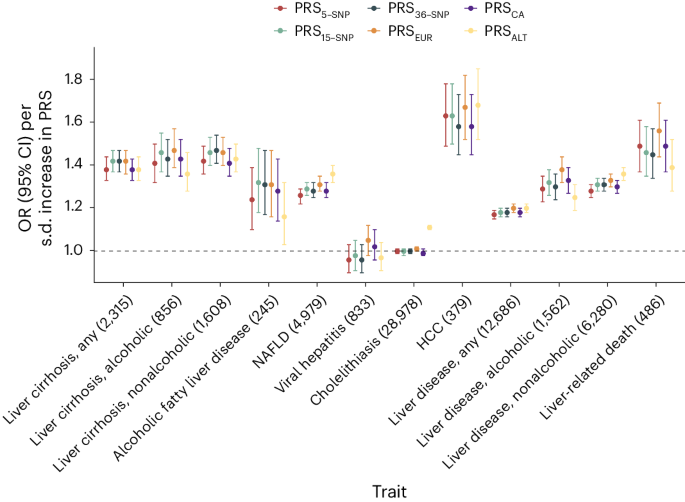
Associations between six different PRS (PRS ALT , PRS 5-SNP , PRS 15-SNP , PRS 36-SNP , PRS EUR and PRS CA ) and hepatobiliary outcomes in the UKB. Points refer to ORs (measure of center) per s.d. increase in PRS; error bars represent 95% CIs. Total number of cases is provided following each outcome. Logistic regression models were used, adjusted for age, sex and ten PCs.
PRSs and disease progression
We evaluated the ability of PRS 15-SNP to classify risk in a sample of 1,796 individuals with cirrhosis from the UKB, of whom 91 developed HCC. We found an association between a higher PRS 15-SNP and an increased risk of HCC after the onset of cirrhosis. Specifically, we found that individuals with cirrhosis and a high PRS 15-SNP (top 20% of the PRS) had a 10-year HCC risk of 15.0% (95% CI: 9.7–22.0) compared with 5.8% (95% CI: 4.3–7.6, P for difference <0.001) for individuals in the bottom 80% of the PRS 15-SNP (Fig. 6a ). A similar pattern was observed in Copenhagen Hospital Biobank (CHB), involving 3,253 individuals with cirrhosis, of whom 172 developed HCC. Individuals in the top 20% of the PRS had an 11% (95% CI: 8.5–14.0) risk of developing HCC, compared to 5.3% (95% CI: 4.4–6.3, P for difference <0.001) for those in the bottom 80% (Fig. 6b ). Correspondingly, the PRS associated with increased risk of progressing to cirrhosis in individuals with registry-defined NAFLD. We identified 4,449 individuals in the UKB with registry-defined NAFLD, of whom 193 progressed to cirrhosis during follow-up. Individuals with a PRS 15-SNP in the top 20% had a 10-year risk of 11.0% (95% CI: 7.1–16.0), whereas individuals in the bottom 80% of the distribution had a 10-year risk of 8.6% (95% CI: 6.8–11.0, P for difference = 0.036; Fig. 6c ). In CHB, among 860 individuals with NAFLD, 95 developed cirrhosis during follow-up. In MVP, of the 18,302 individuals with NAFLD, 280 developed cirrhosis. Those in the top 20% of the PRS 15-SNP distribution had a 10-year cirrhosis risk of 13.0% (95% CI: 7.5–19.0) in CHB and a 5-year risk of 2.8% (95% CI: 2.3–3.5) in MVP, respectively (Fig. 6d ). In contrast, those in the bottom 80% of PRS 15-SNP had a 10-year risk of 9.9% (95% CI: 7.6–12.0, P for difference = 0.032) in CHB and 5-year risk of 1.5% (95% CI: 1.3–1.7, P for difference <0.001) in MVP, respectively.
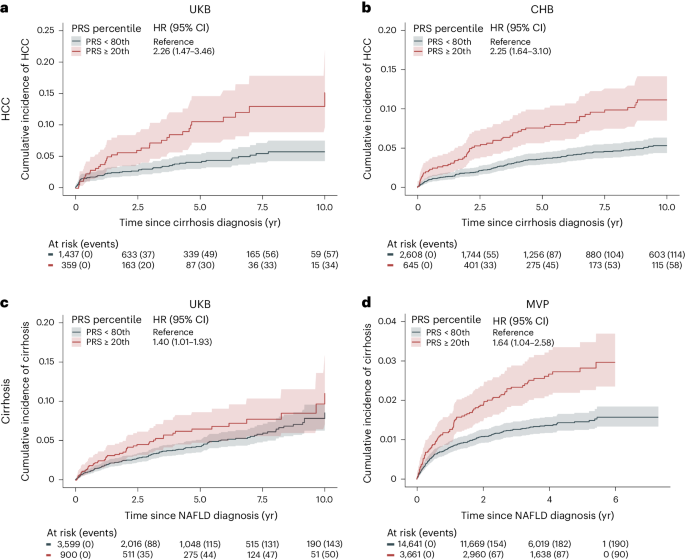
a , b , Risk of HCC in individuals with liver cirrhosis according to polygenic risk percentile. c , d , Risk of cirrhosis in individuals with NAFLD diagnosis, according to polygenic risk percentile. We used the PRS 15-SNP to assign individuals to different risk groups. Cumulative incidence (solid line, measure of center) was estimated using Fine-Gray regression, which takes the competing risk of death into account. The lighter shades represent the respective 95% CIs. Number of individuals at risk according to each exposure group and events are given below each plot.
We report the largest GWAS meta-analysis to date on cirrhosis and its associated endophenotypes, ALT and GGT. Our study included over 18,000 cirrhosis cases and more than 1 million individuals with endophenotypic data sampled from four populations and identified 36 risk variants for cirrhosis, of which 14 replicated in an independent cohort. We found that PRSs were linked to the progression of NAFLD to cirrhosis and of cirrhosis to HCC. In addition, we used molecular QTLs and gene-prioritization methods to identify genes for rare variant burden analyses. This enabled us to investigate the convergence of risk between common and rare genetic variants and identify potential targets for pharmacological intervention.
As expected for an end-stage disease, we found that the risk variants were mainly associated with cirrhosis through known risk factors. The majority of the variants were associated with hepatic lipid metabolism and fatty liver disease, with certain variants (in APOE and TRIB1 ) displaying significantly larger effects on NAFLD compared with cirrhosis. Other variants, such as those in HSD17B13 and MAMSTR , were found to have larger effects on cirrhosis compared with NAFLD, indicating a more dominant role in the progression to clinically advanced stages of chronic liver disease. Variants near HKDC1 were mainly associated with cirrhosis, indicating the involvement of potential profibrotic pathways that do not involve the accumulation of hepatic fat. Conversely, other variants in COBLL1 and SH2B3 were related to body fat distribution traits, indicating that an impaired ability to store adipose tissue in peripheral compartments may contribute to disease 16 , 17 , 18 . Additionally, lead variants at ADH1B and ALDH2 have been shown to cause adverse symptoms with alcohol intake, thus reducing the risk of alcohol-related diseases such as cirrhosis 19 . The variants at HFE and SERPINA1 cause hemochromatosis and α-1 antitrypsin deficiency, respectively, well-known risk factors for cirrhosis.
The discovery of naturally occurring loss-of-function variants associated with protection against liver disease has led to the identification of new therapeutic targets 6 , 9 . We showed that rare loss-of-function variants in GPAM associate with lower plasma ALT levels. This finding aligns with two recent reports on the relationship between rare loss-of-function variants and ALT levels 9 , 20 . GPAM encodes the mitochondrial isoform of glycerol-3-phosphate acyltransferase, an enzyme that catalyzes the first step of glycerolipid synthesis in the liver and adipose tissue. Genetic variation in GPAM has previously been associated with fatty liver disease through GWAS 20 , 21 and with cirrhosis, albeit not at genome-wide significance 21 , 22 . These observations support the inhibition of GPAM as a potential treatment for cirrhosis and related liver diseases like steatosis and steatohepatitis 20 .
The potential use of PRSs for prognostication in individuals at risk of cirrhosis is a topic of major clinical interest. We evaluated the predictive ability of a range of differently constructed cirrhosis PRSs. The main finding of these analyses was that a PRS based on the 15 SNPs that associated with cirrhosis at GWAS significance in our study performed as well as scores based on all 36 SNPs identified in our study, or scores based on more than 1,000,000 SNPs. Of note, a PRS based on the five SNPs with the strongest associations with cirrhosis had only slightly attenuated effects compared to the abovementioned PRSs. Taken together, our PRS analyses indicate that the genetic architecture of cirrhosis is dominated by few, large-effect variants. Such an oligogenic model is consistent with recent GWAS findings, which showed that a 9-variant PRS for chronic ALT elevation, a proxy for NAFLD, had effects similar to those observed for a 77-variant PRS 23 . Interestingly, the cross-ancestry PRS, which was derived from the largest set of cirrhosis cases, performed worse than the European-specific genome-wide PRS in predicting cirrhosis. This contrasts with recent studies on other complex traits that have shown that incorporating a broader set of ancestries can improve prediction, even for European populations 24 , 25 , 26 , 27 . The difference in performance may reflect the diverse underlying causes of cirrhosis, where viral hepatitis is the leading cause in East Asians, while alcohol and obesity are dominant in Europeans 2 . These differences are also reflected in the variants that are mainly driven by specific ancestries, such as the HLA locus association, which contributes to hepatitis persistence and chronicity in East Asians 28 .
Environmental risk factors, such as BMI, exacerbate the risk of liver disease conferred by known genetic risk factors 11 . In alignment with previous observations 4 , 11 , we found that the risk conferred by PNPLA3 rs738409 was significantly amplified by adiposity, alcohol intake and diabetes for a range of liver-related outcomes, including NAFLD, cirrhosis and HCC. These relationships are among the strongest gene–environment interactions seen in man 29 , 30 .
Our study has some limitations that should be considered. First, the cirrhosis phenotype was mainly based on registry-based International Classification of Diseases (ICD) codes, a definition that inevitably suffers from some degree of misclassification. That said, cirrhosis is a hard endpoint with well-defined diagnostic criteria. Supporting the validity of the endpoint, PNPLA3 rs738409 was associated with cirrhosis in each cohort, with effect sizes like those seen in histologically defined cirrhosis cohorts. Second, we included relatively few individuals of African American and Hispanic ancestry. Third, although we included liver tissue in our eQTL analyses, the majority of eQTLs that we report are based on datasets from nonhepatic tissues, some of which had manyfold larger sample sizes. This limits the ability to draw conclusions on liver expression specificity of the reported loci. Nevertheless, we did not solely depend on eQTL signals as standalone evidence but reported potential effector genes when complementary evidence from other gene mapping strategies converged on the same gene.
In conclusion, we identified 36 risk variants for cirrhosis, including 24 that have not been previously linked to this disease. These results provide an expanded catalog of genes to interrogate mechanistically in future studies. A better understanding of the genetic factors that underpin cirrhosis will improve our ability to predict and ultimately treat this deadly disease.
Ethics approval
All human research was approved within each contributing study by the relevant institutional review board (IRB) and conducted according to the Declaration of Helsinki (CHB-CID/DBDS: National Committee on Health Research Ethics; deCODE: National Bioethics Committee; Intermountain Healthcare: Intermountain Healthcare IRB; UKB: Northwest Multicenter Research Ethics Committee; Geisinger DiscovEHR: The GHS project has received ethical approval from the Geisinger Health System IRB under project 2006-0258; FinnGen: The Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa; Estonian Biobank: ethical approval 1.1-12/624 from the Estonian Committee on Bioethics and Human Research, Estonian Ministry of Social Affairs; Biobank Japan: research ethics committees at the Institute of Medical Science, the University of Tokyo, the RIKEN Yokohama Institute and the 12 cooperating hospitals; Copenhagen General Population Study and Copenhagen City Heart Study: IRBs and Danish ethical committees; All of Us: National Institute of Health All of Us IRB; Dallas Liver Cohort: University of Texas Southwestern IRB; and MVP: VA Central IRB). All participants (except for CHB-CID) provided written informed consent. For CHB-CID, patients were informed about the opt-out possibility of having their biological specimens excluded from use in research in general. Since 2004, a national Register on Tissue Application (Vævsanvendelsesregistret) lists all individuals who have chosen to opt-out and whose samples cannot be used for research purposes. Before initiating this study, individuals listed in the Register on Tissue Application were excluded.
Cohorts, association testing and meta-analysis
Cases were defined using hospital or registry records (ICD-9 or ICD-10). Controls were defined as individuals without a known history of cirrhosis. A full description of the cohorts and case and control definitions is provided in Supplementary Information and Supplementary Table 1 . Details on genotyping methods, pre-imputation quality control and imputation methods are provided in Supplementary Table 1 . Each study performed a GWAS of cirrhosis using logistic regression with at least age (or year of birth), sex and PCs used as covariates. Postregression quality control (QC) included the removal of variants with an imputation quality score <0.6, minor allele count <6 or absolute log(OR) or s.e. >10. We conducted two-fixed effect inverse-variance-weighted (IVW) meta-analyses using METAL 31 . The first involved individuals of European ancestry, including nine studies, totaling 15,225 cases and 1,564,786 controls. Only variants that were present in at least three studies were retained. In the second meta-analysis, we included individuals from East Asian (Biobank Japan), African American and Hispanic ancestries (latter two from All of Us), totaling 18,265 cases and 1,782,047 controls. Genomic inflation factors were calculated for each cohort and for the full meta-analysis. To assess any residual confounding due to population stratification, we calculated the LDSC intercept using LD scores calculated in the HapMap3 CEU population 32 . Genome-wide significance was set at P < 5 × 10 −8 .
Risk loci definition
To identify independent variants within each risk locus, LD clumping was performed using PLINK v1.9. We used a 1 Mb window (--clump-kb 1000) and an LD threshold (-- r 2 0.1) to identify independently significant SNPs. Using the independently significant SNPs, distinct genomic loci were defined by starting with the lowest P value variant, excluding other variants within ±1 Mb and iterating until no variants remained. The independently significant variant with the lowest P value that defined each genomic locus is termed the lead variant. Risk loci were defined as a ±1 Mb region around each lead variant. A risk locus was termed new if neither the lead variant nor any variant within 1 Mb had previously reached genome-wide significance for cirrhosis.
Endophenotype analyses
Both ALT and GGT levels are used clinically as biomarkers for liver injury. To increase statistical power for genomic discovery of cirrhosis, we used GWAS summary statistics for ALT and GGT as priors for association with cirrhosis. We first performed meta-analyses on both ALT and GGT summary data using previously published summary statistics 33 , 34 and data from CHB including more than 1 million individuals. We then tested independent variants that reached genome-wide significance for association with ALT or GGT in both the European-only and cross-ancestry cirrhosis meta-analysis. We considered associations significant if their FDR was <0.05.
To validate our findings, we performed replication of cirrhosis variants identified via cirrhosis GWAS and/or endophenotype-informed analysis using summary statistics of 21,689 cirrhosis cases and 617,729 disease-free controls from the MVP. Two variants (rs146650659 and rs113469203) were not available in the MVP, for which we selected suitable proxies ( r 2 ≥ 0.65). rs671 in ALDH2 was not amenable to validation, due to low frequency in non-East Asian populations. Cases were defined as in the primary GWAS analysis (Supplementary Table 1 ). A P < 1.4 × 10 −3 (0.05/35 variants) and consistent direction of effect were considered successful replication.
To gain insight into the potential underlying mechanisms by which the new risk loci contribute to disease, we tested the association between the 36 risk loci and 41 predefined metabolic and hepatobiliary traits using data from deCODE, UKB, FinnGen, Intermountain Healthcare, CHB-CID/DBDS and publicly available summary statistics, where available. The 36 variants were taken forward from the three main analyses (that is, the European-specific analysis, cross-ancestry meta-analysis and endophenotype-driven approach). In instances where risk loci were represented in multiple analyses, we selected the most significant variant (that is, the lowest P value). Binary traits were analyzed using logistic regression, and quantitative traits were inverse-rank normalized and analyzed using linear regression. The models were adjusted for age, sex and ten PCs. We considered associations significant if their FDR was <0.05.
Gene–environment interaction analyses
Environmental factors, such as alcohol consumption and BMI, are known risk factors for cirrhosis, and synergistic effects with genetic risk factors have previously been reported 4 , 11 . Here we systematically investigated for potential effect modification between risk loci and BMI, weekly alcohol intake and T2D in the UKB. BMI was measured at the baseline assessment visit and calculated as weight in kilograms divided by height in meters squared. Information on alcohol consumption was retrieved from questionnaire-based data on alcohol use. Participants who consumed alcohol at least once or twice per week were asked to provide information on their average weekly and monthly alcohol consumption across various alcoholic beverages (red wine, white wine, champagne, fortified wine, spirits and beer/cider). Based on data collected from individuals who consumed alcohol regularly, we calculated the average weekly alcohol intake in units. Information on T2D was retrieved from either from self-reported history of T2D or unspecified diabetes or HbA1c levels >48 mmol mol −1 measured at baseline. We examined a total of 35 variants (excluding the HLA variant) from the three main analyses for interaction with these factors. Potential interactions between the variants and environmental factors were evaluated using likelihood ratio tests, comparing the main-effects model (variant + environmental factor) with a model including an interaction term (variant × environmental factor). We set the significance threshold at P < 4.8 × 10 −4 (0.05/(35 variants × 3 traits)).
We conducted MR analyses on a set of biomarkers that had previously been identified as risk factors for cirrhosis, including BMI, lipids and alcohol intake 35 . To ensure that our analysis did not have overlapping samples, we conducted a meta-analysis on all available cirrhosis cohorts of European ancestry except for the UKB sample set, as all the exposure traits were derived from the UKB. We excluded exposure traits with fewer than ten instrumental variables (IVs) to avoid underpowered tests, resulting in 39 traits being tested. We evaluated instrument strength by calculating the F statistic 36 . To ensure a comparable LD structure between exposure and outcome datasets, only exposures derived from samples of European ancestry were taken forward. We selected independent variants with genome-wide significance ( P < 5 × 10 −8 ) and an r 2 < 0.001 to serve as IVs for our MR analyses using the clumping procedure in the TwoSampleMR software and LD estimates from the European samples from the 1000 Genomes Project. We used the following two different MR methods: IVW model as our primary model and the weighted median model as sensitivity analysis. MR–Egger intercept was used to test for pleiotropy. To test whether the results were driven by individual variants, we conducted leave-one-out analyses. Only associations that passed P < 1.2 × 10 −3 (0.05/39 traits) in the primary analyses (IVW), had a P < 0.05 in our sensitivity analyses (weighted median) and showed no evidence of pleiotropy (MR–Egger intercept P ≥ 0.05) were considered significant. Finally, we explored whether the genetic effects of BMI and alcohol were mediated by the effect of NAFLD, by using the ivw_mvmr() function in the MVMR package. Genetic effects on NAFLD were obtained from a meta-analysis comprising 9,491 cases 20 .
Heritability
We used LDSC v.1.0.0 to estimate the SNP heritability of cirrhosis in Europeans, East Asians, African Americans and Hispanics using ancestry-matched precomputed LD scores obtained at https://gnomad.broadinstitute.org/downloads/ . We reformatted association statistics to LDSC format with the munge tool, which excluded variants that did not match with the LD panel, had strand ambiguity, MAF < 0.01, INFO < 0.9 and variants that resided in long-range LD regions and the major histocompatibility locus on chromosome 6. To convert to liability scale, we used population-specific prevalence estimates, ranging from 0.5% in Europeans to 1.7% in East Asians 2 .
Gene mapping
We used six complementary approaches to annotate lead variants to potentially causal genes. First, we investigated whether the lead variants or proxy variants ( r 2 > 0.8) were annotated as loss-of-function or missense variants using Variant Effect Predictor (VEP) v.95 (ref. 37 ). Second, we used molecular QTLs to investigate the relationship between risk loci and potential downstream effects on gene expression (eQTL), alternative splicing (sQTL) and protein levels (pQTL). We investigated whether lead or proxy variants overlapped with top cis- eQTLs from the following two resources: adipose ( n = 750) and whole-blood eQTL ( n = 17,846) data from deCODE 13 and 54 tissues and cell lines from GTEx (v.8) 12 . Top cis- eQTLs were eQTLs with the strongest association with each gene within a 1 Mb window and a P < 1 × 10 −7 . We used colocalization analyses to detect shared causal variants between cirrhosis and gene expression using COLOC (v.3.2.1) R package 38 . We tested genes with significant cis -eQTL association by analyzing all variants that were located within a ±1-Mb window around the sentinel variant using eQTL and cirrhosis, ALT and GGT meta-analysis summary statistics. We set the prior probabilities to P 1 = 1 × 10 −4 , P 2 = 1 × 10 −4 and P 12 = 5 × 10 −6 , as suggested previously 39 . We report the posterior probability that the association with gene expression and cirrhosis risk is driven by a single causal variant. We consider a PPa ≥ 0.70 as supporting evidence for a causal role for the gene as a mediator of cirrhosis. Data on alternative RNA splicing were derived from whole-blood RNA-seq ( n = 17,846) data available at deCODE 20 . The strongest association for each splice junction with a P < 1 × 10 −8 was deemed top cis- sQTL. Data on protein levels were based on the following two datasets: (1) 4,907 proteins ( n = 35,559) measured using the SomaScan v.4 assay available at deCODE 14 and (2) 1,472 proteins ( n = 47,151) measured using the Olink Explore 1536 platform available at UKB 15 . Top cis -pQTLs were pQTLs that had the strongest association within a 1 Mb window. If the lead or proxy variants were in LD ( r 2 > 0.8) with either a top cis- eQTL, top cis- pQTL or top cis- sQTL, the two signals were considered overlapping. Third, we used the gene that was assigned the highest Variant-to-Gene (V2G) score provided by Open Targets Genetics ( https://genetics.opentargets.org/ ). The V2G score is an ensemble score that combines evidence on variant–gene associations from multiple sources, including molecular cis- QTL data (for example, pQTL and eQTL), interaction-based datasets (for example, promoter capture Hi-C) and genomic distance. For details on specific datasets and corresponding weights, please see https://genetics-docs.opentargets.org/our-approach/data-pipeline . Fourth, we used PoPS, a similarity-based gene-prioritization approach, which integrates GWAS summary statistics with gene-expression data, biological pathways and predicted protein–protein interaction data from more than 50,000 features 40 . We first computed gene-level association statistics and gene–gene correlations from our European-specific and cross-ancestry GWAS summary statistics using MAGMA 41 and LD estimates from 1000 Genomes European Ancestry data. Then, we conducted an enrichment analysis for gene features outlined at https://github.com/FinucaneLab/gene_features with MAGMA. Finally, we determined PoPS for each gene by fitting a joint model that considers the enrichment of all resulting features. Genes with a PoP score in the top 10% of the distribution were considered potential causal genes. We used the sum of the listed approaches and prioritized genes that had at least two lines of evidence. In the event of a tie-break, genes with coding variants in LD with the lead variant were given priority over V2G and/or PoPS.
While common variant associations enable the connection between a specific gene region and a disease, associations with rare coding variants can precisely identify causal genes and offer insights into the potential therapeutic effect and direction of targeting a gene or its product. To investigate the directional concordance between common and rare variants, we conducted rare variant analyses, studying the association between rare pLoF and missense variants in genes, supported by our gene-prioritization analyses, and ALT and cirrhosis, respectively. Details on calling and quality control have been described elsewhere 42 . We used SnpEff to annotate the variants and prioritized those with a minor allele frequency of <0.1%, which were predicted to cause loss-of-function, including stop-gain, frameshift, splice acceptor and splice donor variants and missense variants with a CADD score ≥20. We created the following two masks: pLoF only and pLoF + missense with CADD ≥20. To evaluate the associations between genotypes and outcomes, we used linear regression models for quantitative traits (ALT) and Firth-bias corrected logistic regression models for binary traits (cirrhosis) using REGENIE and individuals of European ancestry 43 . The models were adjusted for age, sex and ten PCs. We set the significance threshold at P < 6.9 × 10 −4 (0.05/(18 genes × 2 masks × 2 traits)).
PRS derivation
The following six PRS were generated to compare the predictive performance in detecting cirrhosis: a cross-ancestry cirrhosis PRS ( n = 1,325,517 (1.1% cases)), a European-only PRS ( n = 1,105,216 (1.1% cases)), an ALT PRS ( n = 257,869) and three weighted scores based on differing numbers of risk variants. Variants selected from the weighted scores were the 36 variants identified in both the cirrhosis GWAS and/or endophenotype-informed analysis, the 15 variants identified through cirrhosis GWAS and 5 known high-effect variants ( rs2642438 in MTARC1 , rs72613567 in HSD17B13 , rs28929474 in SERPINA1 , rs739846 in TM6SF2 and rs738408 in PNPLA3 ). Polygenic weights were calculated using PRS-CSx 44 . This method uses ancestry-specific GWAS weights, paired with LD information from an ancestry-matched external reference panel to estimate the posterior effect size for each SNP. Reference panels from the 1000 Genomes European, East Asian, Admixed American and African American samples were used. For the cross-ancestry PRS, ancestry-specific posterior effect sizes were meta-analyzed using the IVW method. For the genome-wide PRSs, we excluded the UKB dataset from the derivation datasets to ensure nonoverlapping samples. For the weighted scores, only effect estimates derived from meta-analysis excluding the UKB were used.
PRS evaluation
We evaluated the PRSs in the UKB. We first evaluated the proportion of variance explained ( r 2 ) by the six PRSs. We estimated the variance explained on the observed scale using Nagelkerke’s r 2 as the difference in r 2 between a full model (PRS + sex + age + ten PCs) and a null model (sex + age + ten PCs). Estimates were converted to the liability scale as per ref. 45 , assuming a population prevalence of 0.5% 2 . We then compared ORs per s.d. increase in PRS for each of the six PRSs using logistic regression, adjusted for age, sex and ten PCs. Finally, we added each of the six PRSs and compared the change in AUC (and 95% CI) to a baseline model comprising age, sex and the first ten PCs. The 95% CIs were computed using a stratified bootstrap with 1,000 replicates. AUCs were computed using the R package pROC 46 . The best-performing PRS (that is, the highest proportion of variance explained and change in AUC) was taken forward in downstream analyses described below.
PRS and disease progression
We evaluated whether the PRS could aid in identifying individuals who are more likely to progress from one hepatic disease state to another. We used the following two models to evaluate disease progression: (1) from NAFLD to cirrhosis and (2) from cirrhosis to HCC. For each model, we estimated 10-year risks using Fine-Gray regression, which accounts for the competing risk of death from all causes 47 . Time zero corresponded to the first occurrence of the exposure, and individual follow-up time ended in case of the event of interest, death or end of follow-up. The earliest start of follow-up began after the time of enrollment to prevent immortal time bias. The PRS was evaluated in the UKB and validated in both CHB (NAFLD to cirrhosis and cirrhosis to HCC) and MVP (NAFLD to cirrhosis). To avoid overfitting, effects were derived from a meta-analysis that did not include the test dataset.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
GWAS meta-analysis summary statistics are available at the GWAS Catalog ( https://www.ebi.ac.uk/gwas/ ) (GCST90319877 and GCST90319878). The cirrhosis PRS are available at the PGS Catalog ( https://www.pgscatalog.org/ ; PGS004621). Data from the UKB samples are available through UKB ( https://www.ukbiobank.ac.uk/ ). FinnGen GWAS summary statistics are publicly accessible following registration ( https://www.finngen.fi/en/access_results ). German/UK cirrhosis cohort can be accessed at http://gengastro.med.tu-dresden.de/suppl/alc_cirrhosis/ . Summary statistics from Biobank Japan are available at https://pheweb.jp/ . Other individual summary statistics will be made available upon request to study PIs (AllOfUs: S.V., CHB-CID/DBDS: J.G./S.S., deCODE and Intermountain Healthcare: G.S./H.H., Estonian Biobank: T.L.). The GTEx v.8 eQTL data used in this study are available in the GTEx Portal ( https://gtexportal.org/home/datasets ).
Code availability
The following software and packages were used for data analysis: PLINK 2.0 ( https://www.cog-genomics.org/plink/2.0/ ), METAL v.2011-03-25 ( http://csg.sph.umich.edu/abecasis/Metal/download/ ), MAGMA v.1.07 ( https://ctg.cncr.nl/software/magma ), EasyQC v.9.2 ( https://www.uni-regensburg.de/medizin/epidemiologie-praeventivmedizin/genetische-epidemiologie/software/ ), LDSC v.1.0.1 ( https://github.com/bulik/ldsc ), PoPS v.0.1 ( https://github.com/FinucaneLab/pops/tree/add-license-1 ), PRS-CS v.2021-06-04 ( https://github.com/getian107/PRScs/ ), REGENIE v.2.0.1 ( https://rgcgithub.github.io/regenie/ ), TwoSampleMR v.0.5.6 ( https://mrcieu.github.io/TwoSampleMR/ ), MVMR 0.4 ( https://github.com/WSpiller/MVMR ), pROC 1.18.4 ( https://cran.r-project.org/web/packages/pROC/index.html ) and R v.4.1.2 ( https://www.r-project.org/ ).
Ginès, P. et al. Liver cirrhosis. Lancet Lond. Engl. 398 , 1359–1376 (2021).
Article Google Scholar
Huang, D. Q. et al. Global epidemiology of cirrhosis—aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 20 , 388–398 (2023).
Article PubMed PubMed Central Google Scholar
Loomba, R. et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology 149 , 1784–1793 (2015).
Article PubMed Google Scholar
Emdin, C. A. et al. Association of genetic variation with cirrhosis: a multi-trait genome-wide association and gene–environment interaction study. Gastroenterology 160 , 1620–1633 (2021).
Article CAS PubMed Google Scholar
Gellert-Kristensen, H. et al. Combined effect of PNPLA3, TM6SF2, and HSD17B13 variants on risk of cirrhosis and hepatocellular carcinoma in the general population. Hepatology 72 , 845–856 (2020).
Abul-Husn, N. S. et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N. Engl. J. Med. 378 , 1096–1106 (2018).
Article CAS PubMed PubMed Central Google Scholar
Emdin, C. A. et al. A missense variant in mitochondrial amidoxime reducing component 1 gene and protection against liver disease. PLoS Genet. 16 , e1008629 (2020).
Schneider, C. V. et al. A genome-first approach to mortality and metabolic phenotypes in MTARC1 p.Ala165Thr (rs2642438) heterozygotes and homozygotes. Med 2 , 851–863 (2021).
Verweij, N. et al. Germline mutations in CIDEB and protection against liver disease. N. Engl. J. Med. 387 , 332–344 (2022).
Buch, S. et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 47 , 1443–1448 (2015).
Stender, S. et al. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat. Genet. 49 , 842–847 (2017).
GTEX ConsortiumThe GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369 , 1318–1330 (2020).
Mikaelsdottir, E. et al. Genetic variants associated with platelet count are predictive of human disease and physiological markers. Commun. Biol. 4 , 1–13 (2021).
Ferkingstad, E. et al. Large-scale integration of the plasma proteome with genetics and disease. Nat. Genet. 53 , 1712–1721 (2021).
Sun, B. B. et al. Plasma proteomic associations with genetics and health in the UK Biobank. Nature 622 , 329–338 (2023).
Akbari, P. et al. Multiancestry exome sequencing reveals INHBE mutations associated with favorable fat distribution and protection from diabetes. Nat. Commun. 13 , 4844 (2022).
Lotta, L. A. et al. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA 320 , 2553–2563 (2018).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28 , 166–174 (2019).
Cho, Y. et al. Genetic influences on alcohol flushing in East Asian population. BMC Genomics 24 , 638 (2023).
Sveinbjornsson, G. et al. Multiomics study of nonalcoholic fatty liver disease. Nat. Genet. 54 , 1652–1663 (2022).
Jamialahmadi, O. et al. Exome-wide association study on alanine aminotransferase identifies sequence variants in the GPAM and APOE associated with fatty liver disease. Gastroenterology 160 , 1634–1646 (2021).
Hakim, A. et al. Genetic variation in the mitochondrial glycerol-3-phosphate acyltransferase is associated with liver injury. Hepatology 74 , 3394–3408 (2021).
Vujkovic, M. et al. A multiancestry genome-wide association study of unexplained chronic ALT elevation as a proxy for nonalcoholic fatty liver disease with histological and radiological validation. Nat. Genet. 54 , 761–771 (2022).
Koyama, S. et al. Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat. Genet. 52 , 1169–1177 (2020).
Márquez-Luna, C., Loh, P.-R. & Price, A. L. South Asian Type 2 Diabetes (SAT2D) Consortium, SIGMA Type 2 Diabetes Consortium Multiethnic polygenic risk scores improve risk prediction in diverse populations. Genet. Epidemiol. 41 , 811–823 (2017).
Kurniansyah, N. et al. A multi-ethnic polygenic risk score is associated with hypertension prevalence and progression throughout adulthood. Nat. Commun. 13 , 3549 (2022).
Ge, T. et al. Development and validation of a trans-ancestry polygenic risk score for type 2 diabetes in diverse populations. Genome Med. 14 , 70 (2022).
Kamatani, Y. et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat. Genet. 41 , 591–595 (2009).
Westerman, K. E. et al. Variance-quantitative trait loci enable systematic discovery of gene–environment interactions for cardiometabolic serum biomarkers. Nat. Commun. 13 , 3993 (2022).
Lyon, M. S., Millard, L. A. C., Smith, G. D., Gaunt, T. R. & Tilling, K. Hypothesis-free detection of gene-interaction effects on biomarker concentration in UK Biobank using variance prioritisation. Preprint at medRxiv https://doi.org/10.1101/2022.01.05.21268406 (2022).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26 , 2190–2191 (2010).
Bulik-Sullivan, B. K. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47 , 291–295 (2015).
Nielsen, J. B. et al. Loss-of-function genomic variants highlight potential therapeutic targets for cardiovascular disease. Nat. Commun. 11 , 6417 (2020).
Pazoki, R. et al. Genetic analysis in European ancestry individuals identifies 517 loci associated with liver enzymes. Nat. Commun. 12 , 2579 (2021).
El-Serag, H. B. et al. Risk factors for cirrhosis in contemporary hepatology practices—findings from Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology 159 , 376–377 (2020).
Davies, N. M., Holmes, M. V. & Smith, G. D. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362 , k601 (2018).
McLaren, W. et al. The ensembl variant effect predictor. Genome Biol. 17 , 122 (2016).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10 , e1004383 (2014).
Wallace, C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet. 16 , e1008720 (2020).
Weeks, E. M. et al. Leveraging polygenic enrichments of gene features to predict genes underlying complex traits and diseases. Nat. Genet. 55 , 1267–1276 (2023).
De Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11 , e1004219 (2015).
Backman, J. D. et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature 599 , 628–634 (2021).
Mbatchou, J. et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat. Genet. 53 , 1097–1103 (2021).
Ge, T., Chen, C.-Y., Ni, Y., Feng, Y.-C. A. & Smoller, J. W. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat. Commun. 10 , 1776 (2019).
Lee, S. H., Goddard, M. E., Wray, N. R. & Visscher, P. M. A better coefficient of determination for genetic profile analysis. Genet. Epidemiol. 36 , 214–224 (2012).
Robin, X. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12 , 77 (2011).
Aalen, O. O. & Johansen, S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand. J. Stat. 5 , 141–150 (1978).
Google Scholar
Download references
Acknowledgements
This research has been conducted using the UKB resource under application 43247. This work was supported by BRIDGE—Translational Excellence Program (NNF20SA0064340 to J.G.), Beckett Fonden (23-2-10636 to J.G.), Independent Research Fund Denmark (Sapere Aude Research Leader, 9060-00012B to S.S.), Borregaard Clinical Ascending Investigator (NNF22OC0075038 to S.S.), Hallas-Møller Emerging Investigator (Novo Nordisk Foundation; NNF17OC0031204 to M.S.O.), The John and Birthe Meyer Foundation (to M.S.O.), The Innovation Fund Denmark (PM Heart to H.B.), NordForsk (to H.B.), Villadsen Family Foundation (to H.B.) and The Arvid Nilsson Foundation. Research in the MVP, funded by the Office of Research and Development, US Veterans Health Administration was supported by award MVP000 and additional funding from the Department of Veterans Affairs (award I01 BX003362 to K.-M.C.). This publication does not represent the views of the Department of Veterans Affairs, the US Food and Drug Administration or the US Government. Novo Nordisk Foundation (grants NNF17OC0027594 and NNF14CC0001 to K.B. and S.B.). The All of Us is supported by the National Institutes of Health, Office of the Director—Regional Medical Centers (1 OT2 OD026549, 1 OT2 OD026554, 1 OT2 OD026557, 1 OT2 OD026556, 1 OT2 OD026550, 1 OT2 OD 026552, 1 OT2 OD026553, 1 OT2 OD026548, 1 OT2 OD026551 and 1 OT2 OD026555), IAA (AOD 16037), Federally Qualified Health Centers (HHSN 263201600085U), Data and Research Center (5 U2C OD023196), Biobank (1 U24 OD023121), The Participant Center (U24 OD023176), Participant Technology Systems Center (1 U24 OD023163), Communications and Engagement (3 OT2 OD023205 and 3 OT2 OD023206) and Community Partners (1 OT2 OD025277, 3 OT2 OD025315, 1 OT2 OD025337 and 1 OT2 OD025276). In addition, the All of Us Research Program would not be possible without the partnership of its participants. The research was also supported by NIH HHS under grant 5T32GM136651-03 (to J.B.). This work was also supported by the NIH/NIDDK (K08 DK113109 and R01 DK131033-01A1 to S.V., and R01 DK134575 and I01 BX003362 to M.V.) and the Doris Duke Charitable Foundation (grant 2019081 to S.V.). The Estonian Biobank was funded by the European Union through the European Regional Development Fund (project 2014-2020.4.01.15-0012 GENTRANSMED) and by the Estonian Research Council (grant PRG1911). Computations were performed in the High-Performance Computing Center, University of Tartu. JK and Dallas Liver cohort are supported by NIH/NIDDK (DK090066). Finally, we would like to acknowledge the participants and investigators of the FinnGen study.
Author information
These authors jointly supervised this work: Henning Bundgaard, Stefan Stender.
Authors and Affiliations
Department of Cardiology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark
Jonas Ghouse, Søren A. Rand, Morten Salling Olesen & Henning Bundgaard
Cardiac Genetics Group, Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark
Jonas Ghouse, Gustav Ahlberg, Søren A. Rand, Pia Rengtved Lundegaard & Morten Salling Olesen
deCODE Genetics/Amgen, Reykjavik, Iceland
Gardar Sveinbjörnsson, Vinicius Tragante, Egil Ferkingstad, Magnus O. Ulfarsson, Árni Sturluson, Patrick Sulem, Daniel F. Gudbjartsson, Kari Stefansson & Hilma Holm
Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA, USA
Marijana Vujkovic, David E. Kaplan & Kyong-Mi Chang
Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA
Department of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
- Marijana Vujkovic
Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark
Anne-Sofie Seidelin, Helene Gellert-Kristensen, Anne Tybjærg-Hansen & Stefan Stender
Section of Digestive Diseases, Department of Internal Medicine, and Department of Pathology, Yale School of Medicine, New Haven, CT, USA
Joseph Brancale & Silvia Vilarinho
Department of Clinical Immunology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark
Erik Sørensen & Sisse R. Ostrowski
Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark
Christian Erikstrup
Department of Clinical Immunology, Odense University Hospital, Odense, Denmark
Mie Topholm Bruun
Department of Clinical Immunology, Aalborg University Hospital, Aalborg, Denmark
Bitten Aagaard Jensen
Translational Disease Systems Biology, Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Søren Brunak
Department of Obstetrics and Gynaecology, Copenhagen University Hospital Hvidovre, Copenhagen, Denmark
Karina Banasik
Statens Serum Institut, Copenhagen, Denmark
Henrik Ullum
Regeneron Genetics Center, Regeneron Pharmaceuticals Inc, Tarrytown, NY, USA
Niek Verweij, Luca Lotta, Aris Baras & Luca A. Lotta
Department of Molecular and Functional Genomics, Geisinger Health System, Danville, PA, USA
Tooraj Mirshahi & David J. Carey
VA Informatics and Computing Infrastructure (VINCI), VA Salt Lake City Health Care System, Salt Lake City, UT, USA
Julie Lynch
Division of Epidemiology, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, UT, USA
Gastroenterology Section, Veterans Affairs Long Beach Healthcare System, Long Beach, CA, USA
Timothy Morgan & Tae-Hwi Schwantes-An
Department of Medicine, University of California, Irvine, CA, USA
Timothy Morgan
Department of Medical and Molecular Genetics, Indiana University, Indianapolis, IN, USA
Tae-Hwi Schwantes-An
Center for Data and Computational Sciences, VA Boston Healthcare System, Boston, MA, USA
Daniel R. Dochtermann & Saiju Pyarajan
Department of Medicine, Brigham Women’s Hospital and Harvard Medical School, Boston, MA, USA
Saiju Pyarajan
Palo Alto Epidemiology Research and Information Center for Genomics, VA Palo Alto, Palo Alto, CA, USA
Philip S. Tsao
Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA
Estonian Genome Centre, Institute of Genomics, University of Tartu, Tartu, Estonia
Triin Laisk & Reedik Mägi
Eugene McDermott Center for Human Growth and Development, University of Texas Southwestern Medical Center, Dallas, TX, USA
Julia Kozlitina
Precision Genomics, Intermountain Healthcare, Saint George, UT, USA
David Jones & Lincoln Nadauld
Intermountain Medical Center, Intermountain Heart Institute, Salt Lake City, UT, USA
Kirk U. Knowlton
University of Utah, School of Medicine, Salt Lake City, UT, USA
Stanford University, School of Medicine, Stanford, CA, USA
Lincoln Nadauld
Faculty of Medicine, University of Iceland, Reykjavik, Iceland
Einar S. Björnsson
Internal Medicine and Emergency Services, Landspitali—The National University Hospital of Iceland, Reykjavik, Iceland
Faculty of Electrical and Computer Engineering, University of Iceland, Reykjavik, Iceland
Magnus O. Ulfarsson
Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
Ole B. Pedersen, Sisse R. Ostrowski, Henning Bundgaard & Stefan Stender
Department of Clinical Immunology, Zealand University Hospital, Køge, Denmark
Ole B. Pedersen
School of Engineering and Natural Sciences, University of Iceland, Reykjavik, Iceland
Daniel F. Gudbjartsson
You can also search for this author in PubMed Google Scholar
DBDS Genomic Consortium
- Kari Stefansson
- , Erik Sørensen
- , Christian Erikstrup
- , Mie Topholm Bruun
- , Bitten Aagaard Jensen
- , Søren Brunak
- , Karina Banasik
- , Henrik Ullum
- , Ole B. Pedersen
- , Sisse R. Ostrowski
- & Daniel F. Gudbjartsson
Regeneron Genetics Center
- Luca A. Lotta
- , Aris Baras
- & Niek Verweij
Geisinger-Regeneron DiscovEHR Collaboration
- Tooraj Mirshahi
- & David J. Carey
VA Million Veteran Program
- , David E. Kaplan
- , Julie Lynch
- , Timothy Morgan
- , Tae-Hwi Schwantes-An
- , Daniel R. Dochtermann
- , Saiju Pyarajan
- , Philip S. Tsao
- & Kyong-Mi Chang
Estonian Biobank Research Team
- Triin Laisk
- & Reedik Mägi
Contributions
J.G., G.S., H.H., H.B. and S.S. conceived the study. J.G., G.S., M.V., G.A., H.G.K., J.B., T.L., E.F., D.F.G. and S.S. performed analyses in the respective cohorts. H.H., S.V., T.L., K.-M.C., H.B. and S.S. supervised analyses in their respective cohorts. J.G., G.S., G.A., H.H., H.B. and S.S. contributed to writing the manuscript. J.G. performed meta-analysis and created figures and tables. J.G., G.S. and S.S. performed downstream analyses and drafted the manuscript. J.G., G.A., M.V., A.S., H.G.K., G.A., V.T., S.A.R., J.B., S.V., P.R.L., E.S., C.E., M.T.B., B.A.J., S.B., K.B., H.U., T.L., R.M., J.K., A.T.H., D.J., K.U.K., L.N., E.F., E.S.B., M.U., A.S., P.S., O.B.P., S.R.O., D.F.G., K.S., M.S.O., K.-M.C., H.H., H.B. and S.S. interpreted the results, reviewed and commented on the manuscript.
Corresponding authors
Correspondence to Jonas Ghouse or Stefan Stender .
Ethics declarations
Competing interests.
The authors who are affiliated with deCODE genetics/Amgen declare competing financial interests as employees. H.B. receives lecture fees from Bristol-Myers Squibb, Merck Sharp and Dohme. J.G. has received lecture fee from Illumina. S.B. is a board member for Proscion A/S and Intomics A/S. N.V., L.L. and A.B. are employees at Regeneron Genetics Center. All other authors have no conflict of interest to declare.
Peer review
Peer review information.
Nature Genetics thanks Yoichiro Kamatani, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
Supplementary Note, Supplementary Figs. 1–6 and Consortia banners.
Reporting Summary
Peer review file, supplementary tables.
Supplementary Tables 1–24.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ghouse, J., Sveinbjörnsson, G., Vujkovic, M. et al. Integrative common and rare variant analyses provide insights into the genetic architecture of liver cirrhosis. Nat Genet (2024). https://doi.org/10.1038/s41588-024-01720-y
Download citation
Received : 23 June 2023
Accepted : 18 March 2024
Published : 17 April 2024
DOI : https://doi.org/10.1038/s41588-024-01720-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Case Study of Patient with Liver Cirrhosis

Liver cirrhosis is a disease in which normal tissue of liver replaced with scar tissue, liver cirrhosis is the 12 th leading cause of deaths by disease in the world.Liver cirrhosis is caused by any factor that can damage liver tissues, mostly fatty liver and chronic liver diseases are the major cause of liver cirrhosis. We are presenting here the case of an Asian man who was the victim of liver cirrhosis that was complicated by untreated hepatitis c.He was experiencing generalized body weakness, brownish tint in the urine, and sudden weight gain of 8-kgs within a period of three weeks. Blood pressure count was100/60, pulse rate found to be 76 beats/min, temperature 98F, and enlarged umbilical.Laboratory tests including complete blood count, liver function tests, and urea tests came out to be significantly abnormal, complicating the case., ultra sound report revealed that his liver was enlarged, urinary bladder partially filled, umbilical hernia gape reported, ascites present (retent...
Related Papers
Journal of Pharmaceutical Research International
Jaya Khandar
Liver is the second largest organ in human body, more than 5,000 separate bodily functions .including helping blood to clot, cleansing the blood of toxins to converting food into nutrients to control hormone levels, fighting infections and illness, regenerating back after injury and metabolizing cholesterol, glucose, iron and controlling their levels. A 56- years old patient was admitted in AVBRH on date 9/12/2020 in ICU with the chief complaint of abdominal distension, breathlessness on exertion, pedal edema, fever since 8 days. After admitted in hospital all investigation was done including blood test, ECG, fluid cytology, peripheral smear, ultrasonography, etc. All investigation conducted and then final diagnosis confirmed as cirrhosis of liver. Patient was not having any history of communicable disease or any hereditary disease but he has history of hypertension and type II Diabetes mellitus for 12 years. Patient was COVID-19 negative and admitted in intensive care unit. Patient...
Background: Liver cirrhosis is one among most common causes of death throughout the world. Pakistan is known as cirrhotic state with high number of cirrhosis anywhere in the world. Aims: The objectives of this study were to assess risk factors, clinical features and complications of liver cirrhosis. Method: This study was done at medical wards of Khyber Teaching Hospital and Lady Reading Hospital Peshawar. Data was collected from 74 patients with cirrhosis through structured questionnaire. Results revealed that in our results showed that Prevalence of liver cirrhosis is high in age group > 45 years(68.9%) and in male patients(79.7%), low in intermediate age group (29.7%) and infrequent in young age group (1.4%). Out of 74 cases 38 were attributed to HCV only, 12 to HBV and HCV co-infection, 7 to HBV only, 6 to HBV and Alcohol and HCV and Alcohol each, 3 to HBV, HCV and Alcohol acting conjointly and 1 to Fatty liver disease. For 1 patient the cause was unknown. More than 75% hepatitis (B and C) was associated with clinical mal-practices. Most frequent signs and symptoms in decreasing order were anorexia and weight loss (100%), jaundice (90.5 %), abdominal disturbances and spleenomegaly (75%), bleeding tendencies, decreasing mental function, itching and palmer erythema (<20%). Complications were ascites (97.3%), peripheral oedema(73%), recurrent infections (43.2), hepatic encephalopathy (28%), GIT bleeding (4.1%) and hepatocellular carcinoma (1.4%). Serum ALT and total Bilirubin were raised in 80 % and 90.7% patients respectively. Conclusion: Hepatitis C is the most frequent cause of liver cirrhosis mostly acquired through prick by contaminated syringes or blades or through blood transfusion.
The Journal of medical research
Amrendra Yadav
The liver is the multi-functional and vital organ of the body. It is found in the upper abdomen region of the vertebrates. Due to long-term damage, liver stops functioning properly which may lead to cirrhosis. This long-term damage occurred when scar tissue replaces the normal tissue of the liver. This disease develops slowly and has no early symptoms, but when it develops and become worse, then it leads to tiredness, itchiness, weakness, yellow skin, swelling in the lower legs, spider-like blood vessels and an easy bruise on the skin with fluid in the abdomen. The severe complications like bleeding dilated veins in esophagus or stomach, hepatic encephalopathy leading to confusion and unconsciousness and liver cancer may occur in the body. This review article is focusing on the effect of liver damage in the
Faridpur Medical College Journal
Shahin Ul Islam
Liver cirrhosis is an important cause of death and disability globally. This cross-sectional study was carried out in Faridpur Medical College Hospital from November 2018 to April 2019 to see the patterns of clinical presentations and associated factors among admitted liver cirrhosis patients. A total of 89 patients were included. Data were collected by detailed history from patients or their relatives followed by thorough physical examination as well as diagnostic evaluation; then those were checked, verified for consistency and edited for result. Among total respondents, the majority were male (69.7%) with a male to female ratio of 1:0.44. Age of patients ranged between 22-106 years with mean age of 52.33 year. The patients predominantly presented with ascites (49.4%), gastrointestinal bleeding (27%), peripheral edema (24.7%), and encephalopathy (21.3%). The in-hospital case fatality rate was 11.2% and the patients presented with decreased urinary output, peripheral edema and ence...
Nepal Medical College journal : NMCJ
robin maskey
One hundred and five (72 males; 33 females) consecutive patients who met the inclusion criteria were studied. The mean age of the patients was 49.06 +/- 11.27 years (range 23-73 years). Ninety patients were adult cirrhotics (age > or = 35 yrs) and the remaining 15 patients were young (age < or = 35 yrs). Ninety out of 105 patients were having alcohol related cirrhosis. The commonest presenting symptoms were abdomen distension (100% in young cirrhotics vs. 84.4% in adult cirrhotics) and jaundice (93.3% in young cirrhotics vs. 84.4% in adult cirrhotics). The most common presenting signs were ascites (100% in young cirrhotics vs. 84.4% in adult cirrhotics) and icterus (93.3% in young cirrhotics vs. 84.4% in adult cirrhotics), followed by loss of body hair (73.3% vs. 71.1% in young and adult cirrhotics respectively) and spider naevi (46.7% vs. 61.1% in young and adult cirrhotics respectively). Sixty percent of young cirrhotics and 52% of adult cirrhotics were in Child's grade ...
Asian Australasian Neuro and Health Science Journal (AANHS-J)
Hanun Mahyuddin
Liver cirrhosis is a chronic disease characterized by the presence of fibrosis and regeneration of nodules in the liver, the consequence of which is the development of portal hypertension and liver failure. Usually associated with infectious infectious diseases such as viral hepatitis, alcohol consumption, metabolic syndrome, autoimmune processes, storage diseases, toxic substances and drugs. Major complications include gastrointestinal variceal bleeding, ascites, spontaneous bacterial peritonitis infection, hepatorenal syndrome, hepatic encephalopathy, and hepatocellular carcinoma. A 23-year-old woman comes to the ER, dr. Soegiri Lamongan with complaints of vomiting blood. The patient also complained of black bloody stools. Referred patient from Intan Medika Hospital with the initial complaint of vomiting blood more than 5 times (± equivalent to one medium drinking bottle) four days ago. On examination also found anemic conjunctiva and found splenomegaly. On abdominal ultrasound ex...
Fortune Journals , Umair Akram
Cirrhosis results from chronic liver disease, and is characterized by advanced fibrosis, scarring, and formation of regenerative nodules leading to architectural distortion. The main objective of the study is to find the cirrhosis and its complication, other clinical complications except ACLF and critical illness. This descriptive study was conducted at DHQ hospital, Vehari, Pakistan during June 2022 to January 2023. A comprehensive literature search of the published data was performed in regard with the spectrum, diagnosis, and management of cirrhosis and its complications. Data was also collected from OPD of the hospital record. We include all patients suffering from liver cirrhosis and its complication. It is concluded that patients with cirrhosis have progressive disease and suffer from multiple complications like ascites, HE, variceal bleeding, hepatorenal syndrome, cirrhotic cardiomyopathy, pulmonary syndromes, sarcopenia, frailty, and HCC. The prevention, early diagnosis, treatment, and palliation of these complications are essential in comprehensive clinical care plans.
The Professional Medical Journal
Zafar Majeed
Cirrhosis once established has no treatment except liver transplantation. It is mostly caused by chronic hepatitis C virus which is very common in our region. As many of these hepatitis C patients are asymptomatic or have non-specific symptoms, so these patients seek medical advice late in the course of their disease. Objectives: To find out the presence of cirrhosis in newly diagnosed hepatitis C patients. Study Design: Descriptive Study. Setting: Hepatitis Clinic, Sheikh Zayed Medical College/Hospital, Rahim Yar Khan. Period: From March to September 2018. Material & Methods: All newly diagnosed hepatitis C patients previously not known to have chronic liver disease were included in the study. Their demographics, symptoms, baseline CBC, LFT, PT, albumin and ultrasound findings were noted. Cirrhosis was diagnosed on the basis of clinical, laboratory and ultrasonography findings. Results: Three hundred and seventy three patients met inclusion criteria. Cirrhosis was present in 64 (17...
Journal of Antivirals & Antiretrovirals
Muhammad Zeeshan Zafar
Journal of Clinical and Experimental Hepatology
Dr. Anil Chandra Anand
RELATED PAPERS
kaleff machicado
ACTA DE INVESTIGACIÓN PSICOLÓGICA. VOL. 9 NÚMERO 1 · ABRIL 2019
ALBA ELISBETH MUSTACA
Prathibha Rohit
RUDN Journal of Informatization in Education
Elena Dudysheva
Antonio Uriarte
Micromachines
Danish Rehman
Journal of Nursing Ufpe Online
Flávia Ramos
Prof.Dr. Haider Al-Moosawi
Arief Budhyantoro
eva mirza syafitri
Cahiers d'économie Politique
Véronique Dutraive
Alexander Ryabchikov
Acta Médica Grupo Ángeles
HORACIO ZALCE
Sergi Maicas
Journal of Nutrition Education and Behavior
Banafsheh Sadeghi
RiMe - Rivista dell'Istituto di Storia dell'Europa Mediterranea
Enrico Basso
Arab Gulf Journal of Scientific Research
Prof. Adel I . Al-Alawi
Canadian Geotechnical Journal
Roman Hryciw
Flavoprotein Protocols
Sandro Ghisla
Études internationales
Gérard Hervouet
Complementary and Alternative Medicine: Effect of the Consciousness Energy Healing Treatment on Physicochemical Behaviour of Silver Sulfadiazine
Alice Branton , Mahendra Kumar Trivedi , Dahryn Trivedi
Andras Turke
MARIA DE LOS ANGELES ALVAREZ SIERRA
Annalisa Bianchera
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Open access
- Published: 26 September 2022
Critical care hepatology: definitions, incidence, prognosis and role of liver failure in critically ill patients
- Aritz Perez Ruiz de Garibay 1 nAff4 ,
- Andreas Kortgen 2 ,
- Julia Leonhardt 2 ,
- Alexander Zipprich 3 &
- Michael Bauer 2
Critical Care volume 26 , Article number: 289 ( 2022 ) Cite this article
19k Accesses
19 Citations
163 Altmetric
Metrics details
Organ dysfunction or overt failure is a commonplace event in the critically ill affecting up to 70% of patients during their stay in the ICU. The outcome depends on the resolution of impaired organ function, while a domino-like deterioration of organs other than the primarily affected ones paves the way for increased mortality. “Acute Liver Failure” was defined in the 1970s as a rare and potentially reversible severe liver injury in the absence of prior liver disease with hepatic encephalopathy occurring within 8 weeks. Dysfunction of the liver in general reflects a critical event in “Multiple Organ Dysfunction Syndrome” due to immunologic, regulatory and metabolic functions of liver parenchymal and non-parenchymal cells. Dysregulation of the inflammatory response, persistent microcirculatory (hypoxic) impairment or drug-induced liver injury are leading problems that result in “secondary liver failure,” i.e., acquired liver injury without underlying liver disease or deterioration of preexisting (chronic) liver disease (“Acute-on-Chronic Liver Failure”). Conventional laboratory markers, such as transaminases or bilirubin, are limited to provide insight into the complex facets of metabolic and immunologic liver dysfunction. Furthermore, inhomogeneous definitions of these entities lead to widely ranging estimates of incidence. In the present work, we review the different definitions to improve the understanding of liver dysfunction as a perpetrator (and therapeutic target) of multiple organ dysfunction syndrome in critical care.
Graphic Abstract
Introduction
The definition of liver dysfunction is far from a global consensus. Typically, liver failure is divided into two major entities depending on the presence or absence of preexisting liver disease. Acute liver failure (ALF) is rare, occurs in the absence of previous liver damage, has a clear etiology and is classified according to the interval between the appearance of jaundice and the onset of hepatic encephalopathy into acute, subacute and hyperacute processes [ 1 , 2 , 3 ]. ALF is managed according to guidelines, which account for transplant needs [ 1 , 4 ]. In contrast, acute-on-chronic liver failure (ACLF) is triggered by acute hepatic decompensation in patients with preexisting chronic disease [ 5 ]. ACLF definitions vary depending on the issuing consortium [ 6 , 7 , 8 ]. Apart from ALF and ACLF, secondary liver injury without underlying liver disease in response to hypoxic, toxic or inflammatory insults represents the most common form of hepatic dysfunction in the intensive care unit (ICU), and it commonly manifests as cholestasis and hypoxic liver injury [ 9 , 10 ].
In ALF, the liver is triggering clinical deterioration, i.e., extrahepatic organ dysfunction develops due to impaired liver function. The situation in ACLF is more complex: A previous chronic liver disease worsens to liver failure by primary hepatic deterioration [alcoholic hepatitis, viral hepatitis, drug-induced liver injury (DILI)] or by secondary hepatic deterioration due to extrahepatic events (for example, sepsis). Whether alterations are classified as “dysfunction,” “injury” or “failure” depends on the surrogate marker and the score used. For instance, when applying bilirubin as a common marker, the definition of acute liver injury (ALI) by Koch et al. uses a value higher than 3 mg/dl (51.3 µmol/l) to define injury [ 11 ]. The Sequential Organ Failure Assessment (SOFA) Score divides dysfunction and failure using thresholds of 1.2 mg/dl (20.5 µmol/l) and 6.0 mg/dl (102.6 µmol/l) [ 12 ], respectively. Levels above 2 mg/dl are otherwise frequently used as pragmatic cutoffs to assess jaundice and cholestasis [ 6 , 9 ].
Experimental data on immunologic, regulatory and metabolic functions of the liver suggest a role of the liver as a “perpetrator” rather than a “victim” of “host response failure” and multiple organ failure [ 13 , 14 , 15 ]. In any case, liver dysfunction and failure are clearly of utmost importance in the ICU as they affect at least 20% of patients and significantly increase the risk of death [ 16 , 17 ].
The following overview will discuss ALF, ACLF and secondary liver failure. A thorough review and description of the pathophysiology and management can be found elsewhere [ 3 , 9 , 18 , 19 , 20 ].
Acute liver failure (ALF)
Acute liver failure was first defined in 1970 to describe a rare and potentially reversible critical illness caused by “severe liver injury in the absence of prior liver disease with hepatic encephalopathy occurring within 8 weeks from the appearance of first symptoms” [ 21 ]. In general, the duration of the symptoms should not be longer than 26 weeks to be considered ALF [ 22 ].
Since the first definition of ALF, several authors have classified ALF according to the interval between the appearance of jaundice and the onset of hepatic encephalopathy (HE) (Table 1 ), e.g., defining “fulminant” for appearance of hepatic encephalopathy in the first 2 weeks and “subfulminant” when occurring until week 12 [ 23 ]. O´Grady employed the terms “hyperacute,” “acute” and “subacute” for an onset of hepatic encephalopathy within 1, 4 or between 5 and 12 weeks, respectively [ 2 ]. The Japanese consensus classifies ALF into “acute liver failure without hepatic coma” (HE < Grade II) and “acute liver failure with hepatic coma” (HE ≥ Grade II). The latter distinguishes between the “acute type” and “subacute type,” with hepatic encephalopathy developing within 10 days or between 11 and 56 days, respectively [ 24 ]. If hepatic encephalopathy occurs later, the disease is known as late-onset hepatic failure (LOHF) [ 4 ]. All these classifications can even be further complicated if the etiology of ALF [ 22 , 25 ] is taken into consideration, which can lead to lack of comparability of clinical trials [ 26 ].
Incidence and mortality
Estimates based on data from transplant units from the European Union suggest that 8% of liver transplants (LTx) are due to ALF, either caused by viral infection (19%), DILI (18%), toxic insults (4%), traumatic events (3%) or unknown causes (56%) [ 27 ]. However, a trend toward an increase in DILI and a decrease in viral etiologies has been documented [ 1 , 28 , 29 ]. Overall, ALF occurs in less than 10 cases per million inhabitants per year [ 3 ]. In-hospital mortality remains high with rates between 23 and 53% [ 9 ].
Prognostic clinical features
Wlodzimirow et al. identified and characterized different prognostic models for mortality in ALF patients [ 30 ]. The variables more commonly included in the final model of the studies investigated were hepatic encephalopathy (45%), prothrombin time (45%), bilirubin (40%), age (40%) and creatinine (25%) [ 30 ]. A recent study revealed that M30, a cleavage product of cytokeratin-18 caspase, most accurately identified patients who would require LTx or die [ 31 ].
Hepatic encephalopathy HE remains the essential clinical hallmark, and its presence, even at low grade, is indicative of poor prognosis [ 1 ]. HE is primarily a clinical diagnosis. The joint EASL/AASLD guidelines suggest that if ammonia levels are normal, the diagnosis of HE is in question [ 32 ]. EEG provides information on the severity of HE in both cooperative and especially in uncooperative patients but is nonspecific [ 33 ]. Arterial ammonia concentration in whole blood on admission to the ICU is an independent risk factor for both encephalopathy and intracranial hypertension [ 34 ].
Creatinine The presence of extrahepatic organ failure, especially acute kidney injury, has been shown to increase mortality rates [ 3 , 35 , 36 ]. The occurrence of concomitant kidney failure (i.e., multiple organ failure) urges admission to the ICU [ 1 ].
Severity of liver injury (reflected in prothrombin time or bilirubin) Changes in coagulation factors, such as INR, reflecting injury to the hepatocellular synthesis machinery, are of prognostic value. Similarly, serum bilirubin, reflecting injury to the excretory machinery of the hepatocyte, serves as a prognostic marker in ALF of non-paracetamol etiology, but has no value in paracetamol-induced ALF or even other causes of hyperacute liver failure due to the time required for bilirubin levels to build up [ 37 ].
Other factors These may include age (e.g., < 10 or > 40 years) and lactate levels (e.g., > 4 mmol/l), as indicated in the King’s College Criteria or the recent guidelines in the UK for the assessment of the need for LTx [ 2 , 38 ]. Lactate, together with bilirubin and etiology, is part of the BiLE score, which has recently shown a good predictive value in a cohort of 102 ALF patients [ 37 ]. Other scoring systems are available for rare diseases, for example, the TIPS-BSC prognostic index [ 39 ].
Acute-on-chronic liver failure (ACLF)
ACLF is a syndrome affecting multiple organs, including new worsening of liver function, defined by an acute hepatic decompensation in patients with preexisting chronic liver disease [ 5 ]. Even if the definition provided by the European Association for the Study of the Liver (EASL) and the International Chronic Liver Failure (CLIF) Consortium is the most employed [ 6 ], there is no single agreed definition for ACLF. Since the first report in 1995 [ 40 ], different attempts have been made in Europe [ 6 , 41 ], Asia [ 7 , 42 , 43 ] and North America [ 8 ]. A consensus definition has been proposed, but has not yet been clinically validated [ 5 ]. Table 1 summarizes the current definitions of ACLF. A recent paper even highlighted three different stages in acutely decompensated cirrhosis, i.e., stable decompensated cirrhosis, unstable decompensated cirrhosis and ACLF [ 44 ]. In this study, ACLF was differentiated from decompensated cirrhosis by the presence of organ failure and systemic inflammation.
According to an investigation of 1343 patients with decompensated cirrhosis (CANONIC study), the EASL included the presence of at least renal dysfunction (creatinine > 1.5 mg/dl) or hepatic encephalopathy (grade I or II), together with a high risk of death due to multiple organ failure, as criteria to define each of the subgroups of ACLF [ 6 ]. These organ failures were first defined according to the SOFA score (Table 2 ). Depending on the number of failing organs, different subgroups were established (Table 1 ).
Similar to the EASL description, the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) includes extrahepatic organ failure in its definition [ 8 ] (summarized in Table 2 ). This stratification was validated in a study with more than 500 cirrhotic patients [ 45 ].
In contrast, the Asian Pacific Association for the Study of the Liver (APASL) does not include extrahepatic organ failure in its definition, arguing with potential delay in identification of the potential therapeutic window for reversal [ 7 ]. In any case, there is no consistent definition of organ failure, as illustrated in Table 2 . Moreover, neither patients with an extrahepatic insult as a precipitating factor for ACLF nor patients with prior decompensation are considered in the definition [ 46 ].
Data reported from hospital registries indicate that 24–40% of patients with cirrhosis admitted to the hospital develop ACLF [ 19 ]. This accounts for approximately 1–5% of patients admitted to the ICU [ 9 ]. Up to 44% and 53% of patients with ACLF died by day 28 and day 90, respectively. In the first 4 weeks, mild ACLF leads to death in 13–22% of patients, whereas moderate and severe failure results in mortality rates of 32–44% and 77–86%, respectively [ 6 , 7 , 43 , 47 ]. A recent meta-analysis using the EASL definition to assess the global burden of ACLF demonstrated that 35% of patients with decompensated cirrhosis already presented ACLF at hospital admission, showing a 60% mortality at 90 days [ 48 , 49 ]. Proven bacterial infection and severe alcoholic hepatitis accounted for almost all (approximately 97%) of acute decompensation and ACLF [ 50 ].
EASL and APASL use different criteria to predict higher mortality rates. The European Society first used the CLIF-SOFA score, a modified SOFA score for patients with cirrhosis, to define organ “failure” (Table 2 ). Parameters such as bilirubin, creatinine, HE, INR, PaO 2 /FiO 2 or S P O 2 /FiO 2 ratios, mean arterial pressure (MAP) and the need for vasopressors were employed to assess damage to liver and extrahepatic organ systems [ 6 ]. In the CANONIC study, four groups of patients were defined: the occurrence of ≥ 2 organ failures, the presence of kidney failure alone and the combination of a single non-renal organ failure with kidney dysfunction and/or mild-to-moderate HE [ 6 ]. In addition, the impact of the inflammatory response (as reflected in an increased white blood cell (WBC) count) and its early recognition and aggressive management were also recognized to influence the outcome [ 18 ]. Afterward, using the same parameters, the score was adapted into the more specific CLIF-C OF score, which was then combined with age and WBC count into the CLIF-C ACLF score, which improved prediction of mortality [ 51 ].
The Asian Pacific societies developed their own score based on bilirubin, HE grade, INR, lactate and creatinine (Table 2 ). This APASL-ACLF Research Consortium (AARC) score was only thought to stratify the degree of liver failure into mild, severe and very severe [ 47 ]. The definition of kidney failure follows the acute kidney injury network (AKIN) criteria [ 52 ], while no specific reference exists for circulatory or respiratory failures [ 7 ]. Prognostic factors such as bilirubin, HE or creatinine are considered, even if different cutoff values are used (Table 2 ). In the last update, the APASL also included obesity as a risk factor [ 7 ].
The approach of NACSELD is even simpler; only the number of organ failures being ≥ 2 is considered the main prognostic factor [ 8 ]. In fact, this seems to predict increased mortality better than age, WBC count, serum albumin, MELD score and/or presence of infection [ 45 , 53 ]. The definition of “failure” does not follow any specific score and is based on the presence of severe HE, low MAP and the need for hemodialysis or mechanical ventilation (Table 2 ). Hepatic encephalopathy grade and the presence of infection have also been shown to be associated with higher mortality rates [ 54 ].
Secondary acquired liver injury
Acquired liver injury without underlying liver disease represents the most common form of hepatic dysfunction in the ICU [ 9 , 10 ]. It can occur after a hypoxic (e.g., shock), toxic (i.e., hepatotoxic drugs) or inflammatory insult (e.g., sepsis) [ 55 ]. There is no common definition for this syndrome, and its diagnosis is mainly based on the elevation of liver parameters, such as transaminases or bilirubin [ 56 ].
Cholestasis is characterized by altered bile excretion (i.e., extrahepatic cholestasis due to mechanical bile duct obstruction) or impaired conjugation and/or secretion (i.e., intrahepatic cholestasis owing to altered hepatocellular signaling and transport) [ 9 , 55 , 57 ]. This results in an accumulation of bile acids and conjugated bilirubin, together with increased enzymes that indicate cholestasis, such as alkaline phosphatase (AP) or γ-glutamyl transpeptidase (GGT). A consensus on how to define cholestasis in critically ill patients using these routine laboratory parameters does not exist. As defined by the SOFA score [ 12 ], a bilirubin threshold > 2 mg/dl is included in the guidelines of the Surviving Sepsis Campaign [ 58 ] and has been adopted by many authors [ 9 , 14 , 15 , 56 , 59 , 60 ]. However, elevated bilirubin levels are not specific to cholestasis and might also reflect hemolysis. Lyu et al. [ 61 ] showed that 73% of adult cardiac patients supported by veno-arterial ECMO had hyperbilirubinemia (> 3 mg/dl), which may be due to hemolysis in up to 42% of the cases. The combination of AP and GGT with or without bilirubin has been suggested to diagnose cholestasis more specifically [ 62 , 63 ].
Hypoxic liver injury is generally defined based on a clinical setting of circulatory, cardiac or respiratory failure, a substantial increase in transaminases, ranging from > 5 to > 20 times the upper limit of normal (ULN), and the absence of other causes of liver damage [ 9 , 64 , 65 , 66 , 67 , 68 , 69 , 70 ]. It was first reported in 1901 as “central necrosis” [ 71 ] and is known as “ischemic hepatitis,” “shock liver” or “hypoxic hepatitis” [ 67 ]. The cause is an imbalance of oxygen supply and demand in the liver that results in cell death [ 64 ]. More specifically, insufficient hepatic perfusion, including Budd–Chiari perfusion, hypoxemia, poor global oxygen delivery, inadequate oxygen extraction by hepatocytes or an increased metabolic demand, can cause hypoxic liver injury [ 72 , 73 , 74 , 75 , 76 ]. Three subgroups of causes may be distinguished: respiratory failure, cardiac failure and shock/hypotension [ 1 , 67 ].
Due to the absence of a consensus definition, heterogeneous epidemiological data for secondary liver injury exist. Cholestasis is present in 11–36% of ICU patients [ 9 ], while hypoxic liver injury occurs in 1–18% of cases [ 69 , 70 , 77 ]. For the latter, the incidence increases over 20% in patients with shock [ 75 ]. The overall mortality for secondary acquired liver injury is high, ranging between 27 and 48% for ICU patients with cholestasis [ 56 , 76 ] and between 40 and 60% for hypoxic liver injury [ 9 , 69 , 70 , 72 , 74 , 75 ]. Moreover, in a single-center cohort with 1116 critically ill patients, mortality rates were significantly correlated with the magnitude of transaminases (33.2, 44.4 and 55.4% for peak AST 5–10 × ULN, 10–20 × ULN and > 20 × ULN, respectively) [ 70 ].
Serum bilirubin is a stable and prevailing marker of liver impairment in the ICU. Its concentration is influenced by bilirubin synthesis, transport, uptake, conjugation and excretion. Ischemic and sepsis-associated cholestasis, drug-induced liver injury and parenteral nutrition are predominant causes of hyperbilirubinemia in the ICU [ 55 ]. Bilirubin is a marker of liver dysfunction and a powerful prognostic factor. It has been shown to be linked to infections in surgical patients [ 78 , 79 ], to an increased mortality among trauma patients [ 80 , 81 ], sepsis [ 82 ] or hematological malignancies [ 83 ], to a poor prognosis among ARDS patients [ 84 ], or simply to a worse outcome in the ICU [ 56 , 85 ] (summarized in Table 3 ).
In the case of cholestasis, total bile acids are also an independent prognostic factor of disease severity [ 57 , 86 ]. An elevation of 5.2 µmol/l of total bile acids from baseline data demonstrated discriminating value, while mortality was specifically augmented with increases > 10 µmol/l [ 86 ]. Horvatits et al. suggested that bile acids could be a better prognostic factor than bilirubin in the ICU. Nevertheless, it remains unclear whether the elevation of bile acids in critically ill patients is a distinct pathophysiological entity or a compensatory mechanism [ 86 ].
Regarding hypoxic liver injury, the indocyanine green plasma disappearance rate is an effective tool for assessing liver function. In an observational study with 97 patients, a cutoff value of 9% 48 h after ICU admission demonstrated a significant prognostic accuracy for 28-day mortality [ 87 ]. In contrast, bilirubin, due to the later peak time, might not be a reliable marker and is elevated in only one-third of patients with hypoxic liver injury [ 76 ]. The indocyanine green plasma disappearance rate outperforms bilirubin as a more sensitive combined indicator of perfusion and excretory liver function in the ICU [ 88 ].
Finally, underlying and concomitant syndromes will impact the outcome, such as sepsis or need for organ support [ 89 ]. Moreover, the presence of coagulopathy or hepatic encephalopathy has been shown to predict mortality in critically ill patients with hypoxic liver injury [ 74 , 90 ]. As expected, the severity of organ failure results in higher mortality rates [ 9 , 74 ].
Liver injury as a core component of multiple organ failure
The role of the liver in multiple organ failure has been widely studied, especially in sepsis [ 15 ]. Due to the extensive number of immunologic, regulatory and metabolic functions, the liver can take the role of the “perpetrator,” triggering an inflammatory response, or that of the “victim” of the host response [ 13 , 14 , 15 ].
ALF is rare but frequently results in damage to other organ systems. These include the cardiovascular and respiratory systems, the central nervous system, the kidney, coagulation and the immune system [ 20 ]. This also applies to secondary acquired liver injury, since a hypoxic, toxic (e.g., due to commonly used drugs in the ICU [ 91 ]) or inflammatory extrahepatic insult triggers hepatic dysfunction.
Each of the organs affected may act either as a precipitating factor or as a target, both paving the way to the worsening of multiple organ failure. This is especially true for the inflammatory response accompanying infection as a prototypical trigger [ 13 , 15 , 92 ].
During ACLF, the release of both damage-associated molecular patterns (DAMPs) linked to inflammation and pathogen-associated molecular patterns (PAMPs) is common [ 46 ]. The intensity of inflammation directly correlates with the number of failing organs and outcome [ 93 ].
The progressive impairment of circulation is considered as a risk factor for the development of multiple organ failure [ 94 , 95 ]. The “peripheral arterial vasodilation hypothesis” suggests that during liver failure, portal hypertension and splanchnic vasodilatation emerge [ 96 ]. As a result, a reduction in systemic vascular resistance and central hypovolemia arises. Moreover, activation of hormone systems, such as the renin–angiotensin–aldosterone system (RAAS), sympathetic nervous system (SNS) and antidiuretic hormone (ADH), occurs.
Critically ill patients with liver disease are also more likely to develop pulmonary complications, e.g., acute respiratory distress syndrome (ARDS) [ 97 ]. The main mechanisms initiating dysfunction or failure of different organs are further described in [ 15 , 96 , 98 ]. A consensus paper from a North American and European expert panel gives a thorough description of possible organ failures and recommendations for their management [ 99 ] (Fig. 1 ).
The liver is a “perpetrator” of remote organ damage and development of multiple organ dysfunction syndrome during liver injury
The silent period of compensated cirrhosis may turn into a decompensated period (with, e.g., ascites, bleeding from esophageal varices, HE) and is further complicated by cardiopulmonary and renal failure [ 96 ]. As described above, the main mechanism triggering renal failure is an alteration in the arterial circulation and volume, which is combined with increased intrarenal vasoconstriction and impaired renal autoregulation. Factors affecting the circulatory status, such as bacterial infections and gastrointestinal bleeding, can reduce renal perfusion and precipitate HRS-AKI [ 100 ]. In the case of cardiorespiratory failure, the occurring circulatory changes (increased cardiac output, peripheral vasodilatation, decreased systemic vascular resistance and decreased oxygen extraction) are associated with hypovolemia and impaired tissue perfusion, together with water and sodium retention [ 100 ].
The majority of the macrophage population of the body is represented by Kupffer cells in the liver [ 14 ]. They act as the first defensive barrier against gut-derived bacteria [ 101 ]. The activation of Kupffer cells triggers the release of proinflammatory mediators [ 102 ], including TNF-α, IL-1, IL-6 and IL-12. TNF-α and IL-1 can act synergistically to activate the cytokine network, the coagulation cascade, fibrinolysis and neutrophils [ 103 ]. IL-6 triggers the synthesis of acute-phase proteins, the production of immunoglobulins, the proliferation and differentiation of T cells, enhanced activity of natural killer cells and the maturation of megakaryocytes [ 103 ]. Finally, IL-12 induces the production of interferon-γ in T cells and natural killer cells [ 14 ]. These processes can trigger cholestasis [ 14 ]. The maximal reduction in bile flow has been reported to occur within the first 24 h after cytokine challenge and can be accompanied by a posttranscriptional mechanism affecting the expression of the hepatobiliary transporters BSEP and MRP2 [ 104 , 105 ]. In this regard, the development of secondary liver injury, i.e., cholestasis or hypoxic liver injury, is common during sepsis, microcirculatory impairment or drug exposure [ 106 ].
Other systems, such as the hemostatic balance in patients with liver injury, might be altered by hemodynamic instability, endothelial dysfunction, the development of endogenous heparin-like substances, leading to either thrombotic or bleeding events [ 99 , 107 , 108 ].
Neurological dysfunction is mainly caused by alterations in ammonia metabolism, brain and systemic inflammation and changes in cerebral blood flow and oxygenation, due to hepatic encephalopathy and/or concomitant infections and electrolyte abnormalities [ 90 , 109 ]. This might include cerebral edema and intracranial hypertension [ 97 ].
Finally, the liver is also responsible for substrate oxidation, metabolism of organic acids (e.g., lactate, ketones, amino acids), metabolism of ammonium and production of plasma proteins [ 110 ]. Any alteration of these functions may change the acid–base balance by modifying the amount of CO 2 produced, the strong ion difference (SID), the concentration of unmeasured ions, the excretion of H + (i.e., as NH 4 + ) or the synthesis of albumin [ 111 , 112 , 113 , 114 , 115 ]. A retrospective study of 23,795 patients showed that liver “dysfunction” (i.e., SOFA score for liver 1 or 2) was already present on admission in at least 20% of the cases. In the same study, 80% of the non-survivors had an increase in at least one individual organ SOFA score in the four days prior to death [ 17 ]. Among patients with organ failure, the highest risk was associated with liver failure (OR 2.587; CI 2.098–3.189).
The association of liver dysfunction and mortality has been suggested by many authors [ 16 , 56 , 76 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 116 , 117 , 118 , 119 ]. Regarding patterns of organ involvement in sepsis, Seymour et al. defined four phenotypes, among which a “hepatic” phenotype with a 28-day mortality rate of 40% among adult patients with sepsis was prognostically the worst [ 120 ].
Conclusions
The definition of liver injury, dysfunction and/or failure is far from a global consensus. Similarly, the cutoff values of prognostic parameters vary. We provided current definitions, epidemiological data and prognostic factors for acute, acute-on-chronic liver failure and secondary liver injury with a focus on the intensive care unit. The reviewed data show an association of liver impairment with extrahepatic organ failure and present liver dysfunction as an underappreciated component for the development of multiple organ failure.
Availability of data and materials
Not applicable.
Wendon J, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–81. https://doi.org/10.1016/j.jhep.2016.12.003 .
Article PubMed Google Scholar
O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–5. https://doi.org/10.1016/0140-6736(93)91818-7 .
Article CAS PubMed Google Scholar
Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–34. https://doi.org/10.1056/NEJMra1208937 .
Mochida S, Nakayama N, Matsui A, Nagoshi S, Fujiwara K. Re-evaluation of the Guideline published by the Acute Liver Failure Study Group of Japan in 1996 to determine the indications of liver transplantation in patients with fulminant hepatitis. Hepatol Res. 2008;38:970–9. https://doi.org/10.1111/j.1872-034X.2008.00368.x .
Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin H-C, et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4–10. https://doi.org/10.1053/j.gastro.2014.05.005 .
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(1426–37):1437.e1-9. https://doi.org/10.1053/j.gastro.2013.02.042 .
Article Google Scholar
Leao GS, Lunardi FL, Picon RV, Tovo CV, de Mattos AA, de Mattos AZ. Acute-on-chronic liver failure: a comparison of three different diagnostic criteria. Ann Hepatol. 2019;18:373–8. https://doi.org/10.1016/j.aohep.2019.01.001 .
Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250–6. https://doi.org/10.1002/hep.27077 .
Horvatits T, Drolz A, Trauner M, Fuhrmann V. Liver injury and failure in critical illness. Hepatology. 2019;70:2204–15. https://doi.org/10.1002/hep.30824 .
Jenniskens M, Langouche L, Vanwijngaerden Y-M, Mesotten D, van den Berghe G. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med. 2016;42:16–27. https://doi.org/10.1007/s00134-015-4054-0 .
Koch DG, Speiser JL, Durkalski V, Fontana RJ, Davern T, McGuire B, et al. The natural history of severe acute liver injury. Am J Gastroenterol. 2017;112:1389–96. https://doi.org/10.1038/ajg.2017.98 .
Article PubMed PubMed Central Google Scholar
Vincent JL, Moreno R, Takala J, Willatts S, de Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Article CAS Google Scholar
Caraballo C, Jaimes F. Organ dysfunction in sepsis: an ominous trajectory from infection to death. Yale J Biol Med. 2019;92:629–40.
CAS PubMed PubMed Central Google Scholar
Fuchs M, Sanyal AJ. Sepsis and cholestasis. Clin Liver Dis. 2008;12(151–72):ix. https://doi.org/10.1016/j.cld.2007.11.002 .
Strnad P, Tacke F, Koch A, Trautwein C. Liver—guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. https://doi.org/10.1038/nrgastro.2016.168 .
Sakr Y, Lobo SM, Moreno RP, Gerlach H, Ranieri VM, Michalopoulos A, Vincent J-L. Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit Care. 2012;16:R222. https://doi.org/10.1186/cc11868 .
Bingold TM, Lefering R, Zacharowski K, Meybohm P, Waydhas C, Rosenberger P, Scheller B. Individual organ failure and concomitant risk of mortality differs according to the type of admission to ICU—a retrospective study of SOFA score of 23,795 patients. PLoS ONE. 2015;10:e0134329. https://doi.org/10.1371/journal.pone.0134329 .
Article CAS PubMed PubMed Central Google Scholar
Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet. 2015;386:1576–87. https://doi.org/10.1016/S0140-6736(15)00309-8 .
Hernaez R, Sola E, Moreau R, Gines P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–53. https://doi.org/10.1136/gutjnl-2016-312670 .
Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869–81. https://doi.org/10.1016/S0140-6736(19)31894-X .
Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–98.
CAS PubMed Google Scholar
Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965–7. https://doi.org/10.1002/hep.25551 .
Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97–106. https://doi.org/10.1055/s-2008-1040593 .
Sugawara K, Nakayama N, Mochida S. Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol. 2012;47:849–61. https://doi.org/10.1007/s00535-012-0624-x .
Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SHB, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. https://doi.org/10.7326/0003-4819-137-12-200212170-00007 .
Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RAFM. Systematic review: acute liver failure—one disease, more than 40 definitions. England; 2012.
Germani G, Theocharidou E, Adam R, Karam V, Wendon J, O’Grady J, et al. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol. 2012;57:288–96. https://doi.org/10.1016/j.jhep.2012.03.017 .
Hey P, Hanrahan TP, Sinclair M, Testro AG, Angus PW, Peterson A, et al. Epidemiology and outcomes of acute liver failure in Australia. World J Hepatol. 2019;11:586–95. https://doi.org/10.4254/wjh.v11.i7.586 .
Rajaram P, Subramanian R. Acute liver failure. Semin Respir Crit Care Med. 2018;39:513–22. https://doi.org/10.1055/s-0038-1673372 .
Wlodzimirow KA, Eslami S, Chamuleau RAFM, Nieuwoudt M, Abu-Hanna A. Prediction of poor outcome in patients with acute liver failure-systematic review of prediction models. United States; 2012.
Rutherford A, King LY, Hynan LS, Vedvyas C, Lin W, Lee WM, Chung RT. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology. 2012;143:1237–43. https://doi.org/10.1053/j.gastro.2012.07.113 .
Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–35. https://doi.org/10.1002/hep.27210 .
Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–47. https://doi.org/10.1016/j.jhep.2014.09.005 .
Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844–52. https://doi.org/10.1002/hep.21838 .
Bagshaw SM, Uchino S, Kellum JA, Morimatsu H, Morgera S, Schetz M, et al. Association between renal replacement therapy in critically ill patients with severe acute kidney injury and mortality. J Crit Care. 2013;28:1011–8. https://doi.org/10.1016/j.jcrc.2013.08.002 .
Hadem J, Kielstein JT, Manns MP, Kumpers P, Lukasz A. Outcomes of renal dysfunction in patients with acute liver failure. United Eur Gastroenterol J. 2019;7:388–96. https://doi.org/10.1177/2050640618817061 .
Hadem J, Stiefel P, Bahr MJ, Tillmann HL, Rifai K, Klempnauer J, et al. Prognostic implications of lactate, bilirubin, and etiology in German patients with acute liver failure. Clin Gastroenterol Hepatol. 2008;6:339–45. https://doi.org/10.1016/j.cgh.2007.12.039 .
Tavabie OD, Bernal W. How to manage: acute liver failure. Frontline Gastroenterol. 2020;11:70–4. https://doi.org/10.1136/flgastro-2018-101105 .
Hernández-Gea V, de Gottardi A, Leebeek FWG, Rautou P-E, Salem R, Garcia-Pagan JC. Current knowledge in pathophysiology and management of Budd-Chiari syndrome and non-cirrhotic non-tumoral splanchnic vein thrombosis. J Hepatol. 2019;71:175–99. https://doi.org/10.1016/j.jhep.2019.02.015 .
Ohnishi H, Sugihara J, Moriwaki H, Muto Y. Acute-on-chronic liver failure. Ryoikibetsu Shokogun Shirizu. 1995:217–9.
Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252–61. https://doi.org/10.1159/000047017 .
Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269–82. https://doi.org/10.1007/s12072-008-9106-x .
Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–71. https://doi.org/10.1007/s12072-014-9580-2 .
Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842–54. https://doi.org/10.1016/j.jhep.2020.06.013 .
O’Leary JG, Reddy KR, Garcia-Tsao G, Biggins SW, Wong F, Fallon MB, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology. 2018;67:2367–74. https://doi.org/10.1002/hep.29773 .
Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382:2137–45. https://doi.org/10.1056/NEJMra1914900 .
Choudhury A, Jindal A, Maiwall R, Sharma MK, Sharma BC, Pamecha V, et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol Int. 2017;11:461–71. https://doi.org/10.1007/s12072-017-9816-z .
Schulz M, Trebicka J. Acute-on-chronic liver failure: a global disease. Gut. 2021. https://doi.org/10.1136/gutjnl-2020-323973 .
Mezzano G, Juanola A, Cardenas A, Mezey E, Hamilton JP, Pose E, et al. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut. 2021. https://doi.org/10.1136/gutjnl-2020-322161 .
Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097–108. https://doi.org/10.1016/j.jhep.2020.11.019 .
Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Gines P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–47. https://doi.org/10.1016/j.jhep.2014.06.012 .
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. https://doi.org/10.1186/cc5713 .
Rosenblatt R, Shen N, Tafesh Z, Cohen-Mekelburg S, Crawford CV, Kumar S, et al. The North American consortium for the study of end-stage liver disease-acute-on-chronic liver failure score accurately predicts survival: an external validation using a national cohort. Liver Transpl. 2020;26:187–95. https://doi.org/10.1002/lt.25696 .
Bajaj JS, O’Leary JG, Tandon P, Wong F, Garcia-Tsao G, Kamath PS, et al. Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol. 2017;15:565-574.e4. https://doi.org/10.1016/j.cgh.2016.09.157 .
Lescot T, Karvellas C, Beaussier M, Magder S. Acquired liver injury in the intensive care unit. Anesthesiology. 2012;117:898–904. https://doi.org/10.1097/ALN.0b013e318266c6df .
Kramer L, Jordan B, Druml W, Bauer P, Metnitz PGH. Incidence and prognosis of early hepatic dysfunction in critically ill patients—a prospective multicenter study. Crit Care Med. 2007;35:1099–104. https://doi.org/10.1097/01.CCM.0000259462.97164.A0 .
Recknagel P, Gonnert FA, Westermann M, Lambeck S, Lupp A, Rudiger A, et al. Liver dysfunction and phosphatidylinositol-3-kinase signalling in early sepsis: experimental studies in rodent models of peritonitis. PLoS Med. 2012;9:e1001338. https://doi.org/10.1371/journal.pmed.1001338 .
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10. https://doi.org/10.1001/jama.2016.0287 .
Chen H-L, Wu S-H, Hsu S-H, Liou B-Y, Chen H-L, Chang M-H. Jaundice revisited: recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J Biomed Sci. 2018;25:75. https://doi.org/10.1186/s12929-018-0475-8 .
Woznica EA, Inglot M, Woznica RK, Lysenko L. Liver dysfunction in sepsis. Adv Clin Exp Med. 2018;27:547–51. https://doi.org/10.17219/acem/68363 .
Lyu L, Yao J, Gao G, Long C, Hei F, Ji B, et al. Incidence, risk factors, and outcomes of hyperbilirubinemia in adult cardiac patients supported by veno-arterial ECMO. Artif Organs. 2018;42:148–54. https://doi.org/10.1111/aor.12979 .
de Tymowski C, Dépret F, Soussi S, Nabila M, Vauchel T, Chaussard M, et al. Contributing factors and outcomes of burn-associated cholestasis. J Hepatol. 2019;71:563–72. https://doi.org/10.1016/j.jhep.2019.05.009 .
EASL Clinical Practice Guidelines. Management of cholestatic liver diseases. J Hepatol. 2009;51:237–67. https://doi.org/10.1016/j.jhep.2009.04.009 .
Ebert EC. Hypoxic liver injury. Mayo Clin Proc. 2006;81:1232–6. https://doi.org/10.4065/81.9.1232 .
Henrion J. Ischemia/reperfusion injury of the liver: pathophysiologic hypotheses and potential relevance to human hypoxic hepatitis. Acta Gastroenterol Belg. 2000;63:336–47.
Horvatits T, Trauner M, Fuhrmann V. Hypoxic liver injury and cholestasis in critically ill patients. Curr Opin Crit Care. 2013;19:128–32. https://doi.org/10.1097/MCC.0b013e32835ec9e6 .
Henrion J. Hypoxic hepatitis. Liver Int. 2012;32:1039–52. https://doi.org/10.1111/j.1478-3231.2011.02655.x .
Waseem N, Chen P-H. Hypoxic hepatitis: a review and clinical update. J Clin Transl Hepatol. 2016;4:263–8. https://doi.org/10.14218/JCTH.2016.00022 .
Jonsdottir S, Arnardottir MB, Andresson JA, Bjornsson HK, Lund SH, Bjornsson ES. Prevalence, clinical characteristics and outcomes of hypoxic hepatitis in critically ill patients. Scand J Gastroenterol. 2022;57:311–8. https://doi.org/10.1080/00365521.2021.2005136 .
van den Broecke A, van Coile L, Decruyenaere A, Colpaert K, Benoit D, van Vlierberghe H, Decruyenaere J. Epidemiology, causes, evolution and outcome in a single-center cohort of 1116 critically ill patients with hypoxic hepatitis. Ann Intensive Care. 2018;8:15. https://doi.org/10.1186/s13613-018-0356-z .
Mallory FB. Necroses of the liver. J Med Res. 1901;6:264–80.
Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med. 2007;46:1063–70.
Drolz A, Horvatits T, Roedl K, Fuhrmann V. Shock liver and cholestatic liver in critically ill patients. [Schockleber und Cholestase beim kritisch Kranken]. Med Klin Intensivmed Notfmed. 2014;109:228–34. https://doi.org/10.1007/s00063-013-0320-5 .
Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, et al. Hypoxic hepatitis: underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009;35:1397–405. https://doi.org/10.1007/s00134-009-1508-2 .
Henrion J, Schapira M, Luwaert R, Colin L, Delannoy A, Heller FR. Hypoxic hepatitis: clinical and hemodynamic study in 142 consecutive cases. Medicine (Baltimore). 2003;82:392–406. https://doi.org/10.1097/01.md.0000101573.54295.bd .
Jager B, Drolz A, Michl B, Schellongowski P, Bojic A, Nikfardjam M, et al. Jaundice increases the rate of complications and one-year mortality in patients with hypoxic hepatitis. Hepatology. 2012;56:2297–304. https://doi.org/10.1002/hep.25896 .
Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, et al. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011;37:1302–10. https://doi.org/10.1007/s00134-011-2248-7 .
Field E, Horst HM, Rubinfeld IS, Copeland CF, Waheed U, Jordan J, et al. Hyperbilirubinemia: a risk factor for infection in the surgical intensive care unit. Am J Surg. 2008;195:304–6. https://doi.org/10.1016/j.amjsurg.2007.12.010 ( discussion 306–7 ).
Diab M, Sponholz C, von Loeffelholz C, Scheffel P, Bauer M, Kortgen A, et al. Impact of perioperative liver dysfunction on in-hospital mortality and long-term survival in infective endocarditis patients. Infection. 2017;45:857–66. https://doi.org/10.1007/s15010-017-1064-6 .
Harbrecht BG, Zenati MS, Doyle HR, McMichael J, Townsend RN, Clancy KD, Peitzman AB. Hepatic dysfunction increases length of stay and risk of death after injury. J Trauma. 2002;53:517–23. https://doi.org/10.1097/00005373-200209000-00020 .
Saloojee A, Skinner DL, Loots E, Hardcastle TC, Muckart DJJ. Hepatic dysfunction: a common occurrence in severely injured patients. Injury. 2017;48:127–32. https://doi.org/10.1016/j.injury.2016.08.017 .
Juschten J, Bos LDJ, de Grooth H-J, Beuers U, Girbes ARJ, Juffermans NP, et al. Incidence, clinical characteristics and outcomes of early hyperbilirubinemia in critically ill patients: insights from the MARS study. Shock. 2022;57:161–7. https://doi.org/10.1097/SHK.0000000000001836 .
Bisbal M, Darmon M, Saillard C, Mallet V, Mouliade C, Lemiale V, et al. Hepatic dysfunction impairs prognosis in critically ill patients with hematological malignancies: A post-hoc analysis of a prospective multicenter multinational dataset. J Crit Care. 2021;62:88–93. https://doi.org/10.1016/j.jcrc.2020.11.023 .
Dizier S, Forel J-M, Ayzac L, Richard J-C, Hraiech S, Lehingue S, et al. Early hepatic dysfunction is associated with a worse outcome in patients presenting with acute respiratory distress syndrome: a post-hoc analysis of the ACURASYS and PROSEVA studies. PLoS ONE. 2015;10:e0144278. https://doi.org/10.1371/journal.pone.0144278 .
Brienza N, Dalfino L, Cinnella G, Diele C, Bruno F, Fiore T. Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med. 2006;32:267–74. https://doi.org/10.1007/s00134-005-0023-3 .
Horvatits T, Drolz A, Rutter K, Roedl K, Langouche L, van den Berghe G, et al. Circulating bile acids predict outcome in critically ill patients. Ann Intensive Care. 2017;7:48. https://doi.org/10.1186/s13613-017-0272-7 .
Horvatits T, Kneidinger N, Drolz A, Roedl K, Rutter K, Kluge S, et al. Prognostic impact of ICG-PDR in patients with hypoxic hepatitis. Ann Intensive Care. 2015;5:47. https://doi.org/10.1186/s13613-015-0092-6 .
Kortgen A, Paxian M, Werth M, Recknagel P, Rauchfuss F, Lupp A, et al. Prospective assessment of hepatic function and mechanisms of dysfunction in the critically ill. Shock. 2009;32:358–65. https://doi.org/10.1097/SHK.0b013e31819d8204 .
Drolz A, Horvatits T, Roedl K, Rutter K, Staufer K, Haider DG, et al. Outcome and features of acute kidney injury complicating hypoxic hepatitis at the medical intensive care unit. Ann Intensive Care. 2016;6:61. https://doi.org/10.1186/s13613-016-0162-4 .
Drolz A, Jager B, Wewalka M, Saxa R, Horvatits T, Roedl K, et al. Clinical impact of arterial ammonia levels in ICU patients with different liver diseases. Intensive Care Med. 2013;39:1227–37. https://doi.org/10.1007/s00134-013-2926-8 .
Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5:58. https://doi.org/10.1038/s41572-019-0105-0 .
Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol. 2014;33:498–510. https://doi.org/10.3109/08830185.2014.889129 .
Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249–64. https://doi.org/10.1002/hep.28740 .
Gines P, Fernandez J, Durand F, Saliba F. Management of critically-ill cirrhotic patients. J Hepatol. 2012;56(Suppl 1):S13-24. https://doi.org/10.1016/S0168-8278(12)60003-8 .
Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. https://doi.org/10.1055/s-2008-1040319 .
Moller S, Bendtsen F. Cirrhotic multiorgan syndrome. Dig Dis Sci. 2015;60:3209–25. https://doi.org/10.1007/s10620-015-3752-3 .
Damm TW, Kramer DJ. The liver in critical illness. Crit Care Clin. 2016;32:425–38. https://doi.org/10.1016/j.ccc.2016.02.002 .
Olson JC, Karvellas CJ. Critical care management of the patient with cirrhosis awaiting liver transplant in the intensive care unit. Liver Transpl. 2017;23:1465–76. https://doi.org/10.1002/lt.24815 .
Nadim MK, Durand F, Kellum JA, Levitsky J, O’Leary JG, Karvellas CJ, et al. Management of the critically ill patient with cirrhosis: a multidisciplinary perspective. J Hepatol. 2016;64:717–35. https://doi.org/10.1016/j.jhep.2015.10.019 .
Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279–90. https://doi.org/10.1056/NEJMra0809139 .
Katz S, Jimenez MA, Lehmkuhler WE, Grosfeld JL. Liver bacterial clearance following hepatic artery ligation and portacaval shunt. J Surg Res. 1991;51:267–70. https://doi.org/10.1016/0022-4804(91)90105-u .
O’Reilly M, Newcomb DE, Remick D. Endotoxin, sepsis, and the primrose path. Shock. 1999;12:411–20. https://doi.org/10.1097/00024382-199912000-00001 .
van der Poll T, van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin N Am. 1999;13(413–26):ix. https://doi.org/10.1016/s0891-5520(05)70083-0 .
Elferink MGL, Olinga P, Draaisma AL, Merema MT, Faber KN, Slooff MJH, et al. LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1008–16. https://doi.org/10.1152/ajpgi.00071.2004 .
Lee JM, Trauner M, Soroka CJ, Stieger B, Meier PJ, Boyer JL. Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology. 2000;118:163–72. https://doi.org/10.1016/s0016-5085(00)70425-2 .
Dhainaut JF, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med. 2001;29:S42–7. https://doi.org/10.1097/00003246-200107001-00016 .
Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–46. https://doi.org/10.1002/hep.21303 .
Tripodi A, Primignani M, Chantarangkul V, Dell’Era A, Clerici M, de Franchis R, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137:2105–11. https://doi.org/10.1053/j.gastro.2009.08.045 .
Cordoba J, Ventura-Cots M, Simon-Talero M, Amoros A, Pavesi M, Vilstrup H, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol. 2014;60:275–81. https://doi.org/10.1016/j.jhep.2013.10.004 .
Hasan A. Buffer Systems. In: Hasan A, editor. Handbook of blood gas/acid–base interpretation. London: Springer; 2009. p. 143–64. https://doi.org/10.1007/978-1-84800-334-7_5 .
Chapter Google Scholar
Adeva MM, Souto G, Blanco N, Donapetry C. Ammonium metabolism in humans. Metabolism. 2012;61:1495–511. https://doi.org/10.1016/j.metabol.2012.07.007 .
Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y. Ketone body metabolism and its defects. J Inherit Metab Dis. 2014;37:541–51. https://doi.org/10.1007/s10545-014-9704-9 .
Guder WG, Häussinger D, Gerok W. Renal and hepatic nitrogen metabolism in systemic acid base regulation. J Clin Chem Clin Biochem. 1987;25:457–66. https://doi.org/10.1515/cclm.1987.25.8.457 .
Häussinger D, Steeb R, Gerok W. Ammonium and bicarbonate homeostasis in chronic liver disease. Klin Wochenschr. 1990;68:175–82. https://doi.org/10.1007/bf01649081 .
Scheiner B, Lindner G, Reiberger T, Schneeweiss B, Trauner M, Zauner C, Funk G-C. Acid-base disorders in liver disease. J Hepatol. 2017;67:1062–73. https://doi.org/10.1016/j.jhep.2017.06.023 .
Han HS, Park C-M, Lee D-S, Sinn DH, Gil E. Evaluating mortality and recovery of extreme hyperbilirubinemia in critically ill patients by phasing the peak bilirubin level: a retrospective cohort study. PLoS ONE. 2021;16:e0255230. https://doi.org/10.1371/journal.pone.0255230 .
Pierrakos C, Velissaris D, Felleiter P, Antonelli M, Vanhems P, Sakr Y, Vincent J-L. Increased mortality in critically ill patients with mild or moderate hyperbilirubinemia. J Crit Care. 2017;40:31–5. https://doi.org/10.1016/j.jcrc.2017.01.017 .
Gupta T, Puskarich MA, Devos E, Javed A, Smotherman C, Sterling SA, et al. Sequential organ failure assessment component score prediction of in-hospital mortality from sepsis. J Intensive Care Med. 2018. https://doi.org/10.1177/0885066618795400 .
Umegaki T, Ikai H, Imanaka Y. The impact of acute organ dysfunction on patients’ mortality with severe sepsis. J Anaesthesiol Clin Pharmacol. 2011;27:180–4. https://doi.org/10.4103/0970-9185.81816 .
Seymour CW, Kennedy JN, Wang S, Chang C-CH, Elliott CF, Xu Z, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321:2003–17. https://doi.org/10.1001/jama.2019.5791 .
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29.
Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. https://doi.org/10.1053/jhep.2001.22172 .
Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26. https://doi.org/10.1056/NEJMoa0801209 .
Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. https://doi.org/10.1002/bjs.1800600817 .
Guo K, Ren J, Wang G, Gu G, Li G, Wu X, et al. Early liver dysfunction in patients with intra-abdominal infections. Medicine (Baltimore). 2015;94:e1782–e1782. https://doi.org/10.1097/MD.0000000000001782 .
Champigneulle B, Geri G, Bougouin W, Dumas F, Arnaout M, Zafrani L, et al. Hypoxic hepatitis after out-of-hospital cardiac arrest: incidence, determinants and prognosis. Resuscitation. 2016;103:60–5. https://doi.org/10.1016/j.resuscitation.2016.03.021 .
Jung C, Fuernau G, Eitel I, Desch S, Schuler G, Kelm M, et al. Incidence, laboratory detection and prognostic relevance of hypoxic hepatitis in cardiogenic shock. Clin Res Cardiol. 2017;106:341–9. https://doi.org/10.1007/s00392-016-1060-3 .
Iesu E, Franchi F, Zama Cavicchi F, Pozzebon S, Fontana V, Mendoza M, et al. Acute liver dysfunction after cardiac arrest. PLoS ONE. 2018;13:e0206655–e0206655. https://doi.org/10.1371/journal.pone.0206655 .
Download references
Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Author information
Aritz Perez Ruiz de Garibay
Present address: ADVITOS GmbH, Munich, Germany
Authors and Affiliations
University of Strasbourg, CNRS, Immunopathology and Therapeutic Chemistry, UPR 3572, 67000, Strasbourg, France
Department of Anesthesiology and Intensive Care Medicine, Jena University Hospital, Am Klinikum 1, 07747, Jena, Germany
Andreas Kortgen, Julia Leonhardt & Michael Bauer
Department of Internal Medicine IV (Gastroenterology, Hepatology, Infectious Diseases), Jena University Hospital, Jena, Germany
Alexander Zipprich
You can also search for this author in PubMed Google Scholar
Contributions
MB developed the initial idea for the review. All authors were responsible for the literature search, manuscript writing and editing. All authors critically reviewed and approved the final manuscript.
Corresponding author
Correspondence to Michael Bauer .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
Aritz Perez Ruiz de Garibay is affiliated with ADVITOS, GmbH, a company that offers extracorporeal support devices including liver support. No statements regarding the use of devices for liver support are made in this review. None of the other authors reports a link with ADVITOS or any other link to commercial entities that would be perceived as conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Perez Ruiz de Garibay, A., Kortgen, A., Leonhardt, J. et al. Critical care hepatology: definitions, incidence, prognosis and role of liver failure in critically ill patients. Crit Care 26 , 289 (2022). https://doi.org/10.1186/s13054-022-04163-1
Download citation
Received : 01 February 2022
Accepted : 10 September 2022
Published : 26 September 2022
DOI : https://doi.org/10.1186/s13054-022-04163-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute liver failure
- Acute-on-chronic liver failure
- Secondary liver failure
- Intensive care unit
- Multiple organ failure
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
- Case Report
- Open access
- Published: 22 April 2024
Spontaneous clearance of serum HCV-RNA after splenectomy in a patient with HCV-related liver cirrhosis and portal hypertension: a case report
- Toshiro Ogata ORCID: orcid.org/0009-0000-1365-3332 1 ,
- Terufumi Sakai 2 ,
- Sho Shibata 2 ,
- Hiroki Kanno 1 ,
- Hiroyuki Nakane 1 ,
- Takeshi Aoyagi 1 ,
- Kazuhiro Koikawa 1 ,
- Yoshihiko Sadakari 1 ,
- Gentaro Hirokata 1 &
- Masahiko Taniguchi 1
Surgical Case Reports volume 10 , Article number: 94 ( 2024 ) Cite this article
Metrics details
Spontaneous clearance of chronic hepatitis C virus (HCV) is rare in adults. A T-lymphocyte response is thought to be involved in HCV-RNA clearance. Splenectomy reportedly has a beneficial effect on T cell immune function in patients with cirrhosis. To the best of our knowledge, the present report is the first to describe spontaneous clearance of serum HCV-RNA within 1 year after splenectomy in a patient with cirrhosis.
Case presentation
A 55-year-old man with HCV cirrhosis was transferred to our institution with advanced pancytopenia, splenomegaly, and gastric varices. He had a 1-year history of ascites, edema, and general fatigue. The patient had a Child–Pugh score of 8 and serological type 1 HCV; the HCV-RNA level was 4.7 log IU/mL. Contrast-enhanced computed tomography showed gastric varices and marked splenomegaly (estimated spleen volume of 2175 mL). Esophagogastroduodenoscopy revealed enlarged gastric varices with no red color sign, and the varices were larger than those 1 year prior. He was diagnosed with decompensated HCV-related liver cirrhosis and portal hypertension. We considered direct-acting antiviral (DAA) therapy; however, DAA therapy was not approved in Japan for patients with decompensated cirrhosis at that time. Hand-assisted laparoscopic splenectomy was performed to improve the worsening portal hypertension. Further, we planned the initiation of DAA therapy after surgery, when such therapy would become available. DAA therapy was approved 1 year after splenectomy. At that time, we measured the HCV-RNA level before the initiation of DAA therapy; unexpectedly, however, serum HCV-RNA was not detectable, and the virus continued to disappear during the following 4 years. His liver function (total bilirubin, albumin, and prothrombin time) and pancytopenia improved during the 5 years postoperatively. The serum aspartate and alanine aminotransferase levels normalized between 1 and 5 years postoperatively. Esophagogastroduodenoscopy showed no change in the gastric varices during the 5 years after surgery. The patient remained asymptomatic and continued to do well.
Conclusions
We have presented a case of spontaneous clearance of HCV-RNA after splenectomy in a patient with cirrhosis and portal hypertension. Splenectomy may be associated with disappearance of HCV-RNA based on previous reports. More cases should be accumulated and evaluated.
Hepatitis C virus (HCV) spontaneously clears in 20% to 40% of acute infections [ 1 ]. By contrast, spontaneous clearance of serum HCV in patients with chronic infection is rare, with an incidence rate of 0.11 to 0.74 per 100 person-years [ 2 , 3 ]. Spontaneous clearance of HCV-RNA has also been reported after total gastrectomy [ 4 ], use of minimal immunosuppressants and prednisolone in patients with HCV reinfection after liver transplantation [ 5 ], use of immune checkpoint inhibitors in the oncology setting [ 6 ], and initiation of highly active antiretroviral therapy for human immunodeficiency virus (HIV) coinfection [ 7 ]. A T-lymphocyte response is thought to be involved in HCV-RNA clearance [ 3 , 5 , 6 ]. We encountered a case of HCV-related decompensated cirrhosis in which HCV-RNA disappeared spontaneously after splenectomy without antiviral treatment. Splenectomy reportedly has a beneficial effect on T cell immune function in patients with cirrhosis [ 8 , 9 , 10 , 11 ]. The present report describes the patient’s clinical course, changes in laboratory test results and imaging findings from approximately 1.5 years before surgery to 5 years postoperatively, and discusses the effect of splenectomy in patients with cirrhosis and HCV infection based on previous reports.
A 55-year-old man was diagnosed with chronic HCV infection at the age of 38 years, but did not accept interferon therapy for chronic hepatitis C. He was transferred to our institution with advanced pancytopenia, splenomegaly, and gastric varices. He had a 1-year history of ascites and general fatigue. Laboratory tests revealed the following: white blood cell count, 950/μL; red blood cell count, 304 × 10 4 /μL; hemoglobin, 10.6 g/dL; platelet count, 3.7 × 10 4 /μL; albumin, 3.2 g/dL; total bilirubin, 2.5 mg/dL; and prothrombin time activity, 47.8%. Real-time polymerase chain reaction showed that the HCV-RNA level was 4.7 log IU/mL. The virus was serological type 1. Enhanced computed tomography (CT) revealed marked splenomegaly, enlargement of the splenic vein, gastric varices, and development of collateral vessels from the left gastric vein to a gastro-renal shunt (Fig. 1 a, b). The spleen volume estimated by preoperative three-dimensional CT was 2175 mL. Esophagogastroduodenoscopy revealed gastric varices in the fundus of the stomach; these varices were markedly enlarged but white and showed no red color sign, and they were larger than those 1 year prior. The patient was diagnosed with decompensated HCV-related liver cirrhosis and portal hypertension. His Child–Pugh score was 8 (grade B). He was given ursodeoxycholic acid and branched-chain amino acids as liver supportive therapy. The ascites almost disappeared with the diuretic therapy. He also had type 2 diabetes mellitus and schizophrenia, which were controlled by medication. He had no history of alcohol consumption.
Contrast-enhanced CT before splenectomy. a An axial CT scan showing marked splenomegaly (yellow arrow) and gastric varices (red arrow). b A coronal CT scan showing an enlarged splenic vein (yellow arrow) and development of collateral vessels from the left gastric vein to a gastro-renal shunt (red arrow). CT computed tomography
We initially considered direct-acting antiviral (DAA) therapy. However, DAA therapy for patients with decompensated cirrhosis was not approved in Japan at that time. Because the patient had worsening portal hypertension, we decided to perform hand-assisted laparoscopic splenectomy to improve the portal hypertension. Furthermore, we planned initiation of DAA therapy after surgery, at which time DAA therapy would be available. Informed consent was obtained from the patient after giving an explanation of the treatment, including the benefits and risks of splenectomy [ 12 , 13 ]. We then administered a pneumococcal conjugate vaccination more than 2 weeks before the operation. Hand-assisted laparoscopic splenectomy with preoperative splenic artery balloon occlusion was performed because of marked splenomegaly [ 14 ]. The operation time was 358 min, and the blood loss volume was 55 mL. The patient was found to have a small abscess around the pancreatic tail on postoperative day 12, which was treated with antibiotics. The patient was discharged on postoperative day 26. He had no postoperative portal vein thrombosis. He was treated with warfarin as a prophylactic anticoagulant to prevent portal vein thrombosis until 10 months postoperatively.
Postoperative laboratory data showed increases in the platelet count, white blood cell count, and hemoglobin level and improvement in the pancytopenia for 5 years after splenectomy (Fig. 2 ). In terms of liver function, the patient’s total bilirubin, albumin, and prothrombin time also improved for 5 years after splenectomy (Fig. 2 ). We could not measure the prothrombin time at 6 months postoperatively because warfarin was being administered at that time. The serum aspartate aminotransferase and alanine aminotransferase levels had decreased to normal by 1 year postoperatively and were still within normal limits 4 years later (Fig. 3 a). DAA therapy was approved for patients with decompensated cirrhosis at 1 year after splenectomy. At that time, we measured the HCV-RNA level before the initiation of DAA therapy; unexpectedly, however, serum HCV-RNA was not detectable. Furthermore, continuous disappearance of virus was observed during the subsequent 4 years (Fig. 3 b). Esophagogastroduodenoscopy at 1, 3, and 5 years after surgery showed that the gastric varices had not changed in form and did not show the red color sign. The patient continued to do well for 5 years postoperatively.
Changes in laboratory indices of liver function and blood cell counts after splenectomy. The platelet count, white blood cell count, and hemoglobin value increased between 6 months and 5 years after surgery, and pancytopenia improved after splenectomy. Total bilirubin and albumin improved between 6 months and 5 years postoperatively, and the prothrombin time increased between 12 months and 5 years after surgery
Time course of serum AST, ALT, and HCV-RNA levels. a The serum AST and ALT levels had decreased to within normal limits by 1 year postoperatively and remained within normal limits for a further 4 years. b Serum HCV-RNA was not detectable at 1 year after surgery, and disappearance of the virus continued for a further 4 years. ALT alanine aminotransferase, AST aspartate aminotransferase, HCV hepatitis C virus
Spontaneous clearance of chronic HCV is rare, with a reported incidence of 0.11 to 0.74 per 100 person-years [ 2 , 3 ]. Spontaneous disappearance of HCV-RNA is associated with pregnancy [ 15 ], alcoholic hepatitis [ 16 ], hepatocellular carcinoma [ 3 ], the IL28B genotype [ 17 ], human leukocyte antigen-B27 [ 18 ], superinfection with hepatitis B virus [ 19 ], HIV coinfection [ 7 ], and surgical stress [ 4 ]. A T-lymphocyte response is thought to be involved in HCV-RNA clearance, but its mechanisms are not well understood [ 3 , 5 , 6 , 15 ]. Our patient did not have hepatitis B virus, HIV infection, malignant disease such as hepatocellular carcinoma, alcohol-associated liver disease, or a history of ingesting health foods. He was not given Stronger Neo-Minophagen C (Minophagen Pharmaceutical Co., Tokyo, Japan) as liver supportive therapy because he declined the injection.
The only other report of spontaneous elimination of HCV after splenectomy was by Sekiguchi et al. [ 20 ] in 2006. They indicated that splenectomy might diminish or remit the virus burden in HCV-positive patients with cirrhosis by increasing natural killer cell activity [ 20 ]. In our patient, elimination of serum HCV occurred within 1 year after splenectomy. By contrast, Sekiguchi et al. [ 20 ] reported eradication of serum HCV-RNA between 11 and 16 years after splenectomy, which was a longer postoperative follow-up period. To the best of our knowledge, our report is the first to describe spontaneous clearance of serum HCV-RNA in the short term after splenectomy. The patient’s low viral load may have been associated with viral clearance soon after the operation in our case [ 2 ]. Splenectomy in patients with cirrhosis has produced concern over an elevated risk of infection, such as overwhelming pneumococcal sepsis [ 21 ]. However, several reports have described beneficial changes in the immune status after splenectomy in patients with cirrhosis [ 8 , 9 , 10 , 11 ]. According to some reports, splenectomy in patients with cirrhosis might improve their impaired immune status by increasing interferon gamma production and reducing programmed cell death protein-1 (PD-1) expression in peripheral CD4 + T cells or CD8 + T cells [ 8 , 9 ]. The PD-1/programmed cell death ligand 1 (PD-L1) pathway is upregulated in chronic viral hepatitis, potentially attenuating the host’s T cell-mediated or natural killer cell-mediated antiviral immune response [ 6 , 22 ]. Hashimoto et al. [ 8 ] showed that patients with HCV-related liver cirrhosis had higher levels of splenic CD4 + T cells as well as PD-1-expressing and PD-L1-expressing cells, and they suggested that the spleen promotes T cell dysfunction through upregulation of the PD-1/PD-L1 pathway. Splenectomy may restore impaired T cell function and natural killer cell function by blocking PD-1/PD-L1 signaling from the spleen [ 8 , 9 ], which may result in increased interferon gamma production from activated T cells and natural killer cells [ 8 , 9 , 10 , 22 ]. Our previous research also indicates that splenectomy in patients with liver cirrhosis ameliorates the impaired immune status by decreasing the numbers of suppressive cells, such as regulatory T cells and myeloid-derived suppressor cells, and increasing the population and function of effector cells, such as CD8 + T cells and natural killer cells [ 10 ]. Considering the findings described in the published literature [ 8 , 9 , 10 ], the spontaneous clearance of HCV in our patient might have been caused by a beneficial change in his immune status after splenectomy.
DAA therapy achieved a sustained virological response rate of > 95% of patients with chronic hepatitis and compensated cirrhosis in 2014 [ 23 ]. We considered DAA therapy for our patient. However, we were unable to administer this treatment because DAA therapy for patients with decompensated cirrhosis was not approved at that time (March 2018). Our patient had worsening pancytopenia, splenomegaly, and gastric varices due to severe portal hypertension. Therefore, we performed hand-assisted laparoscopic splenectomy to improve the portal hypertension. In Japan, DAA therapy was approved for patients with HCV-related decompensated cirrhosis in February 2019 [ 23 ], 1 year after our patient’s splenectomy. We observed continuous disappearance of serum HCV-RNA from 1 to 5 years after splenectomy. Therefore, the patient no longer needed DAA therapy.
Liver function and pancytopenia improved during the 5 years after splenectomy in this study. In several previous studies, splenectomy was similarly effective in improving liver function and pancytopenia in patients with cirrhosis [ 12 , 24 , 25 ]. Achievement of a sustained virologic response with DAA therapy has also been reported to improve liver functional reserve in patients with decompensated cirrhosis [ 26 ]. The improvement of liver function in this study was suggested to be caused by both splenectomy and elimination of HCV. We consider that the normalization of transaminase levels from 1 to 5 years after surgery was caused by elimination of HCV [ 27 ] because no reports have described long-term liver enzyme normalization by splenectomy in patients with cirrhosis.
DAA agents should be the first-choice antiviral therapy for patients with HCV-related decompensated cirrhosis and portal hypertension. However, DAA therapy is not a perfect treatment. We believe that our study is worthy of reporting because it describes a phenomenon whereby splenectomy may have a potential antiviral effect. As a limitation of this case, HCV-RNA was not measured until 12 months after splenectomy. Additionally, the patient was not examined for IL28B genotype or human leukocyte antigen-27 before the operation. Furthermore, the influence of surgical stress due to splenectomy cannot be ruled out.
Our findings in this case suggest that splenectomy may contribute to elimination of HCV in patients with HCV-positive cirrhosis and portal hypertension. Based on previous reports, we speculate that splenectomy causes beneficial changes in patients’ immune status and is associated with disappearance of HCV-RNA. Further studies and evaluation of more patients are required to fully understand this phenomenon.
Availability of data and materials
The data sets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
Hepatitis C virus
Direct-acting antiviral
Human immunodeficiency virus
Computed tomography
Programmed cell death protein-1
Programmed cell death ligand 1
Bulteel N, Partha Sarathy P, Forrest E, Stanley AJ, Innes H, Mills PR, et al. Factors associated with spontaneous clearance of chronic hepatitis C virus infection. J Hepatol. 2016;65:266–72.
Article PubMed Google Scholar
Scott JD, McMahon BJ, Bruden D, Sullivan D, Homan C, Christensen C, et al. High rate of spontaneous negativity for hepatitis C virus RNA after establishment of chronic infection in Alaska Natives. Clin Infect Dis. 2006;42:945–52.
Article CAS PubMed Google Scholar
Minami T, Tateishi R, Shiina S, Fujiwara N, Mikami S, Sato M, et al. Spontaneous clearance of serum hepatitis C virus RNA during the clinical course of hepatocellular carcinoma in patients with chronic hepatitis C. Hepatol Res. 2014;44:E32–7.
Yoshikawa M, Morimoto Y, Shiroi A, Yoshiji H, Kuriyama S, Fukui H. Spontaneous elimination of serum HCV-RNA after total gastrectomy for early gastric cancer in a patient with chronic hepatitis C. Am J Gastroenterol. 2001;96:922–3.
Kogiso T, Hashimoto E, Ikarashi Y, Kodama K, Taniai M, Torii N, et al. Spontaneous clearance of HCV accompanying hepatitis after liver transplantation. Clin J Gastroenterol. 2015;8:323–9.
Davar D, Wilson M, Pruckner C, Kirkwood JM. PD-1 blockade in advanced melanoma in patients with hepatitis C and/or HIV. Case Rep Oncol Med. 2015;2015:737389.
PubMed PubMed Central Google Scholar
Kaung A, Sundaram V, Tran TT. Spontaneous clearance of hepatitis C virus in a patient co-infected with hepatitis C virus and human immunodeficiency virus: a case report. J Gastrointestin Liver Dis. 2014;23:325–7.
Hashimoto N, Shimoda S, Kawanaka H, Tsuneyama K, Uehara H, Akahoshi T, et al. Modulation of CD4+ T cell responses following splenectomy in hepatitis C virus-related liver cirrhosis. Clin Exp Immunol. 2011;165:243–50.
Article CAS PubMed PubMed Central Google Scholar
Sumida K, Shimoda S, Iwasaka S, Hisamoto S, Kawanaka H, Akahoshi T, et al. Characteristics of splenic CD8+ T cell exhaustion in patients with hepatitis C. Clin Exp Immunol. 2013;174:172–8.
Hirakawa Y, Ogata T, Sasada T, Yamashita T, Itoh K, Tanaka H, et al. Immunological consequences following splenectomy in patients with liver cirrhosis. Exp Ther Med. 2019;18:848–56.
CAS PubMed PubMed Central Google Scholar
Nomura Y, Kage M, Ogata T, Kondou R, Kinoshita H, Ohshima K, et al. Influence of splenectomy in patients with liver cirrhosis and hypersplenism. Hepatol Res. 2014;44:e100–9.
Ogata T, Okuda K, Sato T, Hirakawa Y, Yasunaga M, Horiuchi H, et al. Long-term outcome of splenectomy in advanced cirrhotic patients with hepatocellular carcinoma and thrombocytopenia. Kurume Med J. 2013;60:37–45.
Kawanaka H, Akahoshi T, Kinjo N, Harimoto N, Itoh S, Tsutsumi N, et al. Laparoscopic splenectomy with technical standardization and selection criteria for standard or hand-assisted approach in 390 patients with liver cirrhosis and portal hypertension. J Am Coll Surg. 2015;221:354–66.
Ogata T, Mikagi K, Sakai H, Yasunaga M, Okuda K, Kinoshita H. Hand-assisted laparoscopic splenectomy with preoperative splenic artery balloon occlusion for massive splenomegaly. J Jpn Soc Endosc Surg. 2014;19:21–7 ( in Japanese ).
Google Scholar
Clohessy P, Polis S, Post J. Spontaneous clearance of hepatitis C virus during pregnancy. Obstet Med. 2013;6:28–9.
Article PubMed PubMed Central Google Scholar
Silva MJ, Calinas F. Spontaneous clearance of hepatitis C virus during alcoholic hepatitis: the alcohol killed the virus? BMJ Case Rep. 2015;2015:bcr2015211896.
Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801.
Neumann-Haefelin C, McKiernan S, Ward S, Viazov S, Spangenberg HC, Killinger T, et al. Dominant influence of an HLA-B27 restricted CD8+ T cell response in mediating HCV clearance and evolution. Hepatology. 2006;43:563–72.
Sagnelli E, Coppola N, Pisaturo M, Masiello A, Tonziello G, Sagnelli C, et al. HBV superinfection in HCV chronic carriers: a disease that is frequently severe but associated with the eradication of HCV. Hepatology. 2009;49:1090–7.
Sekiguchi T, Nagamine T, Takagi H, Mori M. Reduction of virus burden-induced splenectomy in patients with liver cirrhosis related to hepatitis C virus infection. World J Gastroenterol. 2006;12:2089–94.
Okabayashi T, Hanazaki K. Overwhelming postsplenectomy infection syndrome in adults - a clinically preventable disease. World J Gastroenterol. 2008;14:176–9.
Wang XF, Lei Y, Chen M, Chen CB, Ren H, Shi TD. PD-1/PDL1 and CD28/CD80 pathways modulate natural killer T cell function to inhibit hepatitis B virus replication. J Viral Hepat. 2013;20:27–39.
Tahata Y, Sakamori R, Takehara T. Treatment progress and expansion in Japan: from interferon to direct-acting antiviral. Glob Health Med. 2021;3:321–34.
Akahoshi T, Tomikawa M, Korenaga D, Ikejiri K, Saku M, Takenaka K. Laparoscopic splenectomy with peginterferon and ribavirin therapy for patients with hepatitis C virus cirrhosis and hypersplenism. Surg Endosc. 2010;24:680–5.
Yamamoto N, Okano K, Oshima M, Akamoto S, Fujiwara M, Tani J, et al. Laparoscopic splenectomy for patients with liver cirrhosis: Improvement of liver function in patients with Child-Pugh class B. Surgery. 2015;158:1538–44.
Atsukawa M, Tsubota A, Kondo C, Toyoda H, Nakamuta M, Takaguchi K, et al. Time-course changes in liver functional reserve after successful sofosbuvir/velpatasvir treatment in patients with decompensated cirrhosis. Hepatol Res. 2022;52:235–46.
Khan ST, McGuinty M, Corsi DJ, Cooper CL. Liver enzyme normalization predicts success of Hepatitis C oral direct-acting antiviral treatment. Clin Invest Med. 2017;40:E73-80.
Download references
Acknowledgements
The authors thank Edanz ( https://jp.edanz.com/ac ) for editing a draft of this manuscript.
Not applicable.
Author information
Authors and affiliations.
Department of Surgery, St. Mary’s Hospital, 422 Tsubukuhonmachi, Kurume, Fukuoka, 830-8543, Japan
Toshiro Ogata, Hiroki Kanno, Hiroyuki Nakane, Takeshi Aoyagi, Kazuhiro Koikawa, Yoshihiko Sadakari, Gentaro Hirokata & Masahiko Taniguchi
Department of Gastroenterology, St. Mary’s Hospital, 422 Tsubukuhonmachi, Fukuoka, Kurume, 830-8543, Japan
Terufumi Sakai & Sho Shibata
You can also search for this author in PubMed Google Scholar
Contributions
TO, HK, HN, TA, KK, YS, GH, and MT performed the surgery, managed the patient in the early postoperative period, performed the literature search, and prepared the manuscript. TS managed the patient preoperatively and in the late postoperative period. SS performed esophagogastroduodenoscopy before and after surgery. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Toshiro Ogata .
Ethics declarations
Ethics approval and consent to participate.
The authors declare that they have obtained permission from the ethics committee of their institution. All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
Written informed consent was obtained from the patient for publication of this report.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ogata, T., Sakai, T., Shibata, S. et al. Spontaneous clearance of serum HCV-RNA after splenectomy in a patient with HCV-related liver cirrhosis and portal hypertension: a case report. surg case rep 10 , 94 (2024). https://doi.org/10.1186/s40792-024-01899-6
Download citation
Received : 02 March 2024
Accepted : 13 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s40792-024-01899-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Liver cirrhosis
- Splenectomy
- Spontaneous HCV-RNA clearance
- Immune function
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 14, Issue 5
- How to manage alcohol-related liver disease: A case-based review
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-1530-5328 James B Maurice 1 ,
- http://orcid.org/0000-0001-5140-517X Samuel Tribich 2 ,
- Ava Zamani 3 ,
- Jennifer Ryan 4
- 1 Department of Gastroenterology and Hepatology, Southmead Hospital , North Bristol NHS Trust , Bristol , UK
- 2 Department of Hepatology, Royal London Hospital , Barts Health NHS Trust , London , UK
- 3 Hammersmith Hospital , Imperial College Healthcare NHS Trust , London , UK
- 4 Department of Hepatology and Liver Transplantation, Royal Free Hospital , Royal Free London NHS Foundation Trust , London , UK
- Correspondence to Dr James B Maurice, Department of Gastroenterology and Hepatology, Southmead Hospital, North Bristol NHS Trust, Bristol BS10 5NB, UK; james.maurice{at}nbt.nhs.uk
https://doi.org/10.1136/flgastro-2022-102270
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- alcoholic liver disease
- chronic liver disease
What is already known on this topic
Alcohol-related liver disease (ArLD) is a major cause of morbidity and mortality.
What this study adds
We present a typical case to illustrate current evidence-based investigation and management of a patient with ArLD.
This case-based review aims to concisely support the day-to-day decision making of clinicians looking after patients with ArLD, from risk stratification and fibrosis assessment in the community through to managing decompensated disease, escalation care to critical care and assessment for liver transplantation.

How this study might affect research, practice or policy
We summarise the evolving evidence for the benefit of liver transplantation in alcoholic hepatitis, and ongoing controversies shaping future research in this area.
ArLD is fundamentally a public health problem, and further efforts are required to implement effective policies to reduce consumption and prevent disease.
Introduction
Alcohol is the leading risk factor for premature death in young adults, of which alcohol-related liver disease (ArLD) is a major contributor. 1 The management of ArLD often requires complex decision-making, raising challenges for the clinician and wider multidisciplinary team. This case-based review follows the typical journey of a patient through the progressive stages of the disease process, from early diagnosis and risk stratification in the outpatient clinic through to alcoholic hepatitis and referral for liver transplantation. At each stage, we discuss a practical approach to clinical management and summarise the underlying evidence base.
Case part 1
A 47-year-old man is referred to the general hepatology clinic from his General Practitioner with abnormal liver function tests, ordered in the community following several episodes of non-specific abdominal pain which subsequently resolved. He is now asymptomatic. The referral states that he drinks one bottle of wine each weekday night and more at the weekends. He is on no regular medication, has no other significant medical history and works in construction. On clinical examination, there are a few spider naevi on the chest wall but no other stigmata of liver disease, and his body mass index is 26 kg/m 2 . The blood results show alanine aminotransferase (ALT) 65 IU/L, aspartate aminotransferase (AST) 92 IU/L, alkaline phosphatase (ALP) 100 IU/L, gamma-GT (GGT) 350 IU/L, bilirubin 15 µmol/L, albumin 45 g/L, platelets 256×10 9 /L, internation normalised ratio (INR) 1.0 and creatinine 50 µmol/L. Abdominal ultrasound reveals a mildly enlarged, hyperechoic liver but normal spleen and no ascites.
How can we risk-stratify patients with ArLD in the outpatient clinic?
Early diagnosis and risk stratification of patients enables appropriate selection of patients for follow-up in secondary care, while also providing an opportunity for preventative interventions in those with mild disease. Emergency admissions for hepatic decompensation, where up to 75% of patients present for the first time, represent a late stage of the disease process when 1-year mortality is very high. 2 It is therefore vital to make an early diagnosis of liver disease.
Hepatic fibrosis has been traditionally staged by liver biopsy; however, non-invasive methods of fibrosis staging have an emerging role in ArLD. Transient elastography (TE) has been validated against liver biopsy to accurately stage both advanced fibrosis and cirrhosis, 3–6 and current NICE guidance recommends TE for the diagnosis of cirrhosis in patients with ArLD.
Serological markers of fibrosis such as FIB-4 and AST-Platelet Ratio Index (APRI) have generally not performed well in ArLD, although the enhanced liver fibrosis (ELF) test, measuring direct markers of fibrosis in blood, has an Area Under the Receiver Operator Curve (AUROC) of 0.92 in diagnosing advanced fibrosis using a cut-off value of 10.5. 7
How can we screen for alcohol use disorder and ArLD?
The primary screening tools for alcohol use disorders are the Alcohol Use Disorders Identification Test (AUDIT) or abbreviated AUDIT-C questionnaires. 8 Although clear documentation of the amount of alcohol consumed is important, a diagnosis of alcohol use disorder is more nuanced than volume of alcohol alone, hence the improved sensitivity through use of validated questionnaires. Identifying increasing risk (AUDIT 8–15), higher risk (AUDIT 16–19) or possible dependence (AUDIT≥20) 8 provides an opportunity for targeted brief interventions in those who would most benefit and is a cost-effective method for reducing alcohol intake. 9 Typically only comprising a 5–20 min single interaction, brief interventions offer personalised advice using a motivational and empathetic style of interview ( Box 1 ). If delivered to all new patients registered in primary care, this could save 2500 alcohol-related deaths over 20 years. 10 Patients identified to have alcohol dependence through screening should be referred for specialist treatment.
Typical features of brief interventions
Feedback on the person’s alcohol use and any related harm.
Clarification as to what constitutes low-risk consumption.
Information on the harms associated with risky alcohol use.
Benefits of reducing intake.
Motivational enhancement to support change.
Analysis of high-risk situations for drinking.
Coping strategies and the development of a personal plan to reduce consumption.
Adapted from Public Health England Review: the public health burden of alcohol and the effectiveness and cost-effectiveness of alcohol control policies. An evidence review. 45
Although routine blood tests may be helpful in supporting a diagnosis of ArLD (eg, AST>ALT, increased GGT, macrocytosis), they are of limited value in determining the severity of liver disease before established cirrhosis has developed with impaired liver synthetic function (low albumin, high INR and bilirubin). Individuals drinking at harmful levels should be screened for liver fibrosis with TE. Hepatology referral should be considered in patients with TE 8–16 kPa, particularly in those who continue to drink at harmful levels. Patients with TE≥16 kPa are at high risk of developing complications of cirrhosis, therefore should be followed up in a specialist hepatology clinic and be screened for hepatocellular carcinoma and oesophageal varices. Screening endoscopy for varices should be offered when TE≥20 kPa or platelets≤150×10 9 /L. 11 In the primary care setting, fibrosis screening of individuals drinking at harmful levels may alternatively be done with the ELF test, although this is not uniformly available ( figure 1 ). 12
- Download figure
- Open in new tab
- Download powerpoint
Screening for cirrhosis in individuals drinking at hazardous and harmful levels. ARFI, Acoustic Radiation Force Impulse; AUDIT, Alcohol Use Disorder Identification Test; AUDIT-C, abbreviated Alcohol Use Disorder Identification Test; ELF, enhanced liver fibrosis; GGT, Gamma-GT; HCC, hepatocellular carcinoma; NICE, The National Institute of Health and Care Excellence. Reproduced with permission from Newsome et al . 46
A high-risk population that should be considered for ArLD screening are the patients admitted to hospital acutely with alcohol-related physical harm, such as acute alcohol withdrawal or alcohol-related trauma. These patients should all be referred to alcohol care teams, and in addition to their expertise in delivering brief interventions, tailored detoxification regimens and vital links to local alcohol support services in the community, some hospitals have trained to perform TE and screen for liver fibrosis. This has provided an opportunity to streamline at risk patients into the hepatology services.
Case part 2
The same patient presents on the acute medical take 1 year later with a 2-week history of jaundice and abdominal swelling. Unfortunately, he has continued drinking alcohol. On examination, he is jaundiced with moderate ascites, tender hepatomegaly and subtle asterixis. He is sarcopenic with arm muscle wasting. He has the following blood results: haemoglobin 100 g/L, mean cell volume 107 fL, white cell count 12×10 9 /L, platelets 135×10 9 /L, INR 2.3, sodium 132 mmol/L, potassium 3.0 mmol/L, creatinine 55 µmol/L, urea 2.0 µmol/L, bilirubin 250 µmol/L, ALT 25 IU/L, AST 60 IU/L, ALP 95 IU/L, GGT 200 IU/L, albumin 35 g/L, c-reative protein (CRP) 45 mg/L. A diagnostic paracentesis reveals an ascitic albumin 16 g/L, white cells 90/mm 3 (80% lymphocytes). An X-ray of the chest is normal. Ultrasound liver demonstrates hepatomegaly 17 cm, splenomegaly 15 cm, moderate ascites and a patent portal vein. A clinical diagnosis of alcoholic hepatitis (AH) is made.
What is the role of liver biopsy in the diagnosis of AH?
AH is a clinical syndrome characterised by jaundice and coagulopathy in the context of recent and prolonged heavy alcohol use. Rapid development of jaundice is accompanied by a systemic inflammatory response with constitutional symptoms and low-grade fever, with or without other features of decompensation.
The diagnosis of AH can be made using a standard consensus definition based on clinical and biochemical parameters ( table 1 ), originally established to allow inclusion in clinical trials without the need for a liver biopsy but now generalised to clinical practice. 13 Neither European Association for the Study of Liver Disease (EASL) nor American College of Gastroenterology (ACG) guidelines recommend liver biopsy in patients meeting the criteria for probable AH, 9 10 and these recommendations have been supported by more recent data, showing that liver biopsy rarely changes the diagnosis when clinical criteria are met for AH. 12 However, if diagnostic uncertainty remains, such as atypical biochemical markers, uncertain alcohol use or a suspected alternative cause of liver injury, a liver biopsy should be undertaken to confirm the diagnosis, particularly if planning to administer AH-directed medical therapies. 14
- View inline
Consensus definition for ‘probable’ alcohol hepatitis 13
In those patients in whom a biopsy is undertaken, specific histological features such as degree of neutrophil infiltration, fibrosis stage and presence of megamitochondria can be useful for prognostication using the Alcoholic Hepatitis Histologic Score, which is independently predictive of 90-day mortality. 15 However, the utility of this is significantly limited by interobserver variability between reporting pathologists. 16
Should this patient be treated with steroids?
Once a diagnosis of AH is established, patients should be risk stratified using a validated scoring system. The modified Maddrey’s discriminant function (mDF) is a commonly used score, which defines a cut-off of ≥32 as severe AH; however, this is very sensitive and risks over-treating patients with mild disease. 17 The Glasgow Alcoholic Hepatitis Score (GAHS) and Model for End-Stage Liver Disease (MELD) are better predictors of 28-day and 90-day mortality than mDF 18 19 and are also now included in EASL and ACG guidelines, with severe AH defined as GAHS≥9 or MELD≥21. 20 , 14
The STeroids Or Pentoxifylline for Alcoholic Hepatitis (STOPAH) study is the largest randomised controlled trial to investigate the efficacy of corticosteroids in the treatment of AH. It included 1103 participants with severe AH and the group that received prednisolone only had a small non-significant improvement in 28-day survival (OR 0.72, 95% CI 0.52 to 1.01, p=0.06), a benefit which was lost by 90 days and 1 year. In the multivariate analysis adjusting for baseline variables, prednisolone was associated with improved 28-day survival compared with placebo (OR 0.61, p=0.015), although not at 90 days or 1 year. 21 Further meta-analyses of pooled data have replicated these findings. 22
The EASL and ACG guidelines advise to take steroid treatment with prednisolone 40 mg per day in patients with severe AH, as defined by either the mDF, GAHS or MELD Score. Steroid responsiveness should be assessed using the Lille Score, typically on day 7, although there is data to support earlier application on day 4, 23 with steroids stopped in non-responders (Lille Score≥0.45); responders should complete a 28-day course. It may be possible to predict Lille response using a baseline neutrophil-to-lymphocyte ratio (NLR), with an NLR of 5–8 predictive of a significant reduction in 90-day mortality with corticosteroid treatment, compared with no reduction when NLR is less than 5 or more than 8. 24 Emerging data is further delineating which patients may derive the greatest benefit from corticosteroids 25 ; however, this remains an area of ongoing research.
Infection is a frequent complication of severe AH, contributing significantly to the high mortality rate and associated in particular with an increased 90-day mortality. 26 Corticosteroids are associated with an increased incidence of infection post-treatment compared with placebo (10% vs 6%), 21 and significantly worse 90-day mortality if patients develop infection within the first week of starting steroids. 26 Therefore, particular caution is required prior to starting prednisolone in patients with active sepsis, bearing in mind that patients with cirrhosis may not mount a classic immune response to infection. 26 Biomarkers to predict risk of incident infection on steroids are an area of research interest; baseline NLR of >8 is also associated with increased infection at day 7 of corticosteroid treatment (OR 2.60, p=0.006), but requires further validation. 24
In clinical practice, the commencement of steroids is delayed until infection is excluded, including negative cultures of blood, urine and ascitic fluid. This period also allows for the assessment of the bilirubin trend which, along with risk stratification scoring, helps to inform the decision to start corticosteroids. 27
What are the considerations in managing alcohol withdrawal in patients with advanced liver disease?
Alcohol withdrawal syndrome (AWS) should be assessed using the Clinical Institute Withdrawal Assessment for AlcoholScore, with a symptom-based regimen rather than fixed dosing in order to reduce drug accumulation. 28 Benzodiazepines reduce withdrawal symptoms and the risk of both seizures and delirium tremens and are considered the gold standard for treatment of AWS. Long-acting benzodiazepines such as chlordiazepoxide and diazepam should only be used with caution in patients with cirrhosis and impaired synthetic function due to their unpredictable half-life and significant accumulation in the presence of hepatic dysfunction, where the use of shorter-acting lorazepam or oxazepam may be preferable if available. In addition, benzodiazepines can both precipitate and worsen hepatic encephalopathy and so should be used with care.
Abstinence from alcohol remains the only independent predictor of long-term survival in patients presenting with severe AH 29 and early intervention from an alcohol liaison service during the hospital admission is of fundamental importance.
What is the role of nutrition in the management of AH?
Patients with both AH and cirrhosis are characterised by an almost universal state of malnutrition, sarcopenia and B vitamin deficiency, alongside increased resting energy expenditure and impaired metabolism of carbohydrates, lipids and proteins. 30 Early involvement of the dietetic team is vital to ensure patients with AH meet their nutritional requirements.
Increased caloric intake has been associated with a reduced incidence of infection, improved liver function and quicker resolution of hepatic encephalopathy in multiple randomised trials. 30 A recent large trial reported lower rates of infections and improved 1-month and 6-month mortality in patients with severe AH treated with corticosteroids who received a calorie intake of ≥21.5 kcal/kg/day compared with those who received<21.5 kcal/kg/day, regardless of Lille response or of the mode by which the calories were delivered. 31
EASL and European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend an aim of 35–45 kcal/kg/day and a daily protein intake of 1.2–1.5 g/kg/day, with the oral route as first line and nasogastric feeding advised if oral intake is inadequate. 30 Intravenous thiamine replacement should be given to all patients with a history of alcohol use to reduce the risk of Wernicke’s encephalopathy. Several clinical trials have failed to demonstrate evidence for the use of various specialised dietary formulas and ESPEN recommend using standard nutritional supplements or feed with a high energy density, with a late evening supplement to reduce overnight starvation duration. 30
Case part 3
A full septic screen including blood cultures did not reveal any evidence of sepsis. The patient is managed with nutritional supplements, lactulose 20 mL three times a day and prednisolone 40 mg once daily. At day 7, his blood results are bilirubin 355, INR 3.0, PT 27, Cr 90, albumin 29. Lille Score is 0.60 (>0.45) indicating a poor prognosis, so prednisolone is stopped. Overall 90-day mortality in patients with AH is approximately 30%, increasing to around 45% in patients with a Lille Score>0.45 after 7 days of corticosteroids. 19
Is liver transplantation an option in severe AH?
In Europe, ArLD is the leading indication for liver transplant (LT), but the timing and selection of patients for liver transplantation with ArLD is controversial. A period of abstinence is vital to understand the extent of hepatic recompensation that can occur without the need to undergo LT and to ensure the patient is engaged with the process. However, although pretransplant abstinence is one important predictor of post-LT sobriety, it is not the only factor, and there is data that the risk of relapse is no higher in carefully selected patients transplanted with severe alcoholic hepatitis (SAH) compared with those with alcohol-related cirrhosis under standard selection criteria. 32
Challenging the traditional exclusion of patients with SAH from consideration for LT, a multicentre cohort study in France offered LT to patients with SAH who met specific stringent selection criteria, including non-response to steroid therapy, a first presentation of liver decompensation and a robust social support network. 33 This study showed significantly improved survival in the group offered LT at 2 years (71% vs 23%), a benefit almost entirely gained in the first 6 months. Long-term follow-up data was recently presented, showing overall survival at 1, 5 and 10 years of 83%, 70% and 56%, respectively. Severe alcohol relapse was evident in 10%, similar to other cohorts transplanted for ArLD using standard selection criteria. 34
The largest study in the USA on LT in SAH is a retrospective review of United Network for Organ Sharing data. In 147 patients transplanted with SAH between 2006 and 2017, with no previous decompensation and abstinence of less than 6 months, 1-year and 3-year survival was 94% and 84%, while return to sustained drinking occurred in 10% at 1 year and 17% at 3 years. 35 Interestingly, in a smaller retrospective case-controlled study comparing patients transplanted with SAH with<6 months abstinence (n=46) with a group transplanted for standard ArLD criteria and>6 months abstinence (n=34), the two groups had comparable 1-year survival (97% vs 100%, p=1) and return to harmful drinking after median follow-up of 532 days (17% vs 12%, p=0.5). 32
The only prospective trial of early liver transplantation in SAH was a non-randomised, non-inferiority, controlled trial recently published by the group in France. Over 2 years of follow-up, patients with SAH offered early liver transplant (n=68) had a small but non-significant increased risk of alcohol relapse compared with those transplanted for alcohol-related cirrhosis after ≥6 months of abstinence (n=93, relative risk 1.45, 95% CI 0.82 to 2.60), but also a greater risk of high levels of alcohol intake (RR 4.10, 95% CI 1.56 to 10.75). The 2-year post-transplantation survival was similar between these groups (89.7% and 88.2% respectively, HR 0.87, 95% CI 0.33 to 2.26), whereas the overall 2-year survival of patients with SAH who were not transplanted (n=47) was significantly reduced compared with those who were (28.3% vs 70.6%, HR 0.27, 95% CI 0.16 to 0.47). 36
The landscape of public and medical opinion on offering transplantation for SAH is changing in light of this data. However, concerns remain that predictive models are suboptimal and over 50% of patients with an unfavourable prognosis based on the Lille Score will survive without LT. 37 As such, the UK pilot on LT in SAH failed to recruit any patients over a 3-year period and was closed. 38 The current UK position recommends that if liver insufficiency persists after 3 months of documented alcohol abstinence in individuals with an index presentation of severe AH, consideration should be given to referral for liver transplantation if their psychosocial risk profile is favourable. 37
Case part 4
The patient’s clinical condition deteriorates over the following week. He becomes febrile, an ascitic tap confirms spontaneous bacterial peritonitis (white cells 700 cells/mL, neutrophils>90%) and he develops an oliguric acute kidney injury with haemodynamic instability requiring regular fluid boluses. His liver function remains poor (UK Model for End Stage Liver Disease (UKELD) 68). You call the intensive care unit (ITU) to review the patient, but questions are raised about his suitability for level 3 care.
Should this patient be escalated to critical care?
This patient now requires organ support with inotropes and likely renal replacement therapy. Historically, patients with ArLD have experienced barriers to timely escalation to ITU due to a perceived poor prognosis. Data in the National Confidential Enquiry into Patient Outcome and Death report in 2013 confirmed this practice, showing that 31% of patients deemed to require and be appropriate for escalation of care on independent review of the case notes did not receive such treatment. 39
In the same report, a review by the treating clinicians identified only 7% of cases who were appropriate for escalation but did not receive it. Subjective judgements detrimentally influenced these decisions and led the report to conclude that failure to escalate was due to clinicians having a prior view that it was not appropriate to escalate care in patients with ArLD.’ 39
Over the last 20 years, there has been a significant improvement in survival of patients admitted to ITU with organ failure complicating decompensated chronic liver disease, with mortality falling from 41.0% to 32.5% over this period, despite comparable scores for severity of illness at presentation. Although ArLD was associated with worse survival, improvements were also reported in this group over the same period (50.9% mortality to 41.9%, mean (Acute Physiology and Chronic Health Evaluation (APACHE) II Score 20 vs 19), such that the majority admitted to ITU will survive. 40
However, mortality rates for patients with ArLD and acute-on-chronic liver failure admitted to the ITU remain high, and escalation decisions require careful discussion and shared decision-making between the medical and critical care teams, alongside patients and their families as required. One of the first questions posed by the ITU team may be whether they are a transplant candidate. This is not a straightforward question to answer and may not be the most pertinent issue at this point in the patient’s care: in his case, the immediate answer would be ‘no’ in the UK, for reasons discussed above. But if he survives this admission, maintains abstinence but continues to have a qualifying UKELD Score he may be considered for a transplant assessment in 3 months.
In this instance, the patient’s age and the fact this is a first presentation with hepatic decompensation strongly support escalation at this stage, but the CLIF-ACLF prognostic scoring system can be helpful to add objective data to discussions between the managing team and ICU. While not including some increasingly recognised prognostic factors such as the presence of sarcopenia, the European Foundation for the Study of Chronic Liver Failure Acute-on-chronic Liver Failure (CLIF-C ACLF) Score has nevertheless been validated in a large dataset of patients with decompensated chronic liver disease and organ failure. 41 Days 3–7 on the ITU may be the optimal time to calculate this score, when an accurate assessment on the trajectory and likely outcome can be made. 42 Applying it in this way can support decision-making between the patient’s primary team and the ITU and provide timescales and goals with which to assess the benefits of level 3 care when there is disagreement or uncertainty.
However, even in the setting of treatment escalation it is important to remember that his overall prognosis is poor at this stage and, therefore, early involvement of the palliative care (PC) team should be considered. This can be done in parallel with full active medical care; it is important to bear in mind that PC is not synonymous with ‘end-of-life care’ and they are excellently equipped to optimise symptom control and begin to address wider holistic issues in the care of a patient at high risk of death. As such, early involvement of PC has been shown to improve symptom control and quality of life. 43
Case part 5
After 2 weeks in the ITU and a prolonged inpatient stay, the patient makes sufficient recovery to be discharged home. He continues to have moderate ascites managed with spironolactone and significant liver synthetic dysfunction (UKELD 60). He begins to attend his local alcohol support group and his partner has removed all alcohol from the house to support his abstinence.
When should you refer for transplant assessment?
Although he has made significant progress, this patient remains very unwell and at high risk of further deterioration. His ‘window of opportunity’ for transplant assessment is small. There is no fixed rule on this, but recent guidelines suggest that referral should be considered after 3 months of abstinence, or even sooner if the patient is actively engaged in alcohol cessation support, if there are issues that may complicate the workup assessment, or if the risk of death within 3 months is high. 44 In parallel to referral, every effort should be made to optimise his physical fitness, including dietician input and a graded exercise plan.
The patient maintains abstinence, is referred and successfully receives a life-saving liver transplant. A multidisciplinary team approach is required for patients admitted with decompensated ArLD, in which a dedicated alcohol care team is vital, but preventative measures on a population (eg, minimum unit pricing) and individual (eg, brief interventions and fibrosis stratification) level early in the disease course will have the greatest impact.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
- Griswold MG ,
- Fullman N ,
- Williams R ,
- Aspinall R ,
- Bellis M , et al
- Kettaneh A ,
- Tengher-Barna I , et al
- Pavlov CS ,
- Casazza G ,
- Nikolova D , et al
- Detlefsen S ,
- Sevelsted Møller L , et al
- Lackner C ,
- Bataller R ,
- Burt A , et al
- Madsen BS ,
- Hansen JF , et al
- National Institute for Health and Care Excellence
- Anderson P ,
- Chisholm D ,
- Gillespie D ,
- Ally A , et al
- de Franchis R , Baveno VI Faculty
- Forrest E ,
- Chalasani NP , et al
- Altamirano J ,
- Katoonizadeh A , et al
- Horvath B ,
- Allende D ,
- Xie H , et al
- Forrest EH ,
- Atkinson SR ,
- Richardson P , et al
- Singal AK ,
- Ahn J , et al
- Thursz MR ,
- Richardson P ,
- Allison M , et al
- Chandar AK , et al
- Garcia-Saenz-de-Sicilia M ,
- Altamirano J , et al
- Sinha R , et al
- Baeza N , et al
- Knapp S , et al
- Cabezas J ,
- Mirijello A ,
- D’Angelo C ,
- Ferrulli A , et al
- Labreuche J ,
- Artru F , et al
- Bischoff SC ,
- Dasarathy S , et al
- Deltenre P ,
- Senterre C , et al
- McCaul ME , et al
- Mathurin P ,
- Samuel D , et al
- Dharancy S ,
- Dumortier J , et al
- Platt L , et al
- Moreno C , et al
- Aldersley H ,
- Leithead JA , et al
- Juniper M ,
- Kelly K , et al
- McPhail MJW ,
- Parrott F ,
- Wendon JA , et al
- Pavesi M , et al
- Fernandez J ,
- Garcia E , et al
- Woodland H ,
- Forbes K , et al
- Millson C ,
- Considine A ,
- Cramp ME , et al
- Newsome PN ,
- Davison SM , et al
Twitter @jamesbmaurice
Contributors JBM conceptualised the original article and the case. JBM, ST and AZ drafted the initial version of the manuscript. JBM and ST contributed further editing of various sections. JR provided senior critical review and edited the manuscript. All authors agreed upon the final version.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Highlights from this issue UpFront R Mark Beattie Frontline Gastroenterology 2023; 14 357-358 Published Online First: 07 Aug 2023. doi: 10.1136/flgastro-2023-102519
Read the full text or download the PDF:
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Paediatr Child Health
- v.21(3); 2016 Apr

Case 1: A 12-year-old boy with acute liver failure
A previously well 12-year-old boy presented with a four-day history of decreased energy and appetite. On day 3 of this illness, his parents noticed yellowing of his sclera and his urine was “dark” in colour. He was found to have elevated total and conjugated bilirubin, and elevated transaminase and gamma-glutamyl transferase levels, with normal alkaline phosphatase. His albumin level was low, along with an elevated international normalized ratio (INR), which did not respond to intravenous doses of vitamin K. He was found to have a low hemoglobin level, requiring packed red blood cell transfusions, with signs of nonautoimmune hemolytic anemia (low haptoglobin, increased lactate dehydrogenase levels and reticulocyte count, and negative Coomb’s test). There was no pruritus and no fever.
He had no sick contacts or pertinent travel history, no history of ingestion of acetaminophen or other drugs or naturopathic remedies, and no family history of liver disease. Serology for hepatitis A, B and C was negative. His urine toxicology screen was negative, total immunoglobulin G level was normal, and he had negative titres for antinuclear, antismooth muscle and antiliver kidney microsomal antibodies.
His blood and urine cultures were negative, and an abdominal ultrasound revealed no focal liver lesions, a normal biliary tree and patent hepatic veins.
CASE 1 DIAGNOSIS: WILSON DISEASE
The patient continued to deteriorate. By day 5 of his hospitalization, he had developed hepatopulmonary syndrome and encephalopathy, and required mechanical ventilation in the critical care unit. He was placed on the wait list for liver transplantation.
Acute liver failure (ALF) is defined by the presence of biochemical evidence of liver injury, with hepatic-based coagulopathy (INR >2.0 after parenteral replenishment of vitamin K, or INR >1.5 if encephalopathy is present) in the absence of evidence of chronic liver disease ( 1 ). It is an uncommon presentation in the previously well child. In general, symptoms are vague and include anorexia, fatigue and jaundice, while the underlying pathophysiology relates to loss of liver function and its subsequent sequelae (ie, coagulation abnormalities, encephalopathy, hemodynamic instability and renal failure) ( 2 ).
The differential diagnosis for ALF includes infective, toxic, autoimmune, metabolic and vascular causes, and varies according to age. In neonates and infants, gestational alloimmune liver disease, inborn errors of metabolism and hemophagocytic lymphohistiocytosis are the main causes of ALF. In older children, viral infections, drug-induced hepatotoxicity and autoimmune hepatitis are more common. Idiopathic ALF represents up to 50% of most reported cohorts ( 2 ).
Wilson disease (WD) is an autosomal recessive disorder of copper metabolism, affecting approximately one in 30,000 to 40,000 individuals. WD may present as nonspecific elevation of liver enzyme levels, cirrhosis or with ALF. It results from mutations in the ATP7B protein, which functions to incorporate copper into ceruloplasmin – a necessary step for biliary copper excretion. As such, serum ceruloplasmin and copper levels are usually reduced, with simultaneous increases in 24 h urinary copper excretion. The pathological features of WD emerge from copper accumulation in various tissues, especially the liver and brain. Diagnosis is aided by identification of Kayser-Fleischer rings in the eyes and typical features on liver biopsy. ALF due to WD is typically lethal without liver transplantation ( 3 ).
Treatment of WD relies on copper chelation with penicillamine or trientine therapy, low copper diet and use of zinc to reduce copper absorption in the gut. Zinc stimulates metallothionein production in enterocytes of the intestinal tract. Metallothionein is a protein with great affinity for copper and, as such, copper is then excreted when the enterocytes naturally shed into the lumen of the intestinal tract ( 3 ).
This patient presented with classic findings of WD ( 3 ): ALF with only a modest rise in liver enzyme levels and bilirubin accompanying a nonautoimmune hemolytic anemia. His ceruloplasmin level was found to be low (67 mg/L [normal 242 mg/L to 396 mg/L]), and he was started on empirical penicillamine and zinc pending definitive diagnosis from examination of liver tissue for total copper content and from genetic testing for ATP7B mutations. He developed kidney failure and atrial fibrillation, likely related to copper toxicity of the cardiac conducting tissue, which improved with plasmapheresis and albumin dialysis. On day 7 of hospitalization, he received a liver transplant.
Although not found on initial examination, as an outpatient, Kayser-Fleisher rings were seen on slit-lamp examination. A liver specimen obtained during transplant demonstrated elevated liver copper levles (622 μg/g [normal 10 μg/g to 35 μg/g], WD likely if >250 μg/g).
Genetic testing found a p.N1270S variant on chromosome 13, consistent with a mutation in the ATP7B gene, and a known causal factor in the development of WD. This patient’s family was screened for WD and the patient’s eight-year-old brother was found to have a low ceruloplasmin level (39 mg/L [normal 242 mg/L to 396 mg/L]), high urine copper level and screened positive on genetic testing. He has been started on zinc therapy and a low copper diet, and is currently doing well.
Our patient has experienced episodes of acute liver rejection, but is maintained on immunosuppressive therapies.
CLINICAL PEARLS
- New-onset jaundice in children should prompt immediate measurement of INR and, if INR is elevated, emergency referral to an expert centre.
- WD should be suspected in a child presenting with ALF, especially in the context of nonautoimmune hemolytic anemia and a modest rise in liver enzyme levels.
- ALF due to WD is frequently fatal and, therefore, immediate referral for evaluation for liver transplantation is required
- Open access
- Published: 16 April 2024
Construction of a nomogram for predicting compensated cirrhosis with Wilson’s disease based on non-invasive indicators
- Jing Ping Wang 1 &
- Xiaoli Zhu 2
BMC Medical Imaging volume 24 , Article number: 90 ( 2024 ) Cite this article
97 Accesses
Metrics details
Wilson’s disease (WD) often leads to liver fibrosis and cirrhosis, and early diagnosis of WD cirrhosis is essential. Currently, there are few non-invasive prediction models for WD cirrhosis. The purpose of this study is to non-invasively predict the occurrence risk of compensated WD cirrhosis based on ultrasound imaging features and clinical characteristics.
A retrospective analysis of the clinical characteristics and ultrasound examination data of 102 WD patients from November 2018 to November 2020 was conducted. According to the staging system for WD liver involvement, the patients were divided into a cirrhosis group ( n = 43) and a non-cirrhosis group ( n = 59). Multivariable logistic regression analysis was used to identify independent influencing factors for WD cirrhosis. A nomogram for predicting WD cirrhosis was constructed using R analysis software, and validation of the model’s discrimination, calibration, and clinical applicability was completed. Due to the low incidence of WD and the small sample size, bootstrap internal sampling with 500 iterations was adopted for validation to prevent overfitting of the model.
Acoustic Radiation Force Impulse (ARFI), portal vein diameter (PVD), and serum albumin (ALB) are independent factors affecting WD cirrhosis. A nomogram for WD cirrhosis was constructed based on these factors. The area under the ROC curve (AUC) of the model’s predictive ability is 0.927 (95% CI: 0.88–0.978). As demonstrated by 500 Bootstrap internal sampling validations, the model has high discrimination and calibration. Clinical decision curve analysis shows that the model has high clinical practical value. ROC curve analysis of the model’s rationality indicates that the model’s AUC is greater than the AUC of using ALB, ARFI, and PVD alone.
The nomogram model constructed based on ARFI, PVD, and ALB can serve as a non-invasive tool to effectively predict the risk of developing WD cirrhosis.
Peer Review reports
Wilson’s disease (WD) is an autosomal recessive genetic disorder. Although it has a low incidence rate, it primarily affects children and adolescents aged [ 1 , 2 , 3 ]. If WD fibrosis is not accurately diagnosed and properly treated, it may progress to cirrhosis and lead to severe complications such as decompensated cirrhosis with portal hypertension or liver failure.
Liver biopsy is considered the gold standard for fibrosis staging. However, the distribution of copper in WD patients is uneven, and the degree of liver fibrosis is also uneven, so liver biopsy cannot accurately represent the overall copper content and fibrosis stage of the whole liver [ 4 – 5 ]. Due to its invasiveness, high cost, potential complications, and ethical issues, liver biopsy is not the preferred method for assessing the degree of liver fibrosis. Therefore, non-invasive methods have become a research hotspot.
Expert consensus suggests that transient elastography (TE) is a reliable method for assessing liver fibrosis in viral hepatitis [ 6 ]. However, TE has limitations and may not provide accurate measurements for patients who are excessively obese, have ascites, or have a small rib cage aperture. Several studies have shown that acoustic radiation force impulse (ARFI) is more effective than TE in patients with ascites and obesity, and also has a lower failure rate [ 7 ]. ARFI has a sensitivity and specificity of 92% and 86%, respectively, for diagnosing cirrhosis [ 8 ]. ARFI has been successfully applied to liver diseases such as chronic hepatitis B and C, and can be recommended as a reliable tool for monitoring liver fibrosis [ 9 , 10 , 11 , 12 , 13 ]. Unfortunately, due to the rarity of WD, there is little research on the application of ARFI in WD.
This study aims to build a predictive model based on ultrasound imaging features and clinical characteristics to predict the risk of developing compensated WD cirrhosis. It evaluates and validates the feasibility and effectiveness of the model in diagnosing WD cirrhosis.
This retrospective study was conducted following the ethical principles of the Declaration of Helsinki and was approved by the hospital’s ethics review committee (registration number: 2021MCZQ02). The study initially included 122 patients diagnosed with Wilson’s disease (WD) from November 2018 to November 2020 based on inclusion criteria and finally included 102 cases after applying the following exclusion criteria. Demographic characteristics, complications, liver function biochemical indicators, copper-related biochemical indicators, zinc-related biochemical indicators, liver fibrosis index, routine ultrasound measurements, and ARFI were extracted from the electronic medical record system.
Inclusion criteria: (1) This study included patients diagnosed with WD, following the diagnostic guidelines for WD published by the European Association for the Study of the Liver (EASL) in 2012 [ 14 ]; (2) Age < 65 years; (3) Body mass index (BMI) range of 18.5 ∼ 28.0 kg/m²; (4) No history of other types of hepatitis or excessive alcohol consumption; (5) No rheumatic diseases that may lead to fibrosis; (6) No diabetes or renal failure.
Exclusion Criteria: Patients were excluded if they met any of the following criteria: (1) Incomplete ARFI examination or ultrasound diagnostic characteristic information; (2) Incomplete clinical biochemical examination data; (3) Poor quality of ARFI images in WD patients due to limb tremors.
According to the inclusion criteria, a total of 122 WD patients were included in this study. However, 5 patients had incomplete ultrasound diagnostic information, 6 patients had incomplete clinical biochemical index data, and 9 patients had poor quality elastography images due to uncontrollable limb tremors. Therefore, these patients were excluded. Finally, the study population consisted of 102 patients. Figure 1 is a flowchart of the case screening and research process.
Flowchart illustrating the selection of the study population and the research
Demographic and laboratory indexes
On the day of the ultrasound measurement, collect the patient’s demographic characteristics and medical history. Laboratory tests included alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum albumin (ALB), prothrombin time (PT), serum copper, ceruloplasmin (CP), serum zinc, 24-hour urinary copper, 24-hour urinary zinc, PLT (platelet count), laminin (LN), hyaluronic acid (HA), collagen IV (CIV), pro-collagen protein III (P III N-P).
The examination of conventional ultrasound
The instrument used is the Siemens Ultrasound System S2000, which is equipped with a convex array probe 4C1 (1–4 MHz) and a linear array probe 9L4 (4–9 MHz). After fasting for 8 h, the patient underwent a routine B-mode ultrasound examination. First, the entire liver was scanned using the low-frequency convex array probe (Fig. 2 A, B), recording the morphological characteristics of the liver, portal vein velocity (PVV), portal vein diameter (PVD), spleen size, and ascites. When measuring the portal vein, the ultrasound beam was required to be as perpendicular to the portal vein as possible, measuring the maximum internal diameter of the portal vein. Take the median of six valid measurements as the final result. Then, the detailed characteristics of the liver capsule were examined using the high-frequency linear array probe (Fig. 2 C).
The measurements of ARFI
The ARFI examinations were conducted by two experienced ultrasound doctors with at least 10 years of abdominal ultrasound diagnostic experience. The ultrasound diagnosis was made by the two doctors, and a consensus on the diagnosis results was reached.
The instrument used is the Siemens Ultrasound System S2000, with a 4C1 probe and a frequency of 4.5 MHz. The instrument is equipped with Acoustic Radiation Force Imaging Virtual Touch Tissue Quantification (ARFI-VTQ) imaging mode.
The patient is placed in a supine position, lifting the right arm to open the intercostal space, and maintaining steady breathing. First, the patient underwent a routine ultrasound examination, documenting information such as the size and shape of the liver, the characteristics of the liver echo (Fig. 2 A, B, C), the diameter and flow velocity in the portal vein, the size of the spleen, and any ascites. Then, the operator selects the S5 and S8 segments of the patient’s liver, with the image depth set at 4–6 cm. When the appropriate liver parenchyma ultrasound section (with fewer blood vessels and clear liver parenchyma) is selected, instruct the patient to hold their breath. When the ultrasound image on the display is still, the trigger key is pressed to capture the image. During measurement, the sampling frame is placed perpendicular to the liver capsule, at least 2–3 cm away from the liver capsule, avoiding large blood vessels and intrahepatic bile ducts (Fig. 2 D). At the same sampling location, 10 sets of values are measured, with at least 6 sets being valid, and the median value is taken.
Quality Control: The IQR/Median (Interquartile Range/Median) ratio ≤ 0.3 is considered the reference for the quality control standard of this technique. During the examination of each patient, ARFI measurements should be taken from the same sampling section and position as much as possible.
The examination of conventional ultrasound and ARFI. A The liver of patients with WD exhibited a patchy, fatty appearance. B Scattered nodular hypoechoic regions were observed in the liver of WD patients. C The liver of WD patients displayed honeycomb-like nodules when observed with a linear array probe, while the liver capsule appeared smooth. D Liver stiffness was assessed using ARFI technology
Staging of WD liver involvement
The staging system for WD liver involvement is as follows [ 15 , 16 ]:
(I) Normal - no signs of laboratory or clinical abnormalities. (II) Elevated ALT with normal liver morphology features. (III) Abnormal liver morphology without cirrhotic manifestations. (IV) Clinical and imaging signs suggestive of compensated cirrhosis (Child-Pugh A). Compensated cirrhosis is diagnosed based on clinical manifestations, medical history, blood biochemistry, and imaging. The final diagnosis of compensated cirrhosis should meet two or more of the following criteria: ① Esophageal and gastric varices identified by endoscopy. ② Ultrasound, CT, and MRI are suggestive of cirrhosis or portal hypertension. ③ Hypersplenism characterized by thrombocytopenia (< 100 × 10 9 /L), after excluding other possible causes. ④ Decreased hepatic synthetic function characterized by prolonged prothrombin time (> 13 s) more than 7 days after discontinuation of thrombolytic or anticoagulant drugs, excluding other causes such as malnutrition or renal disease and decreased serum albumin (< 35 g/l). (V) Decompensated hepatic function (Child-Pugh B and C) or severely impaired cirrhosis. Decompensated cirrhosis is often accompanied by ascites, gastrointestinal bleeding, and hepatic encephalopathy. Combined with history, laboratory findings, and typical portal hypertension manifestations, decompensated cirrhosis can be easily diagnosed.
Data analysis and statistical methods
Statistical analysis was conducted using SPSS 26.0 software and R (R4.1.3). Continuous variables with a normal distribution were presented as mean ± standard deviation (x̄±s), while those with a non-normal distribution were presented as median (interquartile range). Categorical data were presented as counts or percentages. For the comparison of normally distributed data between two groups, two independent sample t-tests were used. For non-normally distributed data, non-parametric Mann-Whitney U tests were employed. The Pearson chi-square test was used to compare categorical data between two groups. In case the conditions for the Pearson chi-square test were not met, either continuous corrected chi-square or Fisher’s exact test were used. Baseline description and analysis of differences were performed using the Compare Groups package in R. Multivariable logistic regression was conducted using the glm package in R. Discrimination analysis was performed using the pROC, ggROC, and fbroc packages in the R language. Calibration analysis was conducted using the calibrate function in the rms package in R, as well as the val. prob and HL test packages. Clinical decision curves were created using the rmda and dcurves packages in R, and nomograms were constructed using the rms package in R. To prevent overfitting in column line plots, bootstrap resampling was performed 500 times to evaluate the internal validation of predictive efficiency. The significance level was set at p = 0.05.
Characteristics of patients
According to the staging system for liver involvement in WD, patients were divided into two groups: the WD cirrhosis group (WD liver involvement stage ≥ IV) and the non-cirrhosis group (WD liver involvement stage: I ∼ III). Table 1 shows the differences in demographic characteristics, complications, liver function biochemical indicators, copper-related biochemical indicators, zinc-related biochemical indicators, liver fibrosis index, routine ultrasound measurements, and ARFI between the two groups. The results revealed statistically significant differences between the groups in terms of age, ascites, ALB, PT, AST, PLT, PVV, PVD and ARFI. However, Other indicators showed no statistically significant difference between groups.
Univariate and multivariable regression analysis of WD cirrhosis and construction of the nomogram model
The assignment of classified variables in Logistic Regression was as follows: Ascites (+) = 1, Ascites (-) = 0, Splenomegaly (+) = 1, Normal Spleen Size = 0, Male = 1, Female = 0. Other indicators in the table were quantitative data and were input by original values. In the univariate regression analysis, factors with a p-value less than 0.1 were included in the multivariable regression analysis.
The results of the multivariable regression analysis indicated that ARFI and PVV were positively correlated with the risk of developing WD cirrhosis, with p-values less than 0.05 and regression coefficients of 6.1 and 0.44, respectively. ALB was negatively correlated with the risk of WD cirrhosis, also with a p-value less than 0.05 and a regression coefficient of -0.28 (Table 2 ). Other factors listed in the table did not show a significant correlation with the risk of cirrhosis, as their p-values were greater than 0.05. ARFI, PVV, and ALB were identified as independent predictors of WD cirrhosis.
A nomogram model was constructed to predict WD cirrhosis based on these factors (Fig. 3 ). In this nomogram, the value of each variable was projected onto the scale at the top to obtain the corresponding score. The scores of all variables were then summed to obtain the total score, which was compared to the total scoreline to determine the final predictive probability.
Construction of a nomogram model for predicting cirrhosis in WD based on ultrasound elastography, serum albumin, and ultrasound measurement of portal vein diameter. Note: ARFI (acoustic radiation force impulse), ALB (albumin), PVD (portal vein diameter)
Verification and evaluation of the prediction model
Discriminating ability of the model.
Figure 4 illustrates the effectiveness of the model in distinguishing hepatic cirrhosis in Wilson’s disease. In the modeling dataset, the area under the receiver operating characteristic (ROC) curve for the predictive ability of the model was 0.927 (95% CI: 0.88–0.978), as depicted in Fig. 4 A. The model’s predictive performance and stability were further validated through 500 iterations of bootstrap internal sampling, with a 95% CI of 0.869–0.97, as shown in Fig. 4 B. The results indicated that this model exhibits excellent discrimination and a strong ability to distinguish hepatic cirrhosis in Wilson’s disease.
Discrimination of the nomogram model for predicting WD cirrhosis. Note A Presented the ROC curve characteristics of the prediction model for diagnosing WD cirrhosis, with an area under the curve of 0.927 (95% CI: 0.88–0.978). B Depicted the ROC curve analysis of the model’s stability, which was validated through 500 iterations of bootstrap internal sampling, with a 95% CI of 0.869–0.97
Calibration of the prediction model
The results of the Hosmer-Lemeshow goodness-of-fit test for the prediction model revealed a chi-square value of 5.15 with a p-value of 0.82. Since the p-value for the Hosmer-Lemeshow test was greater than 0.05, it indicated that the model was well-calibrated. The calibration analysis of the model, depicted in Fig. 5 , showcased the model’s calibration after 500 iterations of Bootstrap internal sampling. The Brier score was 0.102, and the p-value was 0.839(>0.05). The calibration curve demonstrated a strong correlation between the predicted probabilities and the actual occurrences.
Calibration curves for model prediction of WD cirrhosis. Note: The model’s predicted probabilities were plotted on the x-axis, while the actual positive occurrence rate was plotted on the y-axis. The solid line (diagonal line) represented the ideal model, and the dashed line represented the calibration of the model established in this study. After conducting 500 iterations of bootstrap internal sampling, the model’s calibration analysis showed a Brier score of 0.102 and a p-value of 0.839(>0.05). These results indicate a high level of consistency between the predicted and actual outcomes
Analysis of the clinical utility and rationality of the prediction model
To assess the clinical utility of the nomogram, we used the predicted probability from the calibration plot as the test variable and the occurrence of WD cirrhosis in patients as the state variable. We constructed a clinical decision curve (DCA) for the nomogram model, as shown in Fig. 6 .
In the DSA curve, the two dashed lines represent the two extreme cases, with the grey horizontal line indicating that the model predicts there are no cirrhosis in all patients with WD and a clinical benefit of zero. The other grey line with a negative slope indicates that the model predicts there are cirrhosis in all WD patients, and the clinical benefit curve is a negative slope oblique line. The red curve represents the benefit for patients using the predictive model from this study. When the predicted probability is greater than the threshold of 0.05 (with a broader range), the red curve is higher than the grey horizontal line and the negatively sloped grey line, indicating that patients can benefit from the predictive model of this study.
Clinical decision curve analysis of the model. Note A is the decision curve of the model sampled by Bootstrap 500 times and B is the decision curve after cross-validation
Figure 7 demonstrated the rationality of the model, with the results indicating that the area under the curve for the nomogram was greater than the area under the curves for the individual use of ALB, ARFI, and PVD, this suggested that the ability to predict WD cirrhosis using this predictive model was superior to using ALB, ARFI, and PVD alone.
Reasonableness analysis of the predictive model (Nomo-ROC curve). Note The area under the curve for nomogram (model) is larger than the area under the curve for ALB, ARFI and PVD
The liver plays a key role in maintaining copper metabolism balance. When this balance is disrupted, excessive copper accumulation in the liver can lead to liver damage, known as WD. Early changes of WD include hepatic steatosis, and over time, fibrosis around the portal vein progresses to cirrhosis [ 17 – 18 ]. Early diagnosis and timely treatment of cirrhosis are crucial for managing chronic liver diseases.
This study categorized WD patients into two groups: cirrhosis and non-cirrhosis, based on the criteria for WD liver involvement. The differences between the two groups were compared in terms of demographic characteristics, biochemical indicators of liver function, related biochemical indicators of copper and zinc, liver fibrosis index, routine ultrasound measurements, and ARFI. Indicators with significant differences between the groups were included in multivariable logistic regression analysis. Ultimately, the results showed that the independent influencing factors for WD cirrhosis were ALB, ARFI, and PVD, and a nomogram for WD cirrhosis was constructed. Since WD patients are rare, this study did not obtain external validation data from other research centers. However, the study used a method of 500 Bootstrap self-sampling for internal validation to prevent model overfitting. After validation of this model, it was shown to have high discriminative ability, calibration, clinical utility, and rationality.
ARFI and PVD are important influencing factors of WD cirrhosis
ARFI technology is a type of ultrasound elastography technology. It uses focused ultrasonic beams as the excitation mechanism, causing longitudinal compression and lateral vibration in tissues when force is applied. This produces shear waves in the tissue, which are then captured using a specific electronic system to gather signals from the tissue. The propagation speed of shear waves in the region of interest can thus be obtained. The speed of shear waves is closely related to tissue elasticity [ 19 ]. By estimating the tissue elasticity modulus, one can indirectly reflect the degree of elasticity in that area. ARFI is similar to a physical palpation examination of the tissue, providing quantitative measurements of tissue hardness.
Several studies have shown a strong correlation between the results of ARFI (especially Siemens S2000) and liver biopsy results in various liver disease patients [ 20 , 21 , 22 ]. Currently, ARFI has established relatively standardized criteria for the diagnosis of viral hepatitis cirrhosis, but research on WD cirrhosis is limited, and diagnostic criteria have not yet been formulated. One guideline suggests using thresholds of 14.6 kPa and 10 kPa to diagnose or exclude hepatitis C cirrhosis [ 23 ]. A meta-analysis of non-invasive liver fibrosis assessment in patients with chronic hepatitis B and hepatitis C found that ARFI was accurate and reliable for diagnosing viral hepatitis liver fibrosis, and an ARFI value of 1.87 m/s could be used as the cutoff for significant liver fibrosis in hepatitis B [ 24 ], with ARFI values for cirrhosis being higher than those for significant liver fibrosis.
This study indicated that the threshold value for diagnosing WD cirrhosis was 1.88 m/s (E = 10.6 kPa). E represented the absolute value of the elastic modulus, and the conversion equation between E and Cs was: E = 3ρCs 2 , where ρ ≈ 1000 Kg/m 3 ) [ 25 – 26 ], denoted tissue density, and Cs was the shear wave propagation speed in human tissues. The results showed that the threshold value for WD cirrhosis was lower than that for other liver disease cirrhosis. We analyzed the main reasons: (1) WD patients often exhibited thickening, enhancement, and nodular distribution along the portal vein in the liver parenchyma, which was related to higher copper deposition around the portal vein [ 27 ]. However, ARFI measurements should avoid the portal vein and were usually taken far from it, which may have led to an underestimation of LSM in WD patients. Secondly, WD typically began in childhood or adolescence and was treated early, leading to a stable course and relatively mild inflammation [ 28 ]. Thirdly, studies had shown that compared to hepatitis B, WD cirrhosis presented with less severe portal hypertension, lower prothrombin time and transaminase levels, and higher albumin levels [ 29 ]. Transaminases, albumin, and prothrombin time were closely related to the degree of liver inflammation, while liver stiffness was associated with liver fibrosis, inflammation, and portal pressure. Therefore, the inflammatory state of the liver and the pressure of the portal vein could affect LSM measurements, thus, it may affect the accuracy of liver fibrosis assessment [ 30 – 31 ].
In this study, another ultrasonographic indicator that affected WD cirrhosis was the PVD. Ultrasound measurement of the PVD was a non-invasive, simple, and reproducible method, usually used to assess liver blood flow and portal vein pressure. WD cirrhosis was caused by copper deposition in the liver, leading to hepatocyte degeneration, necrosis, fibrosis, and sinusoidal dilation. These pathological changes affected the histological structure of the liver tissue, thereby affecting blood reflux through the portal vein, resulting in an enlarged portal vein diameter. Studies have shown that ultrasound measurement of the PVD had high sensitivity and specificity for diagnosing WD cirrhosis, and the degree of liver inflammation or fibrosis was directly proportional to the width of the PVD [ 32 ]. Changes in the PVD indirectly reflected the degree of cirrhosis and changes in disease conditions.
The value of the nomogram
In our previous study [ 33 ], we only conducted a multivariable regression analysis of the factors influencing WD cirrhosis, but multivariable logistic regression can only analyze the factors affecting positive events and cannot easily predict the probability of positive events. This study established a nomogram model based on our preliminary research. A nomogram can transform complex regression equations into visual graphics, characterized by being simple and easy to understand. Moreover, nomograms have strong visualization and operability. In clinical practice, projecting patients’ PVD, ARFI, and ALB measurement values onto the ruler at the top of the nomogram yields corresponding scores. Adding up all variable scores to obtain the total score, then comparing this score with the total score line, assesses the final predicted probability.
This article’s predictive model construction is based on the R data analysis system, which provides a unified framework for establishing predictive models, while machine learning often offers a variety of algorithms [ 34 – 35 ]. Currently, radiomics is a medical field with broad application prospects [ 36 – 37 ], and in future research on WD cirrhosis, we hope to explore radiomics analysis methods based on WD ultrasound images to further improve the accuracy of predictions.
This study has some limitations. First, it is a retrospective study, and the ideal study design should be a randomized controlled trial. Second, due to the rarity of WD, our research institution’s sample size is not large enough. In addition, the low incidence of WD makes it difficult to obtain relevant data from a large number of external research units, and models without external validation may lead to overfitting of the model, reducing its clinical applicability. However, this problem was partially resolved through internal validation by using 500 Bootstrap independent sampling. Another limitation is liver biopsy, which is the gold standard for diagnosing WD cirrhosis, but is not widely accepted in clinical practice. Therefore, this study could not obtain sufficient pathological reference but used the WD liver involvement staging system as the staging standard. However, this staging system has been cited in multiple studies related to WD.
ARFI, PVD, and ALB are influencing factors in predicting WD cirrhosis. The nomogram model based on these three factors demonstrates high reliability and clinical utility. It can serve as a visual assessment tool for predicting the risk of WD cirrhosis, offering a convenient and user-friendly method.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71. https://doi.org/10.1016/j.jhep.2018.09.014 .
Article PubMed Google Scholar
Wooton-Kee CR, Jain AK, Wagner M, et al. Elevated copper impairs hepatic nuclear receptor function in Wilson’s disease. J Clin Invest. 2015;125(9):3449–60. https://doi.org/10.1172/JCI78991 .
Article PubMed PubMed Central Google Scholar
Członkowska A, Litwin T, Dusek P, et al. Wilson disease. Nat Rev Dis Primers. 2018;4(1):21. https://doi.org/10.1038/s41572-018-0018-3 .
Liggi M, Mais C, Demurtas M, et al. Uneven distribution of hepatic copper concentration and diagnostic value of double-sample biopsy in Wilson’s disease. Scand J Gastroenterol. 2013;48(12):1452–8. https://doi.org/10.3109/00365521.2013.845904 .
Article CAS PubMed Google Scholar
Yang X, Tang XP, Zhang YH, et al. Prospective evaluation of the diagnostic accuracy of hepatic copper content, as determined using the entire core of a liver biopsy sample. Hepatology. 2015;62(6):1731–41. https://doi.org/10.1002/hep.27932 .
Shearer JE, Jones R, Parker R, Ferguson J, Rowe IA. The natural history of Advanced Chronic Liver Disease defined by transient Elastography. Clin Gastroenterol Hepatol. 2023;21(3):694–e7038. https://doi.org/10.1016/j.cgh.2022.03.015 .
Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33(8):1138–47. https://doi.org/10.1111/liv.12240 .
Cui XW, Li KN, Yi AJ, et al. Ultrasound elastography. Endosc Ultrasound. 2022;11(4):252–74. https://doi.org/10.4103/EUS-D-21-00151 .
Chen SH, Lai HC, Chiang IP, et al. Performance of Acoustic Radiation Force Impulse Elastography for staging liver fibrosis in patients with chronic Hepatitis C after viral eradication. Clin Infect Dis. 2020;70(1):114–22. https://doi.org/10.1093/cid/ciz161 .
Friedrich-Rust M, Buggisch P, de Knegt RJ, et al. Acoustic radiation force impulse imaging for non-invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2013;20(4):240–7. https://doi.org/10.1111/j.1365-2893.2012.01646.x .
Friedrich-Rust M, Romen D, Vermehren J, et al. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81(3):e325–31. https://doi.org/10.1016/j.ejrad.2011.10.029 .
Zhang D, Li P, Chen M, et al. Non-invasive assessment of liver fibrosis in patients with alcoholic liver disease using acoustic radiation force impulse elastography. Abdom Imaging. 2015;40(4):723–9. https://doi.org/10.1007/s00261-014-0154-5 .
Friedrich-Rust M, Nierhoff J, Lupsor M, et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19(2):e212–9. https://doi.org/10.1111/j.1365-2893.2011.01537.x .
European Association for Study of Liver. EASL Clinical Practice guidelines: Wilson’s disease. J Hepatol. 2012;56(3):671–85. https://doi.org/10.1016/j.jhep.2011.11.007 .
Article Google Scholar
Karlas T, Hempel M, Tröltzsch M, et al. Non-invasive evaluation of hepatic manifestation in Wilson disease with transient elastography, ARFI, and different fibrosis scores. Scand J Gastroenterol. 2012;47(11):1353–61. https://doi.org/10.3109/00365521.2012.719924 .
Hwang J, Yoon HM, Jung AY, et al. Diagnostic performance of Ultrasound Elastography and Serologic Fibrosis Indices for evaluation of hepatic involvement in Wilson Disease. J Ultrasound Med. 2020;39(11):2231–42. https://doi.org/10.1002/jum.15334 .
Alqahtani SA, Chami R, Abuquteish D, et al. Hepatic ultrastructural features distinguish paediatric Wilson disease from NAFLD and autoimmune hepatitis. Liver Int. 2022;42(11):2482–91. https://doi.org/10.1111/liv.15319 .
Schilsky ML, Roberts EA, Bronstein JM, et al. A multidisciplinary approach to the diagnosis and management of Wilson disease: executive summary of the 2022 Practice Guidance on Wilson disease from the American Association for the study of Liver diseases. Hepatology. 2023;77(4):1428–55. https://doi.org/10.1002/hep.32805 .
Nehring P, Szeligowska J, Przybyłkowski A. Elastography of the liver in Wilson’s Disease. Diagnostics (Basel). 2023;13(11):1898. https://doi.org/10.3390/diagnostics13111898 .
Chinese Foundation for Hepatitis Prevention and Control; Chinese Society of Infectious Disease and Chinese Society of Hepatology, Chinese Medical Association; Liver Disease Committee of Chinese Research Hospital Association. Zhonghua Gan Zang Bing Za Zhi. 2019;27(3):182–91. https://doi.org/10.3760/cma.j.issn.1007-3418.2019.03.004 .
Cassinotto C, Boursier J, de Lédinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63(6):1817–27. https://doi.org/10.1002/hep.28394 .
Goya C, Hamidi C, Yavuz A, et al. The role of Acoustic Radiation Force Impulse Elastography in the differentiation of infectious and neoplastic liver lesions. Ultrason Imaging. 2015;37(4):312–22. https://doi.org/10.1177/0161734614566697 .
Kiani A, Brun V, Lainé F, et al. Acoustic radiation force impulse imaging for assessing liver fibrosis in alcoholic liver disease. World J Gastroenterol. 2016;22(20):4926–35. https://doi.org/10.3748/wjg.v22.i20.4926 .
Article CAS PubMed PubMed Central Google Scholar
Hu X, Qiu L, Liu D, Qian L. Acoustic Radiation Force Impulse (ARFI) Elastography for noninvasive evaluation of hepatic fibrosis in chronic hepatitis B and C patients: a systematic review and meta-analysis [published correction. Appears Med Ultrason. 2017;19(1):23–31. https://doi.org/10.11152/mu-942 .
Marcellin P, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29(2):242–7. https://doi.org/10.1111/j.1478-3231.2008.01802.x .
Zheng J, Guo H, Zeng J, et al. Two-dimensional shear-wave elastography and conventional US: the optimal evaluation of liver fibrosis and cirrhosis. Radiology. 2015;275(1):290–300. https://doi.org/10.1148/radiol.14140828 .
Faa G, Nurchi V, Demelia L, et al. Uneven hepatic copper distribution in Wilson’s disease. J Hepatol. 1995;22(3):303–8. https://doi.org/10.1016/0168-8278(95)80283-5 .
Kazemi K, Geramizadeh B, Nikeghbalian S, et al. Effect of D-penicillamine on liver fibrosis and inflammation in Wilson disease. Exp Clin Transpl. 2008;6(4):261–3. PMID: 19338486.
Google Scholar
Zhong HJ, Sun HH, Xue LF, McGowan EM, Chen Y. Differential hepatic features presenting in Wilson disease-associated cirrhosis and hepatitis B-associated cirrhosis. World J Gastroenterol. 2019;25(3):378–87. https://doi.org/10.3748/wjg.v25.i3.378 .
Xie LT, Gu JH, Chai WL, et al. Pre-operative detection of liver fibrosis in Hepatocellular Carcinoma patients using 2D Shear Wave Elastography: where to measure? Ultrasound Med Biol. 2020;46(6):1412–23. https://doi.org/10.1016/j.ultrasmedbio.2020.02.012 .
Zhang Y, Chen H, Chen S, Li W. Prognostic value of liver stiffness measurement in patients with hepatocellular carcinoma (HCC) treated by radiofrequency ablation: a meta-analysis. Int J Hyperth. 2021;38(1):1052–9. https://doi.org/10.1080/02656736.2021.1947529 .
Article CAS Google Scholar
Tian L, Tang S, Wang N, Deng H, et al. Hepatic and portal vein Doppler ultrasounds in assessing liver inflammation and fibrosis in chronic HBV infection with a normal ALT level. Front Med (Lausanne). 2023;10:1178944. https://doi.org/10.3389/fmed.2023.1178944 .
Li Y, Ma J, Li B, Zhu X, et al. Cirrhosis of Wilson’s disease: high and low cutoff using acoustic radiation force impulse (ARFI) -Comparison and combination with serum fibrosis index. Clin Hemorheol Microcirc. 2021;79(4):575–85. https://doi.org/10.3233/CH-211219 .
Heydarheydari S, Birgani MJT, Rezaeijo SM. Auto-segmentation of head and neck tumors in positron emission tomography images using non-local means and morphological frameworks. Pol J Radiol. 2023;88:e365–70. https://doi.org/10.5114/pjr.2023.130815 .
Salmanpour MR, Hosseinzadeh M, Rezaeijo SM, et al. Fusion-based Tensor radiomics using reproducible features: application to survival prediction in head and neck cancer. Comput Methods Programs Biomed. 2023;240:107714. https://doi.org/10.1016/j.cmpb.2023.107714 .
Hosseinzadeh M, Gorji A, Fathi Jouzdani A, et al. Prediction of Cognitive decline in Parkinson’s Disease using clinical and DAT SPECT Imaging features, and Hybrid Machine Learning systems. Diagnostics (Basel). 2023;13(10):1691. https://doi.org/10.3390/diagnostics13101691 .
Rezaeijo SM, Chegeni N, Baghaei N, et al. Within-modality synthesis and Novel Radiomic evaluation of Brain MRI scans. Cancers (Basel). 2023;15(14):3565. https://doi.org/10.3390/cancers15143565 .
Download references
Acknowledgements
I would like to thank my friend, Libao Qi, for his guidance through each stage of the process.
Provincial key specialty construction projects in 2020.
Author information
Authors and affiliations.
Department of Ultrasound, The first affiliated hospital of Anhui University of Traditional Chinese Medicine, MeiShan Road, 230031, Anhui, P.R. China
Yan Li & Jing Ping Wang
Department of Intervention, The First Affiliated Hospital of Soochow University, 899, The Pinghai Road, 215006, Jiangsu, P.R. China
You can also search for this author in PubMed Google Scholar
Contributions
Y L collected clinical data and drafted the manuscript, Y L is the First Author and Corresponding author. Jp W and Xl Z aided in the design of the study. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Yan Li .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of the first affiliated hospital of Anhui University of Traditional Chinese Medicine (registration number: 2021MCZQ12). The requirement for indi-vidual informed consent was waived by the committee (full name: The Ethics Committee of the first affiliated hospital of Anhui University of Traditional Chinese Medicine) because of the ret-rospective nature of the study. The data are anonymous, and the requirement for informed consent was therefore waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, Y., Wang, J.P. & Zhu, X. Construction of a nomogram for predicting compensated cirrhosis with Wilson’s disease based on non-invasive indicators. BMC Med Imaging 24 , 90 (2024). https://doi.org/10.1186/s12880-024-01265-w
Download citation
Received : 21 September 2023
Accepted : 29 March 2024
Published : 16 April 2024
DOI : https://doi.org/10.1186/s12880-024-01265-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Wilson's disease
- ARFI (acoustic radiation force impulse)
BMC Medical Imaging
ISSN: 1471-2342
- General enquiries: [email protected]
- Search Menu
- Advance Articles
- Editor's Choice
- Braunwald's Corner
- ESC Guidelines
- EHJ Dialogues
- Issue @ a Glance Podcasts
- CardioPulse
- Weekly Journal Scan
- European Heart Journal Supplements
- Year in Cardiovascular Medicine
- Asia in EHJ
- Most Cited Articles
- ESC Content Collections
- Author Guidelines
- Submission Site
- Why publish with EHJ?
- Open Access Options
- Submit from medRxiv or bioRxiv
- Author Resources
- Self-Archiving Policy
- Read & Publish
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Journals Career Network
- About European Heart Journal
- Editorial Board
- About the European Society of Cardiology
- ESC Publications
- War in Ukraine
- ESC Membership
- ESC Journals App
- Developing Countries Initiative
- Dispatch Dates
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, acknowledgements, declarations, data availability, ethical approval, pre-registered clinical trial number, hepatocellular carcinoma in survivors after fontan operation: a case–control study.
- Article contents
- Figures & tables
- Supplementary Data
Yuli Y Kim, Gentian Lluri, Christiane Haeffele, Tami Daugherty, Richard A Krasuski, John D Serfas, R Andrew de Freitas, Avaliese Porlier, Adam M Lubert, Fred M Wu, Anne Marie Valente, Eric V Krieger, Jonathan Buber, Fred H Rodriguez, Scott Gaignard, Anita Saraf, Morgan Hindes, Michael G Earing, Matthew J Lewis, Marlon S Rosenbaum, Ali N Zaidi, Kali Hopkins, Elisa A Bradley, Ari M Cedars, Jong L Ko, Wayne J Franklin, Abby Frederickson, Salil Ginde, Jasmine Grewal, Annique Nyman, Jungwon Min, Charlotte Schluger, Elizabeth Rand, Benjamin E Rosenthal, Moira Hilscher, Jack Rychik, Maarouf A Hoteit, Hepatocellular carcinoma in survivors after Fontan operation: a case–control study, European Heart Journal , Volume 45, Issue 16, 21 April 2024, Pages 1477–1480, https://doi.org/10.1093/eurheartj/ehad788
- Permissions Icon Permissions
Liver injury is universal in patients who have undergone Fontan palliation for complex congenital heart disease and results in fibrosis and cirrhosis. 1 Fontan-associated liver disease (FALD) can be rarely complicated by hepatocellular carcinoma (HCC), 2–4 risk factors for which are not fully elucidated. We aim to identify clinical characteristics associated with the development of HCC in adult single ventricle patients with Fontan circulation.
This is a multi-centre, retrospective case–control study of Fontan patients ≥ 18 years old across 18 North American centres of the Alliance for Adult Research in Congenital Cardiology between 2005 and 2021 and approved by all research oversight boards. Cases were included based on histologic or imaging-based diagnosis of HCC [computed tomography (CT) or magnetic resonance imaging (MRI) that fulfil Liver Imaging Reporting and Data System® (LI-RADS)-5 criteria, 5 plus alpha-fetoprotein (AFP) > 20 ng/mL or evidence of lesion growth ≥ 50% within 6 months or > 100% in a period of over 6 months on serial imaging studies]. Controls were randomly selected in a 3:1 ratio from the centre in which the case was derived. Inclusion criteria were blood work and abdominal imaging (ultrasound, CT, or MRI) within 18 months of the last clinic visit, the latter without a focal liver lesion or if present, imaging evidence of stable size over at least 24 months. Those without imaging or labs, AFP ≥ 10 ng/dL at any time, or history of hepatitis B or C were excluded.
Clinical characteristics and laboratory values were collected by chart review. AST to Platelet Ratio Index (APRI) and Fibrosis-4 Index (FIB-4) (non-invasive methods that correlate with degree of fibrosis on biopsy in other chronic liver disease states) 6 and Model for End-stage Liver Disease excluding INR (MELD-XI) scores were calculated and collapsed into binary (low/high) variables using cut-offs of < 0.5 and ≥ 0.5, < 1.45 and ≥ 1.45, and < 11 and ≥ 11, respectively. Radiology and liver biopsy reports were reviewed by a board-certified hepatologist. Bivariate and multivariate analyses were performed to examine association between variables and HCC. We included characteristics having a significant association with HCC risk ( P <0.05) in the multivariate logistic regression model after evaluating collinearity and area under the curve (AUC) of the receiver operating characteristic (ROC).
There were 58 HCC cases (43% female and 79% White) and 174 controls ( n = 232). Out of a total of 3251 adult Fontan patients followed across all 18 centres, the estimated prevalence of HCC was 1.8%. Median age of HCC diagnosis was 31 (interquartile range 26–38) years. One-year survival after HCC diagnosis was 81%.
There was no difference between cases and controls regarding age, sex, body mass index, underlying congenital heart defect, age at Fontan operation, or time since Fontan; those with HCC more commonly had undergone right atrial to pulmonary artery or right atrial to right ventricle Fontan repair, had higher burden of cardiac comorbidities, including arrhythmia, desaturation (oxygen saturation < 90%), heart failure, and lymphatic disorders, and were more likely to have undergone prior Fontan revision and valve surgery, despite comparable echocardiographic and historical catheterization haemodynamic parameters. Just over half of HCC cases occurred in patients with FIB-4 < 1.45, a threshold which has a 90% negative predictive value for advanced fibrosis in other liver disease populations. 7 Similarly, nearly half of HCC cases occurred in patients with MELD-XI < 11 and with APRI < 0.5.
Bivariate analyses examining the relationship between select variables and HCC were performed ( Table 1 ). There was no relationship between age at Fontan or time since Fontan operation with HCC.
Bivariate analyses for the odds of hepatocellular carcinoma
P -values <0.05 are noted in bold. APRI, AST to Platelet Ratio Index; AST, aspartate transaminase; CI, confidence interval; FIB-4, Fibrosis-4; MELD-XI, Model for End-stage Liver Disease eXcluding International normalized ratio; OR, odds ratio.
We created two multivariable models using select variables from the bivariate analyses. Model A showed desaturation [adjusted odds ratio (aOR) 2.4, 95% confidence interval (CI) 1.1–5.2; P = .02], history of Fontan revision (aOR 2.3, 95% CI 1.1–4.8; P = .02), and thrombocytopenia (aOR 2.3, 95% CI 1.2–4.5; P = .02) were associated with increased odds of HCC. Model B showed desaturation (aOR 2.2, 95% CI 1.0–4.7; P = .05) and FIB-4 ≥ 1.45 (aOR 3.9, 95% CI 2.0–8.0; P < .001) as associated with higher odds of developing HCC. Both models had ROC AUC = 0.72.
We hypothesized that HCC is time-dependent and that those affected would be older than controls, but the data do not suggest that length of exposure to Fontan physiology is the main driver of HCC. Instead, we found that advanced FALD and cardiac comorbidities were more prevalent in HCC cases. Progression of liver fibrosis in FALD is not uniform 8 and is likely driven by circulatory factors, which hypothetically could explain why length of exposure to Fontan physiology alone was not found to be significant. It is important to emphasize that non-invasive fibrosis scores, such as APRI and FIB-4, do not have adequate discrimination for the detection of HCC and should be interpreted in the context of other clinical factors. Our results support the concept that worse FALD (by liver function tests and imaging) is the primary substrate for the development of HCC in the Fontan liver and suggest that ‘sicker’ Fontan patients—those with desaturation and need for cardiac surgery beyond the Fontan operation itself—may be at highest risk.
The numbers studied were relatively small but represent the largest collection of HCC in the Fontan population published to date. This was a study on Fontan patients of adult age and therefore results may not be applicable to the paediatric population. We also acknowledge that the imaging-based inclusion criteria as described are not standard, as LI-RADS is not applicable in ‘cardiac congestion’. By additionally requiring either elevated AFP or evidence of rapid lesion growth, we attempted to improve the diagnostic accuracy of LI-RADS in the Fontan liver.
Hepatocellular carcinoma is diagnosed in up to 2% of adult Fontan patients. Those with more advanced FALD represented by abnormal liver biochemistries and signs of circulatory failure may be at increased risk. Until more clearly defined risk factors are identified, we recommend abdominal ultrasounds and AFP every 6 months for adult Fontan patients in accordance with guidelines for HCC screening. 9
We would like to acknowledge the Alliance for Adult Research in Congenital Cardiology for its support rendered to this study.
Disclosure of Interest
All authors declare no disclosure of interest for this contribution.
No data were generated or analysed for or in support of this paper.
This study was supported by a grant from the Children’s Hospital of Philadelphia Cardiac Center.
Ethical approval was not required.
None supplied.
Rychik J , Veldtman G , Rand E , Russo P , Rome JJ , Krok K , et al. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium . Pediatr Cardiol 2012 ; 33 : 1001 – 12 . https://doi.org/10.1007/s00246-012-0315-7
Google Scholar
Egbe AC , Poterucha JT , Warnes CA , Connolly HM , Baskar S , Ginde S , et al. Hepatocellular carcinoma after Fontan operation: multicenter case series . Circulation 2018 ; 138 : 746 – 8 . https://doi.org/10.1161/CIRCULATIONAHA.117.032717
Ohuchi H , Hayama Y , Nakajima K , Kurosaki K , Shiraishi I , Nakai M . Incidence, predictors, and mortality in patients with liver cancer after Fontan operation . J Am Heart Assoc 2021 ; 10 : e016617 . https://doi.org/10.1161/JAHA.120.016617
Inuzuka R , Nii M , Inai K , Shimada E , Shinohara T , Kogiso T , et al. Predictors of liver cirrhosis and hepatocellular carcinoma among perioperative survivors of the Fontan operation . Heart 2023 ; 109 : 276 – 82 . https://doi.org/10.1136/heartjnl-2022-320940
American College of Radiology . Liver Imaging Reporting and Data System (LI-RADS) v2018 . ACR. 2018. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf? la=en (14 April 2023, date last accessed).
Chou R , Wasson N . Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection . Ann Intern Med 2013 ; 159 : 372 . https://doi.org/10.7326/0003-4819-159-5-201309030-00021
Sterling RK , Lissen E , Clumeck N , Sola R , Correa MC , Montaner J , et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection . Hepatology 2006 ; 43 : 1317 – 25 . https://doi.org/10.1002/hep.21178
Goldberg DJ , Surrey LF , Glatz AC , Dodds K , O’Byrne ML , Lin HC , et al. Hepatic fibrosis is universal following Fontan operation, and severity is associated with time from surgery: a liver biopsy and hemodynamic study . J Am Heart Assoc 2017 ; 6 : e004809 . https://doi.org/10.1161/JAHA.116.004809
Marrero JA , Kulik LM , Sirlin CB , Zhu AX , Finn RS , Abecassis MM , et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases . Hepatology 2018 ; 68 : 723 – 50 . https://doi.org/10.1002/hep.29913
Email alerts
More on this topic, citing articles via, looking for your next opportunity, affiliations.
- Online ISSN 1522-9645
- Print ISSN 0195-668X
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Hosted content
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Online First
- Clinical, experimental and pathophysiological effects of Yaq-001: a non-absorbab le, gut-restricted adsorbent in models and patients with cirrhosis
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Jinxia Liu 1 , 2 ,
- Jane MacNaughtan 1 ,
- Annarein J C Kerbert 1 ,
- Theo Portlock 3 ,
- Javier Martínez Gonzalez 4 , 5 ,
- Frederick Clasen 3 ,
- Abeba Habtesion 1 ,
- Huoyan Ji 6 ,
- Qin Jin 7 ,
- Alexandra Phillips 1 ,
- Francesco De Chiara 1 ,
- Ganesh Ingavle 8 , 9 ,
- Cesar Jimenez 5 ,
- http://orcid.org/0000-0002-3219-6963 Giacomo Zaccherini 10 , 11 ,
- Katherine Husi 12 ,
- Miguel Angel Rodriguez Gandia 13 ,
- Paul Cordero 9 ,
- Junpei Soeda 1 ,
- Lynda McConaghy 14 ,
- Jude Oben 1 ,
- Karen Church 14 ,
- Jia V Li 15 ,
- Haifeng Wu 2 ,
- Aarti Jalan 16 ,
- Pere Gines 17 ,
- Elsa Solà 17 ,
- Simon Eaton 18 ,
- Carrie Morgan 14 ,
- Michal Kowalski 14 ,
- Daniel Green 14 ,
- Amir Gander 19 ,
- http://orcid.org/0000-0002-8222-4555 Lindsey A Edwards 20 , 21 ,
- I Jane Cox 22 , 23 ,
- Helena Cortez-Pinto 24 ,
- Thomas Avery 25 ,
- Reiner Wiest 26 ,
- Francois Durand 27 ,
- Paolo Caraceni 10 , 28 ,
- Roberto Elosua 29 ,
- Joan Vila 29 ,
- Marco Pavesi 30 ,
- Vicente Arroyo 30 ,
- Nathan Davies 1 ,
- Rajeshwar P Mookerjee 1 ,
- Victor Vargas 5 ,
- Susan Sandeman 8 ,
- http://orcid.org/0000-0002-5696-359X Gautam Mehta 1 ,
- Saeed Shoaie 3 ,
- Julian Marchesi 31 ,
- http://orcid.org/0000-0001-9131-2592 Agustín Albillos 32 ,
- Fausto Andreola 1 ,
- http://orcid.org/0000-0002-7747-4015 Rajiv Jalan 1 , 30
- 1 Liver Failure Group , UCL Institute for Liver & Digestive Health, Division of Medicine , London , UK
- 2 Department of Gastroenterology , Affiliated Hospital of Nantong University , Nantong , Jiangsu , China
- 3 Centre for Host-Microbiome Interactions, Faculty of Dentistry, Oral & Craniofacial Sciences , King’s College London , London , UK
- 4 Hospital Ramón y Cajal, IRYCIS, CIBEREHD, Universidad de Alcalá , Madrid , Spain
- 5 Liver Unit, Hospital Vall d’Hebron, Universitat Autónoma, CIBERehd , Barcelona , Spain
- 6 Department of Laboratory Medicine , Affiliated Hospital of Nantong University , Nantong , China
- 7 Department of Pathology , Affiliated Hospital of Nantong University , Nantong , China
- 8 Centre for Regenerative Medicine and Devices, School of Applied Sciences , University of Brighton , Brighton , UK
- 9 Symbiosis Centre for Stem Cell Research (SCSCR), Symbiosis School of Biological Sciences (SSBS), Symbiosis International (Deemed University) , Pune , India
- 10 Department of Medical and Surgical Sciences , University of Bologna , Bologna , Italy
- 11 Unit of Semeiotics, Liver and Alcohol-related Diseases , University of Bologna Hospital of Bologna Sant'Orsola-Malpighi Polyclinic , Bologna , Italy
- 12 Department of Gastroenterology , Inselspital University Hospital Bern , Bern , Switzerland
- 13 Hospital Universitario Ramon y Cajal , Madrid , Spain
- 14 Yaqrit Discovery Limited. The Elms Courtyard, Bromesberrow , Ledbury , UK
- 15 Department of Surgery and Cancer , Imperial College London , London , UK
- 16 King's College Hospital , London , UK
- 17 Liver Unit, Hospital Clinic of Barcelona, IDIBAPS, Faculty of Medicine and Health sciences , University of Barcelona , Barcelona , Spain
- 18 Institute of Child Health , University College London , London , UK
- 19 Tissue Access for Patient Benefit , University College London , London , UK
- 20 Centre for Host Microbiome Interactions, Faculty of Dentistry, Oral & Craniofacial Sciences, Guy's Tower, Guy's Hospital , King’s College London , London , UK
- 21 Institute of Liver Studies, School of Immunology and Microbial Sciences, Faculty of Life Sciences and Medicine , King’s College London , London , UK
- 22 The Roger Williams Institute of Hepatology, Foundation for Liver Research , London , UK
- 23 Faculty of Life Sciences and Medicine, King's College London , London , UK
- 24 Clínica Universitária de Gastrenterologia, Laboratório de Nutrição, Faculdade de Medicina, Universidade de Lisboa , Lisbon , Portugal
- 25 Yaqrit Limited , London , UK
- 26 UVCM Gastroenterology , University Bern , Bern , Switzerland
- 27 Hepatology and Liver Intensive Care , Hospital Beaujon, Clichy, University paris Cité , Paris , France
- 28 Unit of Semeiotics, Liver and Alcohol Related Diseases , IRCCS Azienda Ospedaliero-Universitaria di Bologna , Bologna , Italy
- 29 C/de Joan Güell , Barcelona , Spain
- 30 European Foundation for the Study of Chronic Liver Failure (EF CLIF) , Barcelona , Spain
- 31 Division of Digestive Diseases, Department of Metabolism, Digestion and Reproduction , St Mary’s Hospital, Imperial College London , London , UK
- 32 Department of Gastroenterology and Hepatology, Hospital Universitario Ramon y Cajal, Universidad de Alcalá, Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS) , Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas (CIBEREHD) , Madrid , Spain
- Correspondence to Professor Rajiv Jalan, UCL Institute for Liver & Digestive Health, London NW3 2PF, UK; r.jalan{at}ucl.ac.uk
Objective Targeting bacterial translocation in cirrhosis is limited to antibiotics with risk of antimicrobial resistance. This study explored the therapeutic potential of a non-absorbable, gut-restricted, engineered carbon bead adsorbent, Yaq-001 in models of cirrhosis and acute-on-chronic liver failure (ACLF) and, its safety and tolerability in a clinical trial in cirrhosis.
Design Performance of Yaq-001 was evaluated in vitro . Two-rat models of cirrhosis and ACLF, (4 weeks, bile duct ligation with or without lipopolysaccharide), receiving Yaq-001 for 2 weeks; and two-mouse models of cirrhosis (6-week and 12-week carbon tetrachloride (CCl4)) receiving Yaq-001 for 6 weeks were studied. Organ and immune function, gut permeability, transcriptomics, microbiome composition and metabolomics were analysed. The effect of faecal water on gut permeability from animal models was evaluated on intestinal organoids. A multicentre, double-blind, randomised, placebo-controlled clinical trial in 28 patients with cirrhosis, administered 4 gr/day Yaq-001 for 3 months was performed.
Results Yaq-001 exhibited rapid adsorption kinetics for endotoxin. In vivo , Yaq-001 reduced liver injury, progression of fibrosis, portal hypertension, renal dysfunction and mortality of ACLF animals significantly. Significant impact on severity of endotoxaemia, hyperammonaemia, liver cell death, systemic inflammation and organ transcriptomics with variable modulation of inflammation, cell death and senescence in the liver, kidneys, brain and colon was observed. Yaq-001 reduced gut permeability in the organoids and impacted positively on the microbiome composition and metabolism. Yaq-001 regulated as a device met its primary endpoint of safety and tolerability in the clinical trial.
Conclusions This study provides strong preclinical rationale and safety in patients with cirrhosis to allow clinical translation.
Trial registration number NCT03202498 .
- BACTERIAL TRANSLOCATION
- LIVER CIRRHOSIS
- LIVER FAILURE
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.
https://doi.org/10.1136/gutjnl-2023-330699
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Current strategies to target bacterial translocation in cirrhosis are limited to antibiotics with risk of resistance.
WHAT THIS STUDY ADDS
Yaq-001 rapidly adsorbs endotoxin, ammonia and bile acids and impacts positively on markers of gut permeability, liver injury, fibrosis, portal pressure, brain and kidney dysfunction in animal models of cirrhosis and reduces mortality from acute-on-chronic liver failure.
In models of cirrhosis, Yaq-001 restores microbiome composition and reduces endotoxaemia, ammonia, severity of inflammation, liver cell death, signalling pathways and lipopolysaccharide sensitivity.
Enhanced permeability of intestinal organoids following incubation with faecal water from cirrhosis animals is prevented by Yaq-001.
In a multicentre, double-blind, randomised, placebo-controlled clinical trial of Yaq-001 versus placebo in patients with cirrhosis, Yaq-001 was found to be safe and well tolerated.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The data provide the preclinical rationale and clinical safety to proceed to the next phase of clinical trials in patients with cirrhosis aiming to prevent the occurrence of complications.
Introduction
Gut dysbiosis and gut-derived bacterial ligands, in particular endotoxin, drive a dysregulated inflammatory response, which has been implicated in the development of cirrhosis and its complications such as sepsis, spontaneous bacterial peritonitis, renal dysfunction and hepatic encephalopathy. 1–3 This dysregulated inflammatory response is also central to the development of acute-on-chronic liver failure (ACLF). 4 Markers of bacterial translocation such as endotoxin and bacterial DNA have been shown to be associated with complications of cirrhosis and diminished survival highlighting their pathogenic importance. 5–7 The microbiome in cirrhosis is characterised by reduced diversity and abundance of autochthonous bacteria. 1 While antibiotics have been shown to impact positively on complications of cirrhosis, their use is associated with antibiotic resistance. 8 9 Furthermore, antibiotics reduce bacterial diversity rendering the microbiome less resilient.
One of the consequences of bacterial translocation in cirrhosis is that the endotoxin-sensing pathways in different organs are known to be primed resulting in heightened susceptibility to organ injury. 3 10 Adsorption of free endotoxin without exerting direct effects on bacterial growth kinetics, therefore, has the potential to attenuate susceptibility to organ injury without producing the deleterious effects on the microbiome. Considering this, we developed a synthetic non-absorbable, non-antibiotic, endotoxin sequestrant and generated the hypothesis that this may be a novel therapeutic strategy to restore the microbiome, prevent bacterial translocation, systemic inflammation progression of fibrosis and cirrhosis complications. Yaq-001 is a gut-restricted, non-absorbable, highly engineered, activated carbon of multiple porosities tailored to the micro (<2 nm) and meso-macroporous (30–200 nm) range and has a high surface area. 11–13 These properties confer a high adsorptive capacity for larger biologically relevant molecules such as bacterial toxins in addition to smaller intraluminal targets. The most closely associated experimental oral adsorbent is AST-120, a microporous carbon bead, which has not been shown to be efficacious in patients with hepatic encephalopathy. 14
In this study, we sought to determine the adsorptive capacity of Yaq-001 and its effect on bacterial growth kinetics in in vitro studies. We then evaluated the in vivo biological effects of Yaq-001 in four animal models representing characteristics of fibrosis, cirrhosis and ACLF. We studied the effects of Yaq-001 on measures of multiorgan function, systemic and portal haemodynamics, immune function, multiorgan transcriptomics and microbiome composition. Finally, we performed a phase 2 equivalent, multicentre, double-blind, randomised, placebo-controlled clinical trial to assess the safety and tolerability of Yaq-001 in patients with decompensated cirrhosis.
Methodological details are described in online supplemental section .
Supplemental material
Functional and structural characteristics of yaq-001.
Adsorption of biomolecules of varying molecular weights (albumin, myoglobin and caffeine) was evaluated. Bacterial growth was studied for Staphylococcus aureus and Escherichia coli . Scanning electron microscopy was performed to characterise the beads and pore size distribution was assessed using mercury porosimetry.
Studies in animal models
Study design.
These studies aimed to characterise the therapeutic potential of Yaq-001 in rats and mice models to define its role in prevention of occurrence of cirrhosis, progression of cirrhosis and occurrence of ACLF ( online supplemental figures S1 and S2 ).
Animal models
Four-week bile-duct ligation model of advanced fibrosis.
Cirrhosis: Sham (n=36); Sham-Yaq-001 (n=30); bile duct ligation (BDL) (n=37); BDL-Yaq-001 (n=44).
Prevention of ACLF: Sham-lipopolysaccharide (LPS) (n=9); Sham-LPS-Yaq-001 (n=10); BDL-LPS (n=16); BDL-LPS-Yaq-001 (n=12).
Yaq-001 (0.4 g/100 g body weight per day) was administered for 2 weeks prior to sacrifice. At the time of sacrifice, mean arterial pressure (MAP) and portal pressure were measured.
Carbon tetrachloride treated model of cirrhosis
Advanced fibrosis and early cirrhosis (CCl4 for 6 weeks): control (n=6); control-Yaq-001 (n=6); CCl4 (n=12); CCl4-Yaq-001 (n=12).
Advanced cirrhosis (CCl4 for 12 weeks): control (n=6); control-Yaq-001 (n=6); CCl4 (n=12); CCl4-Yaq-001 (n=12).
Yaq-001 (0.4 g/100 g body weight per day) was administered from 0 to 6 weeks in the 6-week model and from 6 to 12 weeks in the 12-week model.
Collection and analysis of biosamples
Blood, stool and tissue samples were collected for later analysis. Portal venous blood was collected where possible. Peripheral blood cells and Kupffer cell reactive oxidant species (ROS) were measured. H&E, picrosirius red (PSR) staining and TUNEL stains were performed in liver tissues. The mRNA in different organs was analysed by using nSolver V.4.0 software (NanoString Technologies). To define effect on the microbiome, 16s microbiome study was performed. To determine the effect of Yaq-001 on modulating metabolism, urinary 1 H-NMR analysis was performed.
Assessment of gut permeability in intestinal organoids
Permeability of mouse intestinal organoids was detected using established protocols. 15 16 Faecal water generated from the stools obtained from the four groups of 6-week CCl4 mice was incubated with the organoids. 15 16 Permeability of the organoids was assessed.
Clinical trial of Yaq-001 versus placebo, CARBALIVE-SAFETY study
The CARBALIVE-SAFETY clinical trial was a first in man, multicentre, double-blind, randomised, placebo-controlled clinical trial of oral Yaq-001 in decompensated cirrhosis. Details of the study protocol are available in online supplemental section (CONSORT, online supplemental figure S3 ). As Yaq-001 is regulated as a device, it followed both ISO standards and ICH-GCP guidance. Informed consent was obtained from each patient. The study was closely monitored and overseen by an independent data safety monitoring board ( NCT03202498 ).
Study design is described as online supplemental figure S4 . The primary endpoint was assessed at 12 weeks. Blood and stool samples were taken at the time of randomisation, 4 weeks and 12 weeks for assessment of some of the secondary and exploratory endpoints. Safety assessments were performed on weeks 1, 4, 8 and 12 and comprised a physical examination, clinical laboratory tests, urinalysis, 12-lead ECG and an assessment of reported and observed adverse events. ECGs were analysed independently. Nutritional status was assessed by the Royal Free Hospital Global Assessment tool at each safety assessment together with micronutrient analysis at baseline, weeks 4 and 12. Vitamin B 12 , A, D, E, folate, and K1 and, trace elements copper, zinc and selenium were analysed.
Main inclusion and exclusion criteria
The main inclusion criteria were participants aged 18 years or above, clinical diagnosis of diuretic-responsive cirrhotic ascites (Child-Pugh score=7–11 inclusive), abstinence from alcohol for at least 4 weeks prior to screening and written informed consent. The main exclusions were lack of informed consent, use of oral antibiotics, immunosuppressants or antiviral medication within 4 weeks prior to recruitment, change in dose of proton pump inhibitor therapy within 4 weeks before the start of the study treatment, hospital admission for liver-related indication for at least 4 weeks (except paracentesis), body mass index (BMI) >35 or BMI<18 kg/m 2 and the presence of a transjugular intrahepatic portosystemic shunt (see protocol in online supplemental file for details).
Randomisation, dosing and compliance
Patients were randomised 1:1 to receive 4 g of oral Yaq-001 or equivalent placebo nocte after dinner for 12 weeks. The interval between Yaq-001 and concomitant medication administration was 4 hours treatment compliance was assessed by the number of used or unopened sachets returned to the clinical site at each visit. Patients taking ≥70% of study medication were considered compliant.
Endpoints and assessments
Primary endpoints.
The main objective of this clinical investigation was to assess the safety and tolerability of Yaq-001 throughout the 3 months’ treatment period.
Secondary and exploratory endpoints
Blood and stool samples were collected for later analysis for markers of endotoxemia, systemic inflammation, bile acids, short-chain fatty acids, gut permeability and the microbiome (results not reported in this paper).
Statistical analysis
Animal studies.
Based on the in vitro studies, we anticipated a 50% decrease in circulating endotoxin in the treatment groups with an alpha error of 0.05 and power of 80%, resulting in a minimum sample size of 5 animals/group. As this study included several pathophysiological endpoints, multiple experimental groups were included. All the data accrued from these studies are described in this paper. All the rats in eight groups from three independent batches were included in the analysis as shown in online supplemental figure S1 . All the mice studied in eight groups were included in online supplemental figure S2 .
Group comparisons for continuous variables were performed using Man-Whitney U test (no-normal distribution) or unpaired t-test (normal distribution) and for categorical variables by using χ 2 test. The data were analysed using R package (R V.4.4.4). 16s microbiome study and circos correlation were analysed by using Wilcoxon rank sum test and Spearman correlation. Software used Graphpad Prism V.9.0 (GraphPad software, San Diego, California, USA).
CARBALIVE-SAFETY clinical trial
This first-in-man clinical investigation was not powered to demonstrate statistical significance for any endpoint. All statistical analyses of study data were carried out using SAS V.9.3 or a later version. For categorical variables, summary tabulations of the number and percentage of patients within each category (with a category for missing data) of the parameter are presented. Percentage calculations are based on non-missing data unless otherwise specified. Please also see protocol ( online supplemental file ).
Yaq-001 beads exhibited a consistent predefined structure with a bead diameter within the 250–500 µm range and the prescribed porosity ( online supplemental figure S5A ). Yaq-001 rapidly adsorbed albumin (66.5 kDa), myoglobin (16.7 kDa) and caffeine (0.194 kDa) representing different sized biomolecules ( online supplemental figure S5B ). Yaq-001 adsorbed LPS (18 kDa) reducing the concentrations from 2.5 to 1.5 EU/mL (60%) within 30 min. No endotoxin was detected in the control solution (0 EU/mL) ( online supplemental figure S5B ). Yaq-001 also adsorbed a range of bile acids ( online supplemental figure S5C ). Direct coincubation of Yaq-001 with bacterial suspensions of either E. coli or S. aureus indicated that Yaq-001 did not affect bacterial growth kinetics for either species following direct contact in comparison to the antibiotic controls ( online supplemental figure S5D ). Mercury porosimetry showed that Yaq-001 used in the clinical trial had a consistent pore size distribution plot in the meso-macroporous range from 30 to 200 nm ( online supplemental figure S5E ).
Yaq-001 exhibited better performance in adsorptive capacity and effect on endotoxin kinetics than AST-120 (Kremezin, Kureha, Japan) ( online supplemental figure S5 ).
Studies in BDL rat model of advanced fibrosis
Effect of yaq-001 on liver injury and portal pressure.
BDL rat model was used to assess the effect of Yaq-001 in cirrhosis ( figure 1A ). Significant reduction in 4-week body weight was observed in BDL rats (p<0.0001), which was prevented by administration of Yaq-001 (p=0.045) ( figure 1A ). Yaq-001 was associated with a significantly lower plasma ALT (p=0.007). ALP, TBIL and albumin were not impacted by Yaq-001 ( online supplemental figure S6A–C ). Total bile acid concentrations were not different between the BDL and Sham groups and there was no significant impact of Yaq-001 ( online supplemental figure S6E ). Mean arterial pressure (MAP) was lower in BDL animals and no effect of Yaq-001 was observed ( online supplemental figure S6F ). Yaq-001 resulted in a significant reduction in portal pressure compared with untreated BDL rats ((median (IQR) 11.1 mm Hg (10.3–11.7) vs 12.4 mm Hg (10.8–13.3), (p=0.025)) ( figure 1A ). TUNEL assay showed significantly more intense staining in the liver tissue of BDL compared with Sham rats ( figure 1A ) (p<0.0001), which were significantly reduced in Yaq-001-treated BDL rats compared with untreated-BDL rats (p=0.025). Collagen proportionate area (CPA) was significantly higher in BDL rats (p=0.0007) compared with Sham rats, which was unchanged with Yaq-001 (p=0.122) ( online supplemental figure S6D ).
- Download figure
- Open in new tab
- Download powerpoint
Effect of Yaq-001 on organ dysfunction, endotoxaemia and bacterial translocation in BDL and ACLF rats. (A) Rats underwent bile duct ligation for 4 weeks as a model of cirrhosis (n=23–37/group). Treatment groups received Yaq-001 for 2 weeks before sacrifice. (A) 4-week body weight in four groups: Sham (n=31), Sham-Yaq-001 (n=24), BDL (n=31) and BDL-Yaq-001 (n=38). Significantly lower final body weights were observed in BDL compared with Sham controls (p<0.001). Yaq-001-treated BDL rats had a significantly higher body weights compared with untreated-BDL rats (p<0.05). Plasma alanine transaminase (ALT) concentrations in Sham (n=17), Sham-Yaq-001 (n=14), BDL (n=17) and BDL-Yaq-001 (n=26) groups and Portal pressure (PP) measurements in Sham (n=17), Sham-Yaq-001 (n=19), BDL (n=14) and BDL-Yaq-001 (n=26) groups. Significantly higher ALT and PP were observed in BDL compared with Sham controls (p<0.0001). Yaq-001-treated BDL rats had a significantly lower ALT and PP compared with untreated-BDL rats (p<0.01, p<0.05). TUNEL assay of liver tissue with quantification of staining by digital image analysis. Significantly higher TUNEL staining was observed in BDL compared with Sham controls (p<0.0001). Yaq-001-treated BDL rats had a significantly lower TUNEL staining compared with untreated-BDL rats (p<0.05) indicative of a reduction in liver cell death with Yaq-001 treatment. Arterial ammonia concentrations in Sham (n=7), Sham-Yaq-001 (n=5), BDL (n=19), BDL-Yaq-001 (n=21) groups and Portal venous ammonia concentrations in Sham (n=6), Sham-Yaq-001 (n=5), BDL (n=13), BDL-Yaq-001 (n=18) groups. Significantly increased arterial ammonia concentrations and portal venous ammonia concentrations were observed in BDL compared with Sham controls (p<0.0001, p=0.0001). Yaq-001 significantly decreased arterial and portal venous ammonia concentrations in BDL rats (p<0.01 for both). Serum creatinine in Sham (n=19), Sham-Yaq-001 (n=17), BDL (n=20), BDL-Yaq-001 (n=17) and serum urea in Sham (n=28), Sham-Yaq-001 (n=23), BDL (n=30), BDL-Yaq-001 (n=34) groups. Yaq-001 markedly decreased serum creatinine levels in BDL rats (p<0.05). Plasma D-lactate in Sham (n=7), Sham-Yaq-001 (n=8), BDL (n=6), BDL-Yaq-001 (n=7). Plasma D-lactate was significantly increased in the BDL group compared with Sham animals (p<0.05). Yaq-001 resulted in a significant reduction in plasma D-lactate in BDL rats (p<0.05). Portal venous endotoxin (Sham (n=6), Sham-Yaq-001 (n=5), BDL (n=12) and BDL-Yaq-001 (n=7)) and arterial endotoxin (Sham (n=6), Sham-Yaq-001 (n=5), BDL (n=12) and BDL-Yaq-001 (n=7)). Portal venous bacterial DNA positivity (Sham (n=6), Sham-Yaq-001 (n=5), BDL (n=12) and BDL-Yaq-001 (n=13)) and arterial plasma bacterial DNA positivity (Sham (n=6), Sham-Yaq-001 (n=6), BDL (n=12) and BDL-Yaq-001 (n=7)). Significantly higher portal venous endotoxin and arterial endotoxin were observed in BDL rats compared with Sham rats (p<0.0001). Significantly higher portal venous plasma bacterial DNA positivity was observed in BDL rats compared with Sham rats (p<0.05). Yaq-001 administration was associated with a significant reduction of portal venous and arterial endotoxin compared with untreated-BDL rats (p<0.0001, p<0.01). Yaq-001 administration reduced bacterial DNA positivity, which was not statistically different (p>0.05). (B) Rats underwent sham biliary surgery or BDL for 4 weeks. The treated group received Yaq-001 for 2 weeks prior to LPS injection. Animals were sacrificed either at coma stages or 6 hours after LPS injection (n=9–16/group). Kaplan-Meier analysis of BDL-LPS rats with (n=16) or without (n=12) Yaq-001 treatment. Yaq-001 treatment significantly improved the survival of BDL-LPS rats compared with untreated-BDL-LPS rats (log rank test, p=0.003). Plasma ALT concentrations in Sham-LPS (n=7), Sham-LPS-Yaq-001 (n=5), BDL-LPS (n=10) and BDL-LPS-Yaq-001 (n=9) groups and portal pressure measurements in Sham-LPS (n=8), Sham-LPS-Yaq-001 (n=10), BDL-LPS (n=9) and BDL-LPS-Yaq-001 (n=9) groups. Yaq-001-treated BDL-LPS rats had a significantly lower ALT and potal pressure compared with untreated-BDL-LPS rats (p<0.005). Brain water percentage in Sham-LPS (n=4), Sham-LPS-Yaq-001 (n=4), BDL-LPS (n=7), BDL-LPS-Yaq-001 (n=13) groups. Arterial ammonia concentrations in Sham-LPS (n=5), Sham-LPS-Yaq-001 (n=5), BDL-LPS (n=7), BDL-LPS-Yaq-001 (n=7) groups. Portal venous ammonia concentrations in Sham-LPS (n=5), Sham-LPS-Yaq-001 (n=5), BDL-LPS (n=6), BDL-LPS-Yaq-001 (n=5) groups. Yaq-001 decreased brain water percentage and arterial/portal venous ammonia concentrations in BDL-LPS rats compared with untreated rats (p<0.05, p<0.01, p<0.05). Serum creatinine in Sham-LPS (n=4), Sham-LPS-Yaq-001 (n=3), BDL-LPS (n=12) and BDL-LPS-Yaq-001 (n=6) groups. Serum urea in Sham-LPS (n=8), Sham-LPS-Yaq-001 (n=4), BDL-LPS (n=12) and BDL-LPS-Yaq-001 (n=8) groups. Yaq-001 significantly decreased creatinine levels in BDL-LPS rats (p<0.05). Plasma cytokines in Sham-LPS (n=6), Sham-LPS-Yaq-001 (n=9), BDL-LPS (n=8) and BDL-LPS-Yaq-001 (n=8) groups. Yaq-001 significantly decreased plasma IL-1β and IL-10 concentrations in BDL-LPS groups (p<0.01, p<0.05). *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. ACLF, acute-on-chronic liver failure; BDL, bile duct ligation; LPS, lipopolysaccharide.
Effect of Yaq-001 on ammonia, organ dysfunction, endotoxaemia and bacterial translocation
Ammonia: Arterial and portal venous ammonia concentrations were significantly increased in BDL rats (p<0.0001), which were significantly reduced by Yaq-001 ((p=0.003) and (p=0.004), respectively) ( figure 1A ). None of the animals showed signs of overt hepatic encephalopathy.
Kidneys: BDL animals had significantly higher plasma creatinine (p=0.049), which was significantly reduced with Yaq-001 (p=0.025) ( figure 1A ). Urea was higher in BDL group (p=0.092), which was reduced with Yaq-001 treatment (p=0.095) ( figure 1A ).
Gut permeability, endotoxaemia, bacterial DNA and cytokines: The microbial metabolite, D-lactate, a marker of gut-specific intestinal barrier damage and translocation 16 was significantly increased in BDL rats (p=0.032) and was significantly reduced by Yaq-001 (p=0.035) ( figure 1A ). BDL rats exhibited marked endotoxaemia in the portal vein and the artery (p<0.0001 for each), which was significantly reduced with Yaq-001 ((p<0.0001) (p=0.003), respectively) ( figure 1A ). Portal venous bacterial DNA was detectable in significantly higher number of BDL rats (p<0.05), which was markedly reduced in Yaq-001 administered BDL rats (p=0.08) ( figure 1A ). Plasma IL- β concentration was higher in the BDL rats but no significant differences were observed in TNF-a, IL-6 and IL-10. No significant changes were seen with Yaq-001 ( online supplemental table S1 ).
Studies in the BDL model of ACLF
This experiment was performed to determine whether Yaq-001 treatment for 2 weeks prevents the occurrence of ACLF when BDL animals are administered LPS ( online supplemental figure S1 , figure 1B ).
Survival: Animals were sacrificed either at coma stages (considered as a surrogate for mortality) or at 6 hours post-LPS. Yaq-001 significantly impacted on time to coma of BDL-LPS rats compared with untreated controls (p<0.01) ( figure 1B ). All animals in the two Sham groups were alive at 6 hours following LPS (data are not shown).
Liver: Yaq-001 was associated with significantly lower ALT in BDL-LPS rats compared with untreated rats (p=0.004) ( figure 1B ). No significant effect of Yaq-001 was observed on ALP, TBIL and albumin ( online supplemental figure S7A–C ). The severity of fibrosis measured using CPA and the body weight was unchanged ( online supplemental figure S7D,E ).
Systemic and portal haemodynamics: No significant difference in MAP was observed between the groups treated with or without Yaq-001 ( online supplemental figure S7F ) but Yaq-001 produced a significant reduction in portal pressure in BDL-LPS animals compared with the untreated group (p=0.003), ( figure 1B ).
Brain: Yaq-001 significantly reduced brain water in BDL-LPS compared with the untreated group (p=0.017) ( figure 1B ). Arterial and portal venous ammonia concentrations were significantly increased in BDL-LPS rats, which were significantly reduced in Yaq-001-treated animals ((p=0.007) and (p=0.017) respectively) ( figure 1B ).
Kidneys: Creatinine concentration was significantly higher in BDL-LPS animals (p=0.004), which was significantly reduced by Yaq-001 (p=0.03) ( figure 1B ).
Cytokines: BDL-LPS group had a significantly higher plasma IL-1β, which was significantly reduced with Yaq-001 (p=0.003). Plasma IL-10 was higher in BDL-LPS and was significantly reduced with Yaq-001 (p=0.028) ( figure 1B ). No significant differences were observed in IL-6 or TNF-α concentrations between any of the groups ( online supplemental table S1 ).
Effect of Yaq-001 on peripheral blood cells and Kupffer cells
Significant increase in total leucocyte, neutrophil and monocyte counts in the artery and portal vein was observed with BDL rats ( online supplemental figure S8A,B ) (p=0.008 and p=0.016, respectively), which were significantly reduced with Yaq-001 in the arterial blood and insignificantly reduced in the portal vein ( online supplemental figure S8B ). To determine whether Yaq-001 impacts on the response of peripheral inflammatory cells and Kupffer cells to generate reactive oxygen species (ROS) to LPS ex vivo, studies using isolated cells incubated with LPS, were performed. Yaq-001 was associated with significantly lower LPS-induced ROS production in CD163− Kupffer cells in BDL rats (p=0.036) and portal venous CD43 hi monocyte populations of BDL rats (p=0.029) ( online supplemental figure S8C ).
Transcriptomic analysis of gene expression profiles from the liver, colon, brain and kidneys
Multiorgan transcriptomic analysis was performed to determine the possible molecular mechanisms underlying the clinical effects of Yaq-001. The four groups studied were as follows: Sham (n=3), Sham-Yaq-001 (n=3), BDL (n=3) and BDL-Yaq-001 (n=4) ( figure 2A ). All differentially expressed genes (DEGs) and related pathways in the liver, colon, kidney and brain are listed in online supplemental table S2 . The top 20 and significant DEGs are listed in online supplemental table S3 .
Effect of Yaq-001 on gene expression profiles in the liver and extrahepatic organs in BDL rats. (A) Rats underwent BDL for 4 weeks as a model of cirrhosis (n=3–4/group) and the treatment groups received Yaq-001 for 2 weeks before sacrifice. Liver, colon, brain and kidney were collected for transcriptomic analysis. (B, D, F, H) Heatmap of differentially expressed genes (DEGs) in different organs between Sham (n=3), Sham-Yaq-001 (n=3), BDL (n=3) and BDL-Yaq-001 (n=4) groups. DEGs were identified at 1.2-fold change and p=0.1 threshold in three pairwise groups (BDL vs Sham, BDL-Yaq-001 vs BDL, Sham-Yaq-001 vs Sham). (C, E, G, I) Volcano plot of pairwise DEGs in four organs among Sham (n=3), Sham-Yaq-001 (n=3), BDL (n=3) and BDL-Yaq-001 (n=4) groups. The vertical dashed lines indicated the threshold for 1.2-fold change. The horizontal dashed line indicated the adjusted p=0.05 and p=0.1 threshold. The right part indicates upregulation of gene expression, and the left part indicates downregulation of gene expression. For top 20 genes indicated by gene names, please see online supplement Table 3. BDL, bile duct ligation.
Effect of Yaq-001 on gene expression profiles in the liver and gut in BDL rats
Liver: Analysis of liver tissue showed 82 DEGs at the threshold of 1.2-fold change and p=0.1 in the four groups ( figure 2B ). Compared with the Sham group, expression of 62 genes was upregulated, and 15 genes were downregulated in BDL. These significantly changed genes were associated with inflammation, cell death and senescence. Compared with the untreated BDL group, the expression of 7 genes was upregulated and 12 genes were downregulated in the Yaq-001-treated BDL group, indicating the potential role of Yaq-001 in reducing inflammation, cell death and cellular senescence. Furthermore, two genes were upregulated, and four genes were downregulated in Sham-Yaq-001 group in comparison to Sham group ( figure 2C ). Functional analysis demonstrated that BDL rats had enriched pathways related to inflammation, cellular senescence, cell death, TLR signalling and other related signalling pathways in comparison with Sham ( online supplemental figure S9A ). Yaq-001 treatment targeted the altered pathways compared with untreated BDL group. Additionally, Yaq-001 treatment also changed the pathways in the liver when compared with Sham group, demonstrating its effect in rats even without cirrhosis ( online supplemental figure S9A ).
Colon: 43 DEGs were identified from the colonic tissue ( figure 2D ). Five genes that correlated with inflammation and cell death were upregulated and 15 genes were downregulated in BDL compared with the Sham group. Moreover, the expression of 10 genes were upregulated, and 13 genes were downregulated with Yaq-001 treatment. Only 1 gene was upregulated in the Sham-Yaq-001 group, and 16 genes were downregulated with Yaq-001 compared with the untreated Sham group ( figure 2E ). Functional analysis indicated that inflammation, cellular senescence, cell death, TLR signalling and intracellular signalling were associated with BDL in comparison with the Sham group ( online supplemental figure S9B ). Yaq-001 targeted the altered pathways, indicating the potential mechanisms in the prevention of gut dysfunction and permeability ( online supplemental figure S9B ).
Effect of Yaq-001 on gene expression profiles in the brain and kidney in BDL rats
Brain: 17 DEGs were identified from the brain tissue ( figure 2F ). Compared with Sham group, expression of 2 genes were upregulated and 13 genes were downregulated in BDL animals. These significantly changed genes were associated with inflammation, cell death and cellular senescence. Compared with the untreated-BDL group, the expression of five genes were upregulated and two genes were downregulated in the Yaq-001-treated BDL group ( figure 2G ). Functional analysis demonstrated that BDL rats had enriched pathways related to inflammation, cellular senescence, cell death, TLR signalling and intracellular signalling ( online supplemental figure S9C ). Yaq-001 targeted cytokine–cytokine receptor interaction, cytosolic DNA-sensing pathway, TLR signalling pathway, NOD-like receptor signalling pathway, neutrophil extracellular trap formation, TGF-beta signalling pathway and cytokine-cytokine receptor interaction pathways compared with untreated-BDL group ( online supplemental figure S9C ).
Kidneys: 30 DEGs were identified from kidney tissue ( figure 2H ). Nine genes that correlated with inflammation were downregulated in BDL. The expression of five genes was upregulated and four genes were downregulated with Yaq-001 treatment compared with untreated-BDL group. Five genes were upregulated in Sham-Yaq-001 group, and three genes were downregulated with Yaq-001 compared with untreated-Sham group ( figure 2I ). Functional analysis indicated that inflammation and TLR signalling were associated with BDL in comparison with Sham ( online supplemental figure S9D ). Compared with the untreated-BDL group, Yaq-001 targeted the altered pathways, indicating the potential mechanisms in the prevention of renal dysfunction ( online supplemental figure S9D ).
Effect of Yaq-001 on the gut microbiome profile
The effects of Yaq-001 on the microbiome bacterial composition were assessed by metataxonomics. At the family level, an abundance of six bacteria was significantly changed at the threshold of twofold change and Porphyomonadaceae was significantly changed (p<0.05) comparing BDL with Sham ( figure 3A ). At genus level, 19 bacteria were significantly changed in abundance. Barnesiella was significantly changed (p<0.05) comparing BDL with Sham group ( figure 3B ). These changes were reversed with Yaq-001 treatment compared with untreated-BDL rats ( online supplemental figure S10A,B , online supplemental table S4 and online supplemental figure S10C,D ). For between-groups sample diversity, PERMANOVA analysis revealed a significant difference in beta diversity between groups (R 2 =0.32, p=0.001). Yaq-001 appeared to moderately restore the beta diversity in the BDL group especially in PCoA2 axis ( online supplemental figure S10E,F ).
Effect of Yaq-001 treatment on the microbiome composition. (A) Heatmap of gut microbiome associated with the effect of Yaq-001 as determined by 16S PCR at the family level. The family Porphyromonadaceae with asterisk was statistically differently abundant between BDL (n=7) vs Sham (n=6), and between BDL-Yaq-001 (n=7) vs BDL groups (n=7) (Wilcoxon rank sum test, p<0.05). The abundance of this family was statistically higher in BDL group than in Sham group, and its abundance statistically decreased in the BDL-Yaq-001 group than in the BDL group. The other six families in the heatmap were with marked fold changes between BDL vs Sham, and between BDL-Yaq-001 vs BDL groups (|log2FC|>2). Of these, five were more abundant in the BDL group than in the Sham group. The abundance largely decreased in the Yaq-001-treated group. In addition, to these, one family was less abundant in the BDL group than in the Sham group. The abundance increased in the Yaq-001-treated group. (B) Heatmap of gut microbiome at the Genus level. The Genus Barnesiella with asterisk was statistically differently abundant between BDL vs Sham, and between BDL-Yaq-001 vs BDL groups (Wilcoxon rank sum test, p<0.05). The abundance of this genus was statistically higher in BDL group than in the Sham group, and its abundance statistically decreased in the BDL-Yaq-001 group. The other 19 genera in the heatmap represent those with significant fold change values between BDL vs Sham, and between BDL-Yaq-001 vs BDL groups (|log2FC|>2). Of these, 14 were more abundant in the BDL group compared with the Sham group. The abundance decreased in the Yaq-001-reated BDL group. In addition, five genera were less abundant in the BDL group than in the Sham group. Their abundance increased in the Yaq-001-treated BDL animals. (C, D) Correlation plots between markedly changed genes and gut microbiome at family/genus. The genes were from among the top 20 changed genes in BDL animals with Yaq-001 treatment. Nodes represent either genes (lower semi-circular part) or bacteria (upper semicircular part) at the family and genus level. The nodes are coloured based on the log-fold change for the differential gene expression and differences in the bacterial abundance. The red nodes indicate an increase and blue nodes indicate a decrease. Edges represent the correlation coefficient calculated between genes and microbial genus or family with red indicating a positive correlation and blue a negative correlation. Correlation coefficients greater or equal to 0.4 were plotted in plot C (Spearman’s coefficient≥0.4), and D shows all correlations. *p<0.05. BDL, bile duct ligation.
To further investigate the potential importance of the changes in the microbiome induced by Yaq-001, we correlated these with all significantly changed DEGs and the top 20 DEGs in the four organs. Circos plots indicated a significant correlation between them ( figure 3C,D and online supplemental figure S11A–C ). Porphyromonadaceae was observed to positively correlate with three DEGs—TGFB2 and CASP1 in liver tissue, and FOS in colonic tissue. Also, it correlated negatively with five DEGs—TGFB2, IL-18 and CCR5 in brain tissue, CXCL10 in colon tissue and CCL24 in kidney tissue.
Effect of Yaq-001 on metabolomic profile
Significant difference in acetate/creatinine, glycine/creatinine, lactate/creatinine, betaine/creatinine, trimethylamine oxide/creatinine and bile acid/creatinine ratio were observed in BDL compared with Sham. Treatment of BDL rats with Yaq-001 resulted in significant resolution of acetate/creatinine, glycine/creatinine and lactate/creatinine compared with the untreated BDL animals ( online supplemental figure S12 ).
Studies in CCl4 mice
Effect of yaq-001 on liver injury and fibrosis.
CCl4 mice models (6 weeks and 12 weeks) were used to further confirm the effect of Yaq-001 on liver injury and fibrosis in models of cirrhosis ( figure 4A,B ). Yaq-001 was associated with a significantly lower plasma ALT (p<0.0001, p<0.0001) in both 6-week and 12-week CCl4 models. ALP and TBIL were reduced by Yaq-001 in 6-week CCl4 mice (p=0.040, p=0.001) ( figure 4A,B ). CPA was significantly higher in both CCl4 mice compared with control animals (p=0.0001, p=0.0001), which were significantly reduced with Yaq-001 (p=0.024, p=0.012) ( figure 4A,B ). TUNEL assay showed significantly more intense staining in the liver tissue of CCl4 compared with control mice (p<0.001, p<0.001), which were significantly reduced in Yaq-001-treated CCl4 mice compared with untreated-CCl4 mice (p=0.021, p=0.017) ( figure 4A,B ).
Effect of Yaq-001 on organ dysfunction, ammonia and endotoxaemia in CCl4 mice. (A) Mice underwent CCl4 injection for 6 weeks as a model of cirrhosis (n=6–12/group) and the treatment groups received Yaq-001 for 6 weeks before sacrifice. Plasma ALT, ALP and TBIL concentrations in control (n=6), control-Yaq-001 (n=6), 6-week CCl4 (n=12) and 6-week CCl4-Yaq-001 (n=12) groups. Significantly higher ALT, ALP and TBIL were observed in CCl4 compared with controls (p=0.0001, p=0.0007, p=0.012). Yaq-001-treated CCl4 mice had a significantly lower ALT compared with untreated-CCl4 mice (p<0.0001, p=0.040, p=0.001). H&E and PSR staining of liver tissue. CCl4 mice were associated with a significant increase in collagen proportionate area (CPA) compared with controls (p=0.0001). Yaq-001 had significant effect on CPA in CCl4-Yaq-001 compared with CCl4 mice(p=0.024). TUNEL staining liver tissues. Significantly greater staining was observed in CCl4 compared with controls (p=0.0001). Yaq-001-treated CCl4 mice had a significantly lower TUNEL staining compared with untreated-CCl4 (p=0.021) with Yaq-001 treatment. Venous ammonia concentrations and serum creatinine levels in control (n=6), control-Yaq-001 (n=6), 6-week CCl4 (n=12) and 6-week CCl4-Yaq-001 groups (n=12). Significantly increased ammonia concentrations were observed in CCl4 compared with controls (p=0.0020). Yaq-001 significantly decreased venous ammonia concentrations and serum creatinine levels in CCl4 mice (p=0.025, p=0.005). Endotoxin concentrations in control (n=3), control-Yaq-001 (n=3), 6-week CCl4 (n=10) and 6-week CCl4-Yaq-001 groups(n=10). Significantly higher endotoxin was observed in CCl4 mice compared with control mice (p=0.007). Yaq-001 administration was associated with a significant reduction of venous endotoxin compared with untreated-CCl4 mice (p=0.007). (B) Mice underwent CCl4 injection for 12 weeks as a model of cirrhosis (n=6–12/group) and the treatment groups received Yaq-001 for 6 weeks before sacrifice. Plasma ALT, ALP and TBIL concentrations in control (n=6), control-Yaq-001 (n=6), 12-week CCl4 (n=12) and 12-week CCl4-Yaq-001 (n=12) groups. Significantly higher ALT, ALP and TBIL were observed in CCl4 compared with controls (p=0.0001, p=0.0008, p=0.007). Yaq-001-treated CCl4 mice had a significantly lower ALT compared with untreated-CCl4 mice (p<0.0001). H&E and PSR staining of liver tissue in CCl4 mice. CCl4 mice were associated with a significant increase in collagen proportionate area (CPA) compared with controls (p=0.0001). Yaq-001 had significant effect on CPA in CCl4-Yaq-001 compared with CCl4 mice(p=0.012). TUNEL staining of liver tissues. Significantly higher TUNEL staining was observed in CCl4 compared with controls (p=0.0001). Yaq-001-treated CCl4 mice had a significantly lower TUNEL staining compared with untreated-CCl4 (p=0.017) indicative of a reduction in liver cell death with Yaq-001 treatment. Venous ammonia. Significantly increased ammonia concentrations were observed in CCl4 compared with controls (p=0.001). Yaq-001 significantly decreased venous ammonia concentrations in CCl4 mice (p=0.035). Serum creatinine: Yaq-001 significantly decreased serum creatinine levels in CCl4 mice (p=0.003). Endotoxin concentrations in control (n=3), Control-Yaq-001 (n=3), 12-week CCl4 (n=10) and 12-week CCl4-Yaq-001 groups(n=10). Significantly higher venous endotoxin was observed in CCl4 mice compared with control mice (p=0.007). Yaq-001 administration was associated with a significant reduction of venous endotoxin compared with untreated-CCl4 mice (p=0.043). *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. ALT, alanine aminotransferase; ALP, alkaline phosphatase; PSR, picrosirius red; TBIL, total bilirubin.
Effect of Yaq-001 on ammonia, organ dysfunction and endotoxaemia
Ammonia: Ammonia concentrations were significantly increased in the 6-week and 12-week CCl4 mice compared with controls (p=0.002, p=0.001), which were significantly reduced by Yaq-001 (p=0.025, p=0.035) ( figure 4A,B ). None of the animals showed signs of overt hepatic encephalopathy.
Kidneys: Higher plasma creatinine was significantly reduced by Yaq-001 treatment (p=0.005, p=0.003) in 6-week and 12-week CCl4 animals ( figure 4A,B ).
Endotoxaemia: Both 6-week and 12-week CCl4 mice exhibited marked endotoxaemia compared with controls (p=0.007, p=0.007), which were significantly reduced with Yaq-001(p=0.007, p=0.043) ( figure 4A,B ).
In vitro studies in intestinal organoids to assess gut permeability
Intestinal organoids were successfully derived and cultured from small intestine of C57BL/6 mice. Intestinal organoids underwent eversion into apical-out polarity in the first 12 hours of suspension culture and collected for identification and subsequent experiments ( figure 5A ). Immunostaining of the microvilli (mv; F-actin) demonstrated that intestinal organoids in suspension had reversed polarity such that the apical surface faced outward ( figure 5A ). Apical-out intestinal organoids possessed goblet cells, which were identified with MUC2 staining ( figure 5B ). Gut permeability of apical-out intestinal organoids was significantly increased by coculturing with faecal water from CCl4 group compared with the control group (p=0.003) ( figure 5C,D ). The gut permeability was significantly decreased with faecal water from Yaq-001 treated CCl4 animals compared with the CCl4 group (p=0.001) ( figure 5C,D ).
Effect of Yaq-001 on gut permeability in intestinal organoids. (A) Intestinal organoids derived and cultured from small intestine of C57BL/6 mice underwent eversion into apical-out polarity in the first 12 hours of suspension culture. Immunostaining of the microvilli (mv; F-actin) demonstrated that intestinal organoids in suspension have reversed polarity from basolateral-out to apical-out. (B) Apical-out intestinal organoids in suspension culture generate goblet cells (MUC2). (C) Gut permeability of apical-out intestinal organoids was significantly increased by coculturing with faecal water from CCl4 group than control group (p=0.003). Gut permeability was notably decreased in faecal water from CCl4-Yaq-001 group compared with CCl4 group (p=0.001). (D) Quantification of the integrated density/area of each group. **p<0.01.
The data regarding safety and tolerability are reported here. Other secondary and exploratory endpoints will be described elsewhere.
Patient characteristics
34 patients were screened for this study at 8-European centres. 28 patients met the study entry criteria and were randomised to either active or placebo groups. Six patients screened did not meet the study entry criteria. Dosing was not initiated in two patients randomised to placebo due to withdrawal of consent ( online supplemental figure S3 , CONSORT). Three patients were included for the second dosing cohort of 8 g. This part of the study was terminated prematurely due to the coronavirus pandemic with none of the patients completing the study duration (data are not included).
In accordance with study entry criteria, all patients had cirrhosis with diuretic-responsive ascites and Child-Pugh score of 7–8. The baseline demographics were similar across treatment groups. The ratio of male to female patients was reflective of the disease state. Compliance in the active and placebo groups was 92.9% and 66.7%, respectively ( table 1 ).
- View inline
Safety and tolerability
Of the 14 patients enrolled in the Yaq-001 treatment group, 13 (93%) completed 12 weeks of therapy. The median duration of exposure was 83 (6–94) days. 10 of the 12 (83%) patients who received placebo completed the treatment. The median duration of exposure was 83 (14–86) days. No deaths or serious adverse events were reported in the study. The difference in treatment-emergent adverse events (TEAEs) in patients treated with Yaq-001 and those treated with placebo is presented in table 2 . The most frequently reported TEAEs were gastrointestinal in nature in both the active and placebo groups. Of these, only constipation and diarrhoea were evaluated by the clinical investigator as possibly related to the investigational product. One placebo-treated patient withdrew from the study due to diarrhoea.
Adverse and serious events
Across both treatment groups, 40/51 (78%) of the reported TEAEs were evaluated by the clinical investigator as not related or unlikely related (32/38; 84% for the active treatment group; 8/13; 62% for the placebo group). The incidence of adverse events reported was reflective of the targeted subject population for this clinical investigation. The majority of the TEAEs reported were not considered by the clinical investigator to be related to treatment and were mild in intensity. Systemic antibiotics were administrated for the following TEAEs in the active arm: amoxicillin—acute bronchitis; clarithromycin—acute bronchitis; phosphomycin—urinary tract infection. None of these infections were related to the administration of the investigational product. Drugs received by the patients at the time of randomisation and during follow-up are listed in online supplemental tables S5 and S6 . Treatment-emergent, clinically significant laboratory abnormalities are listed in table 3 . None were deemed treatment related by the investigator.
Safety parameters: clinical laboratory assessments, royal free global assessment and micronutrient concentrations
Clinical, haematological and biochemical variables
The data are summarised in table 3 . No significant changes in any of the clinical parameters were observed in any of the groups. Although there was a trend towards a reduction in the white cell count and C reactive protein in the Yaq-001 group, the differences were not statistically significant.
Nutritional status
The data are summarised in table 3 . No significant differences were observed in either treatment group with regard to global nutritional status, vitamin B 12 and folate, vitamin A or E, or copper zinc, and selenium. Median vitamin A, zinc and baseline vitamin D concentrations were below the limit of normal range but no differences between treatment groups were observed. No changes were observed in any of the micronutrient parameters with treatment and these were evenly matched between groups. Any baseline abnormalities were attributable to the underlying cirrhosis.
The results of the study showed that Yaq-001 prevented progression of liver injury and fibrosis in animal models of cirrhosis and significantly reduced the mortality of ACLF animals. This effect of Yaq-001 on ACLF mortality will need to be confirmed in patients. This was associated with positive impact on markers of gut permeability, liver injury, portal pressure, brain and kidney dysfunction. These pleiotropic effects of Yaq-001 were associated with partial restoration of the composition of the microbiome bacterial community, reduction in the severity of endotoxaemia, ammonia, inflammation, cell death, signalling pathways and LPS sensitivity. A phase 2 equivalent, multicentre, double-blind, randomised, placebo-controlled clinical trial in patients with cirrhosis confirmed regulatory compliance and, safety and tolerability of Yaq-001, thereby, providing evidence of clinical translatability. The data provide the rationale to proceed to further clinical trials.
Translocation of bacteria, its products and metabolites are critically important in the progression of hepatic fibrosis and pathogenesis of complications of cirrhosis. 1 17–20 Indeed, selective gut decontamination using norfloxacin or rifaximin is the current standard of care for secondary prophylaxis of patients with spontaneous bacterial peritonitis and hepatic encephalopathy, respectively. 21 22 However, the use of these antibiotic strategies is limited to patients with advanced cirrhosis and induces the risk of antibiotic resistance. 23 The data presented here provide a safe, gut-restricted, non-antibiotic strategy, Yaq-001, which has the potential to diminish translocation and prevent the progression of hepatic injury, fibrosis and prevent extrahepatic organ injury in models of cirrhosis. The in vitro studies demonstrate that Yaq-001 has the optimal pore size distribution to bind intraluminal factors such as free endotoxin. We also tested in vitro bacterial growth kinetics of two species, which were not affected by Yaq-001, an observation that was subsequently confirmed in the studies in the BDL animal model where no change diversity was observed.
Endotoxaemia has been implicated in immune dysfunction resulting in a dysregulated systemic inflammatory response, which is strongly associated with the progression of fibrosis, cirrhosis and occurrence of ACLF. 24 25 Yaq-001 reduced the severity of endotoxaemia and bacterial DNA positivity, which was associated with attenuated systemic inflammation. Significant improvements in LPS-induced ROS production were observed in trafficking portal venous monocytes suggesting that Yaq-001 had attenuated the primed state of monocyte/macrophage populations within the gut–liver axis. This observed reduction in LPS-induced ROS production may be important in explaining the reduction in plasma IL-1β in LPS-treated BDL rats.
Plasma D-lactate, a marker of increased gut permeability was reduced in the Yaq-001 treated BDL rats. 26 Elevated plasma D-lactate levels in cirrhosis are associated with decompensation. 22 Transcriptomic analysis of colonic tissue demonstrated upregulation of genes associated with necroptosis, apoptosis and inflammation in BDL animals. Functional analyses pointed to the modulation of colonic inflammation by Yaq-001, IL-17 signalling, which is known to have diverse biological functions, promoting protective immunity against many pathogens, neutrophil recruitment, antimicrobial peptide production and enhanced barrier function. 27 28 To further validate the potential effect of Yaq-001 in modulating gut permeability, we performed experiments in intestinal organoids that were incubated with faecal water. 29 The data confirmed that even in in vitro settings, faecal water obtained from the faeces of CCl4-induced cirrhosis animals enhanced permeability of the organoids, which was prevented in the faecal water obtained from animals that were treated with Yaq-001. The data support the hypothesis that Yaq-001 impacts on the factors in the gut responsible for increasing gut permeability in cirrhosis.
Yaq-001 significantly reduced the severity of liver injury and portal hypertension in both the BDL and BDL-LPS models of cirrhosis and ACLF. The lack of significant differences in CPA between untreated and Yaq-001-treated BDL groups suggests that the reduction in portal pressure is possibly due to modulation of the dynamic component of portal hypertension, in which inflammation is known to play a role and proposes Yaq-001 as a potential treatment for portal hypertension. 30 31 Reduction in ALT levels and TUNEL staining confirmed a reduction in liver injury in the Yaq-001 treated animals. The reduction in liver injury in the LPS treated BDL animals suggests that Yaq-001 has a particular effect on endotoxin sensitivity in vivo . This hypothesis was tested in isolated Kupffer cells, which confirmed that LPS-induced ROS production was significantly impacted by Yaq-001 treatment.
Transcriptomic analysis of liver tissue demonstrated that the upregulated genes, CXCL16, CASP1 and TGFB2 in BDL rats was prevented by Yaq-001 administration. Silencing of CXCL16 alleviates hepatic ischaemia reperfusion injury and CXCL16 variant is also associated with hepatitis B virus-related acute liver failure. 32 CASP1 mediates proinflammatory cytokine release and pyroptotic cell death in cirrhosis and its inhibition has been shown to prevent ACLF. 33 TGFB2 is an important mediator of cellular senescence. 34 35 Of note, Yaq-001 also modified necroptosis and cytosolic DNA-sensing pathways representing cell death, which are known to be activated by LPS and can trigger systemic inflammation. 36 These effects of Yaq-001 potentially explain the effect of Yaq-001 in reducing liver injury. 33 37
Yaq-001 administration had a significant impact on time to coma of ACLF rats, which is considered a surrogate for mortality compared with untreated controls. Whether it impacts truly on mortality of ACLF animals will require more confirmation. Yaq-001 also significantly lowered portal venous and arterial ammonia levels, which was associated with reduced brain water. Transcriptomic analysis of brain tissue showed that IL-18, TGFB2, CCR5 and IL-23A were dysregulated in BDL rats and these were corrected by Yaq-001. IL-18 is released during pyroptosis by activation of the inflammasome complex in neuroinflammatory and neurodegenerative diseases. 38 The effect of Yaq-001 on TGFB2 may mean that it has an impact on cellular senescence, which is known to be associated with hepatic encephalopathy. CCR5 has been implicated in neuroprotection and is a novel therapeutic target in stroke. 39 The impact of Yaq-001 on IL-23A indicates possible reduction in neuroinflammation.
In both cirrhosis and ACLF models, Yaq-001 reduced renal dysfunction. Transcriptomic analysis of kidney tissue showed that CCL24 was downregulated in BDL rats, which was prevented in the Yaq-001-treated animals. CCl4 protects renal function in the development of early diabetic nephropathy by exerting an anti-inflammatory effect. 40 Yaq-001 impacted, on the cytokine-cytokine receptor interactions and chemokine and toll-like signalling pathways, which were abnormal in the BDL rats.
BDL animals become sarcopenic and lose weight, which were significantly abrogated by Yaq-001. 41 The possible mechanisms underlying this effect are likely multifactorial. 42 Yaq-001 reduced ammonia significantly, which has been shown to induce sarcopenia. 43 Weight loss in cirrhosis is also attributed to increased catabolic state in the context of systemic inflammatory response and thus the observed improvement in body weight may reflect the diminished catabolic state with reduced inflammation. 42
The clinical effects of Yaq-001 observed in the BDL models were validated in the CCl4-induced liver injury animal models. Two models were studied. In the first (6-week model), Yaq-001 was administered in a preventative mode starting its administration with the onset of liver injury during administration of CCl4. The results showed significant reduction in the severity of liver injury, fibrosis and progression to cirrhosis, endotoxaemia, creatinine and ammonia levels. In the second (12-week model), Yaq-001 was administered starting at 6 weeks when the animal already had advanced fibrosis/cirrhosis. Again, significant reduction in markers of liver injury, fibrosis, endotoxaemia, creatinine and ammonia were observed. Extrapolating these observations to humans, the results from the 6-week model suggest that Yaq-001 may be useful to prevent the progression of fibrosis in patients without cirrhosis and, from the 12-week model, the possibility of prevention of progression of liver disease in those with well-compensated cirrhosis.
Gut microbiota are important in modulating intestinal health, permeability, bacterial translocation, systemic inflammation and complications of cirrhosis. 44–46 BDL was associated with marked changes in the abundance of microbiota, which were reversed by Yaq-001. In particular, the abundance of Porphyromonadaceae and Barnesiella were significantly elevated in BDL rats and significantly decreased with Yaq-001. This change is potentially important as Porphyromonadaceae is a proinflammatory bacterium that has been positively correlated with hepatic encephalopathy and, Barnesiella and Porphyromonadaceae have been associated with liver cancer. 47–49 Urinary nuclear magnetic resonance (NMR) analysis reflects the combined metabolic status of both host and microbiota. Yaq-001 was associated with a distinct shift of acetate, glycine and lactate in metabolomic profile in BDL rats. These metabolites are generated by mixed acid fermentation (MAF), typically by bacteria such as Enterobacter. MAF is not the preferred metabolic pathway for facultative anaerobes and may be indicative that Enterobacter populations are under conditions of metabolic stress in Yaq-001 treated BDL animals. As these species are often pathogenic in cirrhosis, this may represent a beneficial change. However, the exact mechanisms by which the change in the microbiome results in improvement in distant organ function and gene expression cannot be directly inferred from the data derived from this study. One possibility is that alongside LPS adsorption and modulation of other unmeasured toxins, the milieu of the gut is changed allowing proliferation of more autochthonous bacteria, which impacts on gut inflammation and reduces gut permeability. 46 This hypothesis is supported by the organoid experiments. Reduction in permeability would result in a reduction in endotoxaemia, systemic inflammation, improvement of organ function and LPS-sensitivity. In this study, most of these changes have been described individually but whether this is happening in sequence has not been investigated.
As Yaq-001 is completely excreted unchanged in the stool, it is regulated in Europe as a device. Therefore, the clinical trial was performed both according to ISO standards and ICH-GCP guidance. The results of this first-in-man, multicentre, double-blind, randomised, placebo-controlled clinical trial suggested that oral Yaq-001 at a dose of 4 g nocte was well tolerated with a favourable safety profile. Despite the rapid adsorption kinetics for bacterial toxins and metabolites, Yaq-001 treatment had no negative impact on micronutrient levels or on nutritional profile as assessed by the gold standard Royal Free Global Assessment tool. These data must be interpreted keeping in mind that Yaq-001 was administered postprandially, separated from drugs by 4 hours as necessitated by the protocol. It is important to note that the studies were performed in stable cirrhosis patients, many of whom had minimal evidence of systemic inflammation and therefore, any clinical effect of this intervention was difficult to gauge. However, future analysis of the available samples from the blood and stool will provide answers as to whether Yaq-001 modulates the gut microbiome, inflammation and endotoxaemia.
These results must be considered in view of some limitations. First, the rodent microbiome is not directly analogous to the human and further clinical studies will be required to verify the effects on the gut microbiome’s bacterial composition. Second, although Yaq-001 was effective in adsorbing a variety of bile acids in vitro and reduced bile acids significantly in Sham animals, no impact on bile acids was seen in BDL animals. This possibly reflects the effect of the BDL model, where no increase in bile acids was observed. Also, no changes in bile acids were observed in CCl4 animals but these animals did not have elevated bile acid either. Further studies on effect of Yaq-001 bile acids both in the circulation and stool need to be performed to confirm safety. Third, although, Yaq-001 was observed to impact positively on the gene expression profiles of multiple pathways, their exact relevance at the protein or cellular level has not been explored. Additionally, the limited genes in the different organs assessed in this study are a potential source of bias as they focused on pathways likely to be impacted by Yaq-001. Thus, further studies using unbiased approaches should be performed to define the true effect of Yaq-001. Fourth, although survival benefit was observed with prior Yaq-001 administration in the ACLF model, further studies are needed to confirm this finding. Fifth, as only one dose of Yaq-001 was tested in the clinical trial, further dose-ranging studies will be needed to define optimal dosing for safety and efficacy. However, the animal toxicity studies that were performed by an independent laboratory for regulatory purposes, showed evidence of safety in much larger doses than that administered in the present studies (summary in online supplemental file ). Finally, although Yaq-001 was safe and tolerable, further analyses are needed to clarify its clinical effects in patients.
In conclusion, the data provide compelling evidence for the potential of Yaq-001 as a novel gut-restricted adsorbent targeting endotoxin and ammonia that impacts on the gut microbiome, gut permeability, systemic inflammation, liver injury and fibrosis and, organ function in models of cirrhosis and improves survival in ACLF. The placebo-controlled clinical trial of Yaq-001 in cirrhosis patients provides evidence of safety and tolerability allowing translation to the next phase of clinical studies to define its potential as a novel therapeutic for patients with cirrhosis.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
This study involves human participants and was approved by UK: South West—Exeter Research Ethics Committee; Ref No. 17/SW/0144France: Le Comité de Protection des Personnes Sud Méditerranée II, agréé par arrêté: Ref No. 17/SW/0144Portugal: Assunto: Estudo Clinico com Intervenao de Dispositivo Medico com o Protocolo n2 Yaq001-S- 001 e Codigo CEIC 1712GK893. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We would like to thank Fraser Simpson (Department of Genetics, Evolution and Environment, University College London) for his support to perform the Nanostring. The urinary NMR studies were facilitated by a Medical Research Council research grant under the High-throughput 'omic' Science and Imaging funding scheme (MC_PC_13045). University of Brighton coauthors acknowledge Prof Sergey Mikhalovsky and Dr Carol Howell in discussion and research leading to the CARBALIVE grant. The authors would like to thank all the patients and public who provided input into designing the study and those that took part in the study.
- Engelmann C ,
- Adebayo D ,
- Oria M , et al
- Michelena J ,
- Martínez-Arranz I , et al
- Sharma S , et al
- Aguilar F , et al
- Albillos A ,
- Martin-Mateos R ,
- Van der Merwe S , et al
- Namisaki T ,
- Sato S , et al
- Thacker LR ,
- Fagan A , et al
- Fernández J ,
- Trebicka J , et al
- Caraceni P , et al
- Mohamed FE ,
- Jover-Cobos M , et al
- Macnaughtan J ,
- Ranchal I ,
- Soeda J , et al
- Kerbert A , et al
- Sheikh MY ,
- Chojkier M , et al
- den Daas SA ,
- Soffientini U ,
- Chokshi S , et al
- Azarian S , et al
- Fernandez J ,
- Wiest R , et al
- Salerno F ,
- Piantoni L , et al
- Singh V , et al
- Habtesion A ,
- Hassan M , et al
- Praharaj DL ,
- Premkumar M ,
- Roy A , et al
- McPhail MJW , et al
- Shenep JL ,
- Barton RP ,
- Wasmuth HE ,
- Yagmur E , et al
- Scarpellini E ,
- Abenavoli L ,
- Cassano V , et al
- Grootjans J ,
- Thuijls G ,
- Verdam F , et al
- Song W , et al
- Puschhof J ,
- Pleguezuelos-Manzano C ,
- Martinez-Silgado A , et al
- Mookerjee RP ,
- Davies NA , et al
- Mookerjee RP , et al
- Dao D , et al
- Macdonald S ,
- Engelmann C , et al
- Tominaga K ,
- Dewidar B ,
- Dooley S , et al
- Bertheloot D ,
- Franklin BS
- Baweja S , et al
- Srinivasan S ,
- Lamkanfi M , et al
- Ben Assayag E ,
- Shabashov-Stone D , et al
- Geng M , et al
- Colares JR ,
- da Fonseca SRB , et al
- Zanetto A , et al
- Yang T-C , et al
- Trebicka J ,
- Krag A , et al
- de Gottardi A ,
- Ahluwalia V ,
- Betrapally NS ,
- Hylemon PB , et al
- Peng Y-M , et al
- Jin C , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
- Data supplement 3
- Data supplement 4
JL and JM are joint first authors.
X @Zac_MD, @DrLAEdwards, @Ijcox_nmr, @drgautammehta
JL and JM contributed equally.
Deceased Dr Soeda is deceased
Contributors JL: performed CCl4 animal experiments; data acquisition and analysis; intestinal organoid experiments; paper organisation, drafting and writing; JMacNaughtan and RJ: concept; study design; performed animal experiments; data acquisition and analysis; clinical trial lead; paper organisation, drafting and writing; AJCK, JMG, CJ, GZ, KH, MARG, PG, ES, HC-P, FD, PCordero, VV, GM and DG: Clinical trial; TP and YJ: Analysis of the microbiome data and correlation analyses; FC and SS: analysis of the microbiome data, correlation analyses and transcriptomics analyses; AH: performed all the bile duct ligation animal studies; sample analyses; HJ, QJ and HW: performed CCl4 animal experiments; data acquisition and analysis; AP: BDL animal experiments; data acquisition; FDC: BDL animal experiments; data acquisition and analysis; GI: BDL animal experiments; data acquisition and analysis; PCaraceni and JS: data acquisition and cell studies; LM, TA, MK and DG: Yaq-001 characterisation and manufacture; JO: concept; study design; data acquisition and cell studies; KC: regulatory and clinical trial design; JVL: analysis of metabolomics data; AJ: data acquisition and analysis; data organisation; SE: analysis of metabolic effects of Yaq-00; CM: clinical trial; study design; AG: data acquisition and analysis biobanking; LAE: data acquisition and analysis; IJC: analysis of metabolic effects of Yaq-001; RW and VA: clinical trial; study design; RE, JV and MP: statistics; ND: concept; study design; performed animal experiments; data acquisition and analysis; RM: concept; study design; paper organisation, drafting and writing; SSandeman: concept; study design; in vitro studies of Yaq-001; SShoaie: analysis of the microbiome data, correlation analyses and transcriptomics analyses; JMarchesi: microbiome sequencing and analysis of the microbiome data; AA: clinical trial and trial design; FA: concept; study design; data acquisition and analysis. RJ accepts full responsibility for the work presented in this paper. the conduct of the study, has access to all the data and controlled the decision to publish the study.
Funding This study was performed with support from a grant from the EU H2020, Grant Agreement number: 634579—CARBALIVE—H2020-PHC-2014-2015/H2020-PHC-2014 programme. JMarchesi and the Division of Digestive Diseases at Imperial College London receives financial support from the NIHR Imperial Biomedical Research Centre.
Competing interests JMacNaughtan: Shareholder in Yaqrit—no payments received; LM: Yaqrit Employee; KC: Yaqrit consultant; CM: Full-time employee of Yaqrit—salary, Share options in Yaqrit Discovery—no payment received; TA: Full-time employee of Yaqrit—Salary; MK: Full-time employee of Yaqrit—salary, Shares and Share options in Yaqrit Discovery—no payment received; DG: Share options—Yaqrit; AG: Shareholder—Yaqrit; RPM: Shareholder in Yaqrit —No payments received; SShoaie: Co-founder of Gigabiome, Bash Biotech and DAS Microbiome; JMarchesi: JMarchesi has received consultancy fees from EnteroBiotix and Cultech, and speaker fees from Falk Forum; RJ: RJ is the inventor of OPA, which has been patented by UCL and licensed to Mallinckrodt Pharma. He is also the founder of Yaqrit Discovery, Hepyx (spin out companies from University College London), and Cyberliver. He has research collaborations with Yaqrit Discovery. Yaq-001 was licensed by Yaqrit from UCL.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
In the PDF this Figure is extremely squashed. It meeds to be on a full page or even 2 pages to allow full visibility
This Figure is very squashed in the PDF I saw. Should be expanded to a full page or even 2 pages please to allow readability
Once these have been corrected, please share the revised version with me to recheck please.
Read the full text or download the PDF:

IMAGES
VIDEO
COMMENTS
Diffuse liver disease includes etiologies such as the various viral and immune hepatitides, storage diseases, hepatic steatosis, fibrosis, and cirrhosis. 38 Ultrasonography findings are often non-specific in the case of diffuse liver disease, and biopsy is usually required to differentiate between the various etiologies. 38 In cases of acute ...
Case Report • 2014 • vol.3 •13-16 CASE STUDY OF PATIENT WITH LIVER CIRRHOSIS Muhammad Rashad, ZubairAhmad, Hassan Ali , shanulqadir , Muhammad umair Faculty of Pharmacy, University of Sargodha. [email protected] Abstract Liver cirrhosis is a disease in which normal tissue of liver replaced with scar tissue, liver cirrhosis is ...
Accepted 16 September 2021. Published 29 November 2021. ABSTRACT. Liver is the second largest organ in human body, more than 5,000 separate b odily functions. .including helping b lood to c lot ...
Educational Objective. •Discuss case review of patient with complications of cirrhosis. •Profile. -55 yr old female being evaluated in clinic for new onset ascites and lower extremity edema -Hospitalized 2 weeks ago for UGI bleed: EGD with grade 1 esophageal varices and a 1cm clean-based antral ulcer -Pt was on naproxen 220 mg TID for ...
Case Scenario 2 58-year-old male with BMI 34, diabetes and hypertension had his yearly labs that showed mildly elevated liver enzymes diagnosed with cirrhosis recently. (AST=40, ALT=55) Hb 14.5, WBC 4.8, platelet count 120. You saw him in clinic and ordered quality of care investigations. His lab work did not reveal any other etiology of liver ...
The first edition of the clinical practice guidelines for liver cirrhosis was published in 2010, and the second edition was published in 2015 by the Japanese Society of Gastroenterology (JSGE). The revised third edition was recently published in 2020. This version has become a joint guideline by the JSGE and the Japan Society of Hepatology (JSH).
suppression of the aetiological factor(s) that has caused liver inflammation and cirrhosis development, whereas the second Table 1. Level of Evidence and Grade of Recommendations. Level of evidence I Randomised, controlled trials II-1 Controlled trials without randomisation II-2 Cohort and case-control analytical studies
Recently published guidelines by European Association for Studies of Liver (EASL) 3 and European Society for Clinical Nutrition and Metabolism (ESPEN) 13 provide comprehensive overviews of nutrition in cirrhosis. Herein, we provide a case-based practical guide of the causes of malnutrition, assessments tools, daily requirements and dietary ...
Vol 392 July 28, 2018. Professor of Pathology at Harvard Medical School, drew on new clinical and pathological work to redefine cirrhosis as a syndrome: "a chronic, progressive, destructive lesion of the liver combined with reparative activity and contraction on the part of the connective tissue". By the early 20th century, clinicians knew ...
in abnormal liver function as a consequence of chronic (long-term) liver injury. Cirrhosis occurs when the normal structure of the liver is disrupted by bands of scar tissue. One of the normal functions of the liver is to filter blood returning to the heart from the digestive system. When cirrhosis is present, the presence of
Liver cirrhosis results from prolonged, widespread but patchy hepato-cellular necrosis due to various reasons. Objectives: To determine the frequency and severity of hyponatremia in patients with liver cirrhosis. Study Design: Descriptive case series study. Period: Six months. Setting: Liaquat University Hospital Hyderabad. Methods: The cirrhotic
This case study examines the physiological nature of alcohol-induced cirrhosis and its pathogenesis, external and internal clinical presentations, and treatment options. Treatments for alcohol-induced cirrhosis include liver transplant for permanent correction as well as varied options to manage symptoms.
symptoms are overlooked, liver disease can go undetected and further complications can arise. The patient in this case study has sustained several major complications attributed to chronic alcoholic cirrhosis. They were acute renal failure, bacterial infections, and edema. Acute renal failure is common in patients with cirrhosis. In
Tel.: 01019673646. Fax: +20-082-2334551. Liver cirrhosis is a common sequel to divers e chronic liver injuries of. different etiologies and represents an elev. mortality worldwide. Identification ...
study used a systematic literature review and various adjusting models to split cirrhosis incidence into spe - cific causes: hepatitis B, hepatitis C, non-alcoholic fatty liver disease, alcohol-related liver diseases, and oth - ers. The literature review included published case series that reported the proportion of cases due to these aetiologies.
We report a multi-ancestry genome-wide association study on liver cirrhosis and its associated endophenotypes, alanine aminotransferase (ALT) and γ-glutamyl transferase. Using data from 12 ...
Sixty-six (95.65%) patients had anemia and 47 (68.12%) patients had thrombocytopenia. Conclusions: From this study, we can conclude that, in cirrhosis of liver patients, various hematological changes are very common which need to be identified and corrected early to reduce morbidity and mortality. Download Free PDF.
Introduction. Hepatic encephalopathy is a known complication of liver cir-. rhosis. It generally presents with altered sleep w ake cycle and be-. havioural changes in early stage. Case Report. A ...
Here, we report the case of a patient presenting with cirrhosis of the liver due to autoimmune hepatitis along with systemic sclerosis. Case presentation A 51-year-old non-obese female with no history of alcoholism was admitted with complaints of gradually progressive abdominal distension and swelling over both lower limbs for the last month.
December 30, 2014 Case Report • 2014 • vol.3 •13-16 CASE STUDY OF PATIENT WITH LIVER CIRRHOSIS Muhammad Rashad, Zubair Ahmad, Hassan Ali , shanulqadir , Muhammad umair Faculty of Pharmacy, University of Sargodha. [email protected] Abstract Liver cirrhosis is a disease in which normal tissue of liver replaced with scar tissue ...
Definition. ACLF is a syndrome affecting multiple organs, including new worsening of liver function, defined by an acute hepatic decompensation in patients with preexisting chronic liver disease [].Even if the definition provided by the European Association for the Study of the Liver (EASL) and the International Chronic Liver Failure (CLIF) Consortium is the most employed [], there is no ...
Spontaneous clearance of chronic hepatitis C virus (HCV) is rare in adults. A T-lymphocyte response is thought to be involved in HCV-RNA clearance. Splenectomy reportedly has a beneficial effect on T cell immune function in patients with cirrhosis. To the best of our knowledge, the present report is the first to describe spontaneous clearance of serum HCV-RNA within 1 year after ...
Alcohol-related liver disease (ArLD) is a major cause of morbidity and mortality. Effective management requires multi-disciplinary input at every stage of the disease trajectory. We present a typical case to illustrate current evidence-based investigation and management of a patient with ArLD. This case-based review aims to concisely support the day-to-day decision making of clinicians looking ...
Liver transplantation is an effective treatment for small, unresectable hepatocellular carcinomas in patients with cirrhosis and after four years, the actuarial survival rate was 75 percent and the rate of recurrence-free survival was 83 percent. Expand. 6,449. PDF.
Acute liver failure (ALF) is defined by the presence of biochemical evidence of liver injury, with hepatic-based coagulopathy (INR >2.0 after parenteral replenishment of vitamin K, or INR >1.5 if encephalopathy is present) in the absence of evidence of chronic liver disease ( 1 ). It is an uncommon presentation in the previously well child.
Background Wilson's disease (WD) often leads to liver fibrosis and cirrhosis, and early diagnosis of WD cirrhosis is essential. Currently, there are few non-invasive prediction models for WD cirrhosis. The purpose of this study is to non-invasively predict the occurrence risk of compensated WD cirrhosis based on ultrasound imaging features and clinical characteristics. Methods A ...
Apr 24, 2016 • Download as DOCX, PDF •. 124 likes • 109,857 views. education4227. case study on liver cirrhosis. Health & Medicine. 1 of 48. Download now. A case study on cirrhosis of liver - Download as a PDF or view online for free.
Introduction. Liver injury is universal in patients who have undergone Fontan palliation for complex congenital heart disease and results in fibrosis and cirrhosis. 1 Fontan-associated liver disease (FALD) can be rarely complicated by hepatocellular carcinoma (HCC), 2-4 risk factors for which are not fully elucidated. We aim to identify clinical characteristics associated with the ...
Objective Targeting bacterial translocation in cirrhosis is limited to antibiotics with risk of antimicrobial resistance. This study explored the therapeutic potential of a non-absorbable, gut-restricted, engineered carbon bead adsorbent, Yaq-001 in models of cirrhosis and acute-on-chronic liver failure (ACLF) and, its safety and tolerability in a clinical trial in cirrhosis. Design ...