Jump to navigation

Search form
The Graduate School
- Faculty/Staff Resources
- Programs of Study Browse the list of MSU Colleges, Departments, and Programs
- Graduate Degree List Graduate degrees offered by Michigan State University
- Research Integrity Guidelines that recognize the rights and responsibilities of researchers
- Online Programs Find all relevant pre-application information for all of MSU’s online and hybrid degree and certificate programs
- Graduate Specializations A subdivision of a major for specialized study which is indicated after the major on official transcripts
- Graduate Certificates Non-degree-granting programs to expand student knowledge and understanding about a key topic
- Interdisciplinary Graduate Study Curricular and co-curricular opportunities for advanced study that crosses disciplinary boundaries
- Theses and Dissertations Doctoral and Plan A document submission process
- Policies and Procedures important documents relating to graduate students, mentoring, research, and teaching
- Academic Programs Catalog Listing of academic programs, policies and related information
- Traveling Scholar Doctoral students pursue studies at other BTAA institutions
- Apply Now Graduate Departments review applicants based on their criteria and recommends admission to the Office of Admissions
- International Applicants Application information specific to international students
- PhD Public Data Ph.D. Program Admissions, Enrollments, Completions, Time to Degree, and Placement Data
- Costs of Graduate School Tools to estimate costs involved with graduate education
- Recruitment Awards Opportunities for departments to utilize recruitment funding
- Readmission When enrollment is interrupted for three or more consecutive terms
- Assistantships More than 3,000 assistantships are available to qualified graduate students
- Fellowships Financial support to pursue graduate studies
- Research Support Find funding for your research
- Travel Funding Find funding to travel and present your research
- External Funding Find funding outside of MSU sources
- Workshops/Events Find opportunities provided by The Graduate School and others
- Research Opportunities and programs for Research at MSU
- Career Development Programs to help you get the career you want
- Teaching Development Resources, workshops, and development opportunities to advance your preparation in teaching
- Cohort Fellowship Programs Spartans are stronger together!
- The Edward A. Bouchet Graduate Honor Society (BGHS) A national network society for students who have traditionally been underrepresented
- Summer Research Opportunities Program (SROP) A gateway to graduate education at Big Ten Academic Alliance universities
- Alliances for Graduate Education and the Professoriate (AGEP) A community that supports retention, and graduation of underrepresented doctoral students
- Recruitment and Outreach Ongoing outreach activities by The Graduate School
- Diversity, Equity, and Inclusion Funding Funding resources to recruit diverse students
- Graduate Student Organizations MSU has over 900 registered student organizations
- Grad School Office of Well-Being Collaborates with graduate students in their pursuit of their advanced degree and a well-balanced life
- Housing and Living in MI MSU has an on and off-campus housing site to help find the perfect place to stay
- Mental Health Support MSU has several offices and systems to provide students with the mental health support that they need
- Spouse and Family Resources MSU recognizes that students with families have responsibilities that present challenges unique to this population
- Health Insurance Health insurance info for graduate student assistants and students in general at MSU
- Safety and Security MSU is committed to cultivating a safe and inclusive campus community characterized by a culture of safety and respect
- Why Mentoring Matters To Promote Inclusive Excellence in Graduate Education at MSU
- Guidelines Guidelines and tools intended to foster faculty-graduate student relationships
- Toolkit A set of resources for support units, faculty and graduate students
- Workshops Workshops covering important topics related to mentor professional development
- About the Graduate School We support graduate students in every program at MSU
- Strategic Plan Our Vision, Values, Mission, and Goals
- Social Media Connect with the Graduate School!
- History Advancing Graduate Education at MSU for over 25 years
- Staff Directory
- Driving Directions
IMA EXAMPLE (Chemistry) 21 College Street, City, State, Zip 123-456-7890 | [email protected]
EDUCATION AND TRAINING Michigan State University, Department of Chemistry PhD Candidate in Chemistry, September 20XX - present
Michigan State University, Department of Chemistry Bachelors of Science, (Honors) Biochemistry and Molecular Biology, May 20XX
CERTIFICATES AWARDED Graduate Certificate in College Teaching, MSU, 20XX International Communication Skills Certificate, MSU 20XX Intel Certificate of Project-based Technology, Ann Arbor, 20XX
RESEARCH ACTIVITES Enzyme Kinetics Kinetic Isotope Effects Protein Expression and Purification Organic and Enzymatic Synthesis (mirco scale)
RESEARCH APPLICATIONS HPLC; FPLC; Cell Culture; LSC (Liquid Scintillation Counting); UV-Vis spectrometer; NMR; IR; Mass spectrometry; Multivariate analysis (PCA, PLS, and NAS); MATLAB; Mini'Tab; Sigma Plot; RefWorks; Mathematica
FELLOWSHIPS Graduate School Summer Fellowship (MSU), June - July 20XX
GRADUATE TEACHING APPOINTMENTS Graduate Research Assistant, Department of Chemistry, MSU, Fall 20XX - Present Graduate Teaching Assistant, Department of Chemistry, MSU, January 20XX - May 20XX
HONORS AND AWARDS Distinction in Sciences Scholarship, (MSU), 2 years Outstanding Teaching Award (Dept. of Chemistry, MSU), 1 year Travel Award, Graduate Student Senate, (MSU), 1 year Future Scientist Scholarship, American Scientists Society, 4 years
SCHOLARSHIP Publications Sample, I., Mousee, N. N., Example, I. The Inhibition of Oxidase Activity of Flavin-Dependent Thymidylate Synthase . [Journal of Physical Chemistry], 59(14), 687-871, 20XX.
Recent Presentations Ima Example and Mouse, N. N. Kinetic Isotope Effects on H-Transfers Catalyzed by Thymidlyate Synthase , 20XX Gordon Research Conference: Isotopic Probes for Mechanisms In the Chemical and LIfe Sciences, Galveston, TX, February 20XX.
Posters Kinetic Isotope Effects on H-transfers Catalyzed by Thymidylate Synthas e. Poster Session, 69th National Conference, Boston, MA (March 20XX)
Contributed Talks Ima Example, Exploring the Role of Dynamics in Enzymatic Catalysis: Kinetic Isotope Effect Studies of E. coli Thymidylate Synthase , 44th ACS Midwest Regional Meeting, Chicago, IL (October 20XX)
SERVICE At-Large-Member, Council of Graduate Schools, 20XX - Present Volunteer, Red Cross Disaster Team, East Lansing area, 20XX - Present Tutor, America Reads, Lansing School District, 20XX - 20XX
AFFILIATIONS American Chemical Society Center for Biocatalysis and Bioprocessing
For more examples of CVs visit our friends at UC Davis .

- Call us: (517) 353-3220
- Contact Information
- Privacy Statement
- Site Accessibility
- Call MSU: (517) 355-1855
- Visit: msu.edu
- MSU is an affirmative-action, equal-opportunity employer.
- Notice of Nondiscrimination
- Spartans Will.
- © Michigan State University
CONNECT 1:1

Analytical Chemist Ph.D. Resume/CV example (CSU Career Center)
View this document to see a sample analytical chemist Ph.D. resume and CV example with qualifications.
Report Resource
" * " indicates required fields
- CV / Curriculum Vitae , Resume
Identities/Populations
Career interests.
- Life Sciences & BioTech
Related Resources

Larimer County Workforce Center
You don’t have to leave Colorado to find your dream job. Find jobs in Larimer County with this website.

Using USAJobs to find government jobs
Find step-by-step instructions on navigating the USAJobs website.
Women’s Sports Foundation
The Women’s Sports Foundation was established in 1974 to advance the lives of women and girls through sports and physical activity. Their mission is to
Dentist School Prerequisites
Read this article to see if you have the prerequisites to enter dental school.
OFFICE HOURS
8:00 a.m. – 5:00 p.m. (970) 491-5707
Lory Student Center 1101 Center Ave Mall Campus Delivery 8033 Fort Collins, CO 80523-8033
We are located in room 120 in the lower level of the Lory Student Center, next to Student Media.
QUICK LINKS
- Meet Your Career Team
- Request A Presentation
- Campus Employment Opportunities
- Attire Fund Information
- Equity Initiatives Fund
- Annual Award Processes
- Report an Employer Concern Form
- Career Center Staff Portal
- Career Outcomes & Data
- Graduation Statistics
- Academic Programs
- Report Your Plans

- Contact CSU
- Equal Opportunity
- Privacy Statement
- Accessibility Statement
© 2022 Colorado State University
Resume Worded | Proven Resume Examples
- Resume Examples
- Research & Science Resumes
6 Chemistry Resume Examples - Here's What Works In 2024
Want to work in chemistry in 2023 we’ve compiled three resume templates for you that’ll help you land your dream chemistry role, along with other tips and knowledge you’ll need to gain an extra edge against the competition. (google docs and pdfs attached)..

Chemists are patient, logical, and analytical. You’re curious about how the world works and love to tinker with something until you get it just right. You pay meticulous attention to detail and have a passion for the molecular. Sound like you? If you’re recruiting in this industry, you’ve probably gone through several years of schooling or working in labs. Chemistry is a highly specialized and ever-evolving field, and demand is never scarce for chemists. That’s not to say you can phone it in on your application. Competition can be fierce in the science world, and recruiters are looking for those who are not only book-smart, but who have the practical ability to apply their knowledge to a variety of disciplines. Whether that’s biochemistry, neurochemistry, or forensic chemistry, there’s no shortage of jobs for those with a background in this unique science. Landing a chemistry job shouldn’t be nerve-wracking and confusing -- we’re here to demystify and destress the chemistry job hunting process. We’ve researched what works in 2023, and below we’ve compiled three chemistry resumes that you can use to stand out from the crowd.
Chemistry Resume Templates
Jump to a template:
- Chemistry Lab Assistant
- Chemistry Lab Technician
- Chemistry Research Student
Jump to a resource:
- Keywords for Chemistry Resumes
Chemistry Resume Tips
- Action Verbs to Use
- Writing a Resume Summary
- Related Research & Science Resumes
- Similar Careers to a Chemistry
- Chemistry CV Examples
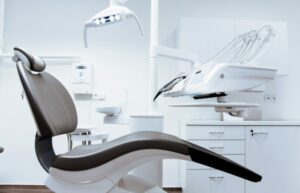
Template 1 of 6: Chemistry Lab Assistant Resume Example
A chemistry lab assistant is someone who supports a senior-level scientist in running and monitoring experiments in a laboratory. They may work at a university, private company, or public company. Chemistry lab assistants conduct and oversee experiments that relate to chemical interactions, such as food grade testing. As a chemistry lab assistant, you will monitor safety standards within the lab, conduct research, record findings, and more. To become a chemistry lab assistant, you will need a bachelor’s degree in chemistry. Many master’s programs in chemistry and related fields might require lab assistant experience as a part of the educational program. You will need knowledge of lab safety, such as an OSHA certificate and/or a CCT (certified chemical technician) credential. Hiring managers will look for someone with scientific research experience. They will also look for someone with great attention to detail and developed organizational abilities.
We're just getting the template ready for you, just a second left.
Tips to help you write your Chemistry Lab Assistant resume in 2024
highlight your experience following lab safety procedures.
It’s extremely important that a prospective chemistry lab assistant has a profound understanding of how to safely execute experiments in a lab setting. Therefore, you should highlight any certifications and experiences you have with following safety procedures.

Showcase your ability to perform effective research
Chemistry lab assistants must understand the scientific method and proper methodologies of research. It’s important to highlight any knowledge and experience you have researching, collecting data, writing reports and performing analysis on your resume.

Skills you can include on your Chemistry Lab Assistant resume
Template 2 of 6: chemistry lab assistant resume example.
A chemistry lab assistant is usually the next level up from a chemistry research student assistant. You will likely support more complex chemical procedures, while also performing administrative and clerical duties like cleaning and sterilizing equipment. When crafting your chemistry lab assistant resume, keep this in mind as you choose what kind of examples to include in your professional experience section. Emphasize your numerical achievements and relevant skills in the field.

Emphasize numerical achievements in chemistry and science
When crafting your chemistry lab assistant resume, it’s important to quantify your past achievements with clear numerical metrics. You’ll be required to assist other chemists and scientists in the lab to perform extensive tests and maintain laboratory equipment, all the while ensuring compliance of safety rules. Recruiting managers want to see concrete evidence that you can perform such responsibilities. Show your skills by including different numerical metrics -- for example, a time when you saved budget by cutting supply costs, or when you reduced experimental error by a certain percentage.
Tailored skill section to chemistry skills
Chemists use a diverse array of equipment and software. Some employers may have specific requirements that they utilize to conduct experiments or model outcomes. To demonstrate to recruiters that you are serious about the job you’re applying to, it’s important to carefully read through the job description and ensure that you tailor your skills accordingly. For example, if a lab emphasizes their use of quantitative analysis techniques, include details of what sort of techniques you have experience in.

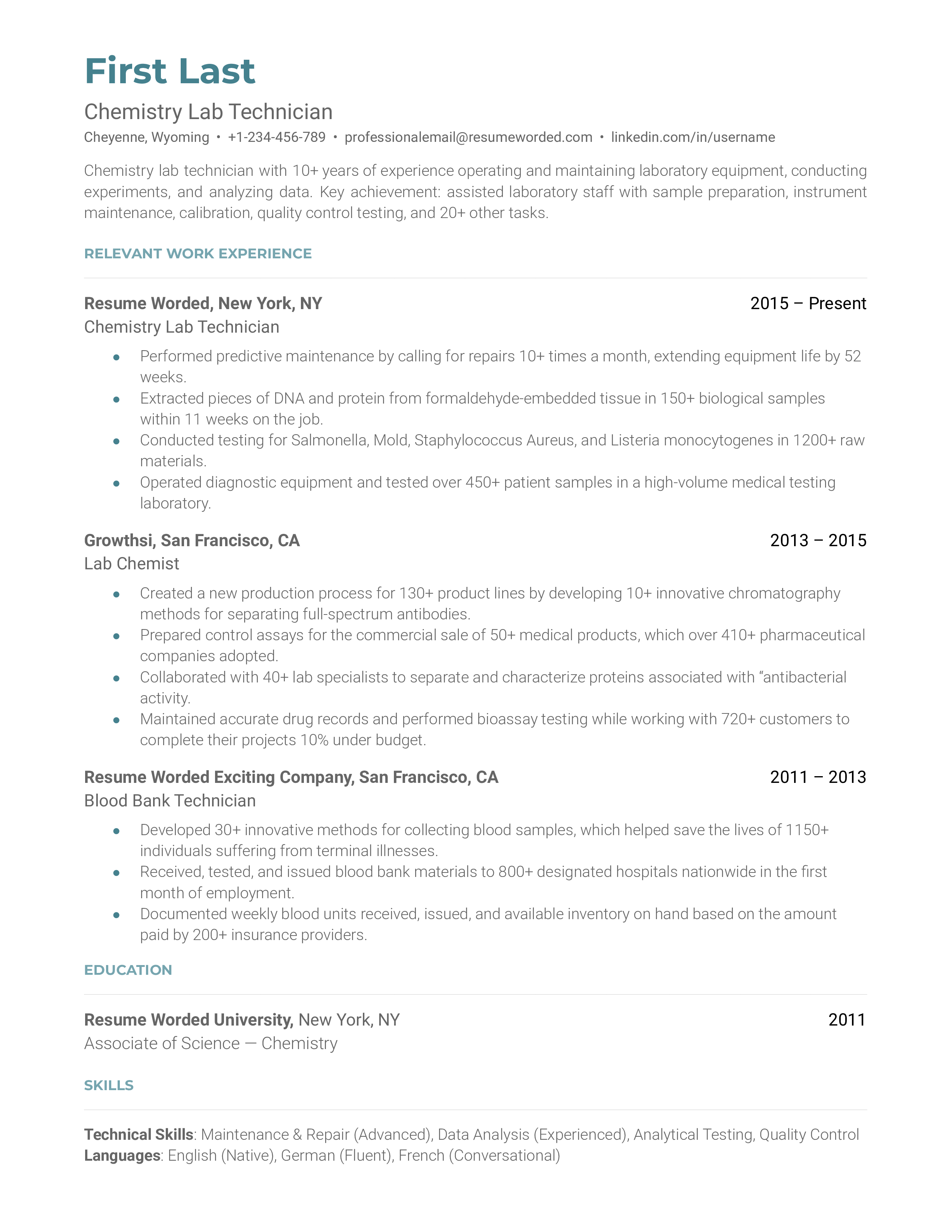
Template 3 of 6: Chemistry Lab Technician Resume Example
A chemistry lab technician is someone who is responsible for carrying out tests as prescribed by the lead scientist. Typically, chemistry lab technicians are responsible for performing important tests such as ink testing. A chemical lab technician will be tasked with carrying out such procedures in a measured and highly effective way, ensuring an accurate test result each time. To become a chemistry technician will need to have an educational background in chemistry, with a minimum of an associate's degree. Previous experience in chemical testing, procedures, and research will be valuable for landing this role. Hiring managers may look for someone who has held previous roles such as a blood bank technician, phlebotomist, or lab technician. All in all, ideal candidates for this role will be well-versed in chemical testing procedures with a keen eye for detail.
Tips to help you write your Chemistry Lab Technician resume in 2024
highlight your ability to accurately and efficiently record information.
While conducting testing, chemical lab technicians must also accurately report their results and findings. Showcasing you have the ability to not only test for, but accurately report on, chemical test findings will help you land the role.
Detail your experience with effectively handling high-volumes of testing
Many labs will expect chemical lab technicians to efficiently carry out as many tests per day as possible. This requires superb organizational skills and multi-tasking skills. Highlighting times where you have effectively managed a large workload can help you land this role.

Skills you can include on your Chemistry Lab Technician resume
Template 4 of 6: chemistry lab technician resume example.
After you’ve worked for a few years in a chemistry lab assistant role, you may be ready to apply to the next position up -- a chemistry lab technician. You’ve demonstrated your ability and skills in lab environments, and can take on more demanding responsibilities, like carrying out routine tests and procedures. As you’ll be taking on more authority and duties, it’s key to highlight your leadership skills on your resume and show your career path.

Highlight leadership skills as a lab technician
As you brainstorm examples and skills to add to your professional experience section, make a point of stressing your ability to manage others. That may include training younger research chemistry student assistants, or working smoothly with lab assistants to implement new systems or testing procedures. Great leadership is as crucial in chemistry as it is in other industries, and recruiters want to know that they can rely on you to supervise others effectively.

Showcase your career trajectory in chemistry and science
Chemists are uniquely dedicated to their craft -- it takes many years of hard work and commitment to perfect the skills needed to become a professional chemist. No amount of schooling or knowledge of scientific theory can replace hours in the laboratory. That’s why it’s key to leverage your years of lab experience and detail your career path through the years. Point out where you took on duties outside of your job scope, or got promoted ahead of schedule.

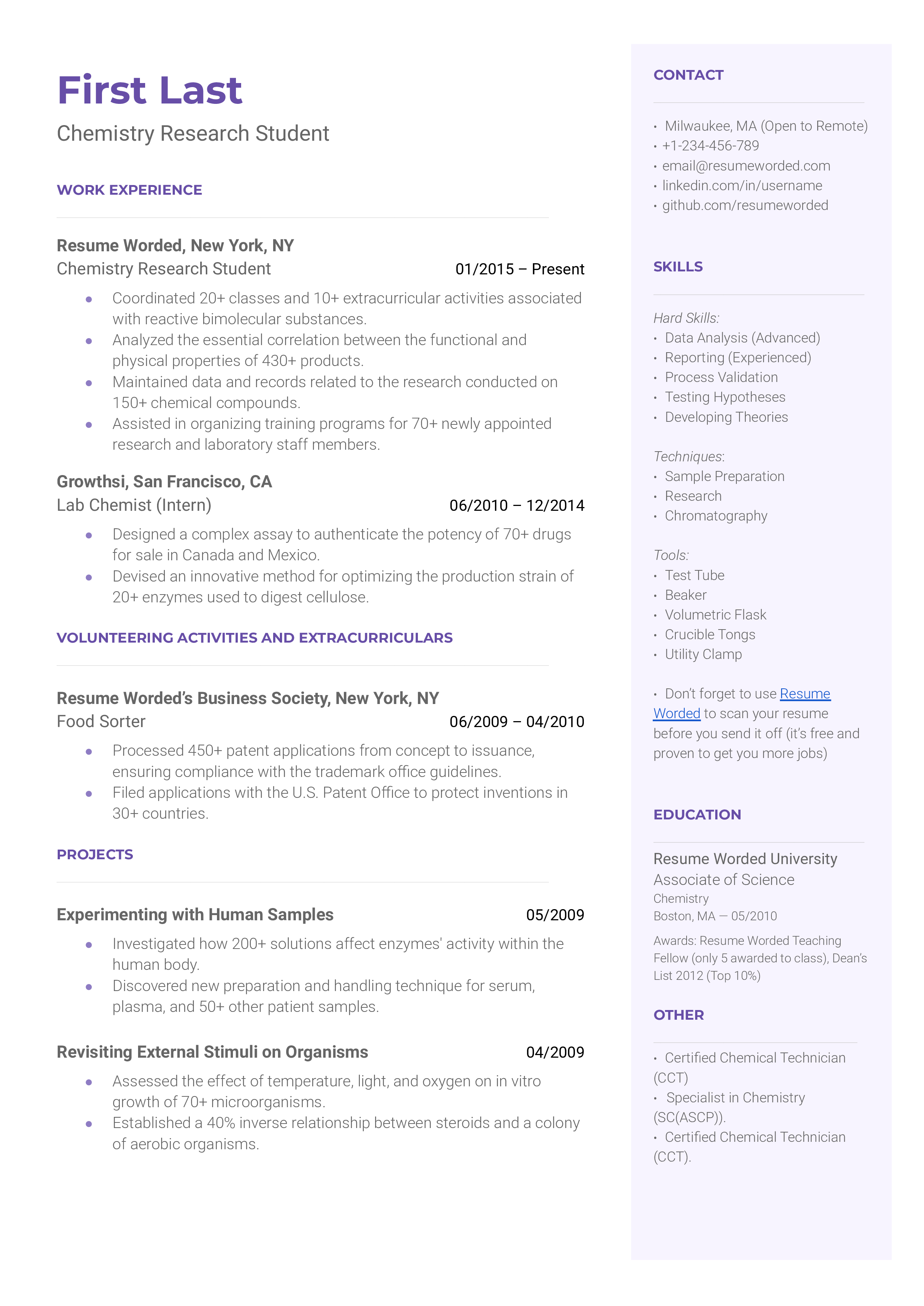
Template 5 of 6: Chemistry Research Student Resume Example
A chemistry research student performs self-directed experimentation under the management of a lead scientist or researcher. They may help the lead scientists prove a hypothesis and carry out tests for them. In some cases, they may work to prove their own hypothesis with the guidance of a senior. In a university setting, they may also help teach undergraduate students in chemistry courses. To become a chemistry research student, you will need a bachelor’s degree in chemistry. This role is a popular one for those pursuing a higher education degree in chemistry. Candidates for this role are expected to display academic excellence in their undergraduate careers. They must also have a deep understanding and experience in conducting effective research and experiments.
Tips to help you write your Chemistry Research Student resume in 2024
detail your academic successes in science and chemistry.
To earn a role as a chemistry research student, you must show you excelled in your undergraduate coursework. You should highlight any successes and honors you have received, as well as interesting research you may have started in your undergraduate studies.
Consider additional certifications to land a role as a chemistry research student
Certifications such as the CCT (certified chemical technician), ASCP (specialist in chemistry) or OSHA, can show hiring managers you have the foundational laboratory knowledge needed to be a successful chemistry research student.

Skills you can include on your Chemistry Research Student resume
Template 6 of 6: chemistry research student resume example.
When applying to be a chemistry research student assistant, emphasize your past research experience and chemistry skills in your resume. Chemistry is a specific and precise discipline, and your resume should reflect these qualities. Aim to choose instances that detail your expertise in hands-on lab procedures or with relevant software, as opposed to simply listing out the responsibilities you were assigned. Use strong action verbs and be deliberate with what you include.
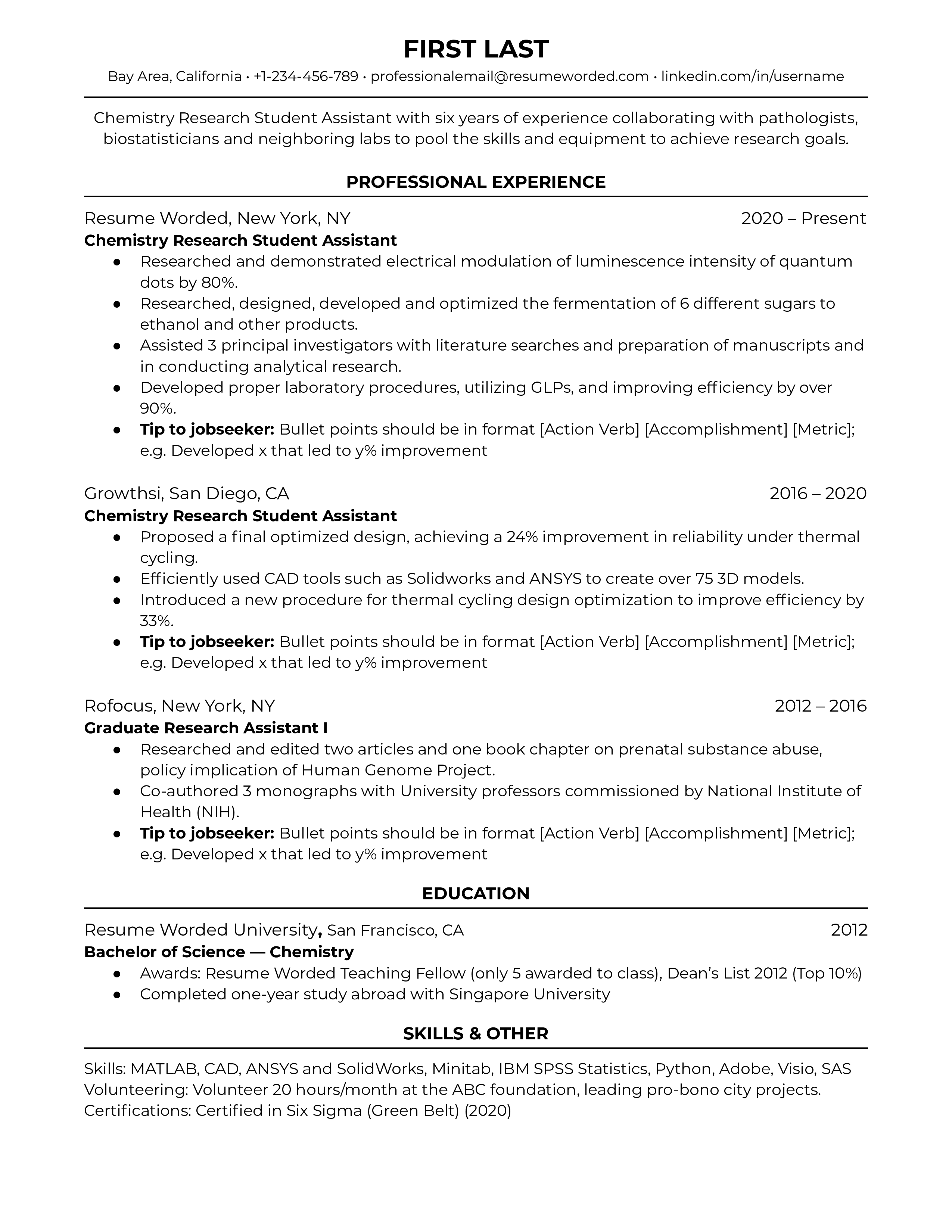
Emphasize hard skills with metrics relevant to chemistry
As mentioned above, chemistry is a precise discipline -- you’re often working in the lab with dangerous chemicals or complex equipment. That means that the employers reading your resume -- labs, government agencies, or academic institutions -- are looking for evidence of your experience and skills in those areas. Do your research to find what types of software the job posting notes, whether that’s MATLAB, Solidworks, or ANSYS. Emphasize the hard skills you’ve learned through your past experience with powerful action verbs, and highlight your achievements with quantifiable metrics.

Concise, informational chemistry resume summary
This resume makes great use of a concise, information-packed elevator pitch that is well-written and to the point. Chemistry recruiting managers often don’t have time to carefully read through every detail of your resume, so a resume summary is a great way for them to get a high-level overview of your work history. When brainstorming what to put in your chemistry resume elevator pitch, include your personal strengths as a chemist, or even the types of people you’ve learned to work with (i.e., biostatisticians, pathologists, professors).

Action Verbs for Chemistry Resumes
Skills for chemistry resumes.
When choosing what to include in your chemistry resume, follow these tips to ensure that you’re picking the best skills you can to help your application stand out. After finding the chemistry roles that you want to apply to, carefully read the job description from front to end. Required skills and experience with certain types of equipment vary widely, depending on if you’re applying to a lab, government agency, or academic institution. Take time to research the employer and figure out what techniques they are known for using so you have a better idea of what skills to include. For example, if a job posting requires knowledge of certain analytical techniques such as Mass spectrometry or HPLC Chromatography, make sure you list that in your skills section. Better yet, you can further expand on instances you’ve applied this knowledge within experiments in your professional experience section.
- High-Performance Liquid Chromatography (HPLC)
- Organic Chemistry
- Nuclear Magnetic Resonance (NMR)
- Analytical Chemistry
- UV/Vis Spectroscopy
- Organic Synthesis
- Liquid Chromatography-Mass Spectrometry (LC-MS)
- Spectroscopy
- Chromatography
- NMR Spectroscopy
- Biochemistry
- Gas Chromatography
- Medicinal Chemistry
- Mass Spectrometry
- IR Spectroscopy
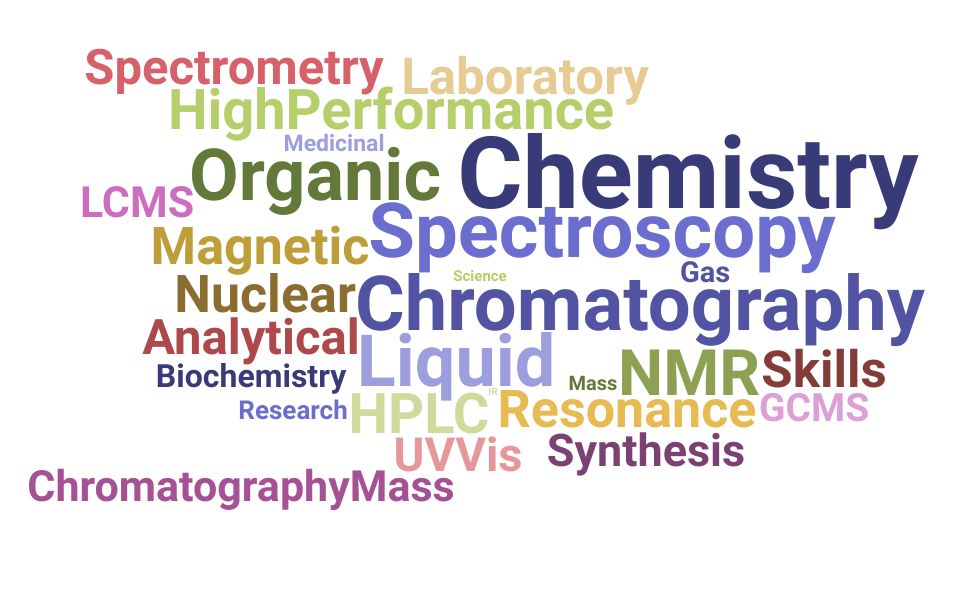
Skills Word Cloud For Chemistry Resumes
This word cloud highlights the important keywords that appear on Chemistry job descriptions and resumes. The bigger the word, the more frequently it appears on job postings, and the more 'important' it is.
How to use these skills?
We spoke with hiring managers at top chemical companies like DuPont, BASF, and Dow Chemical to gather their best advice for creating a strong chemistry resume. Based on their insights and our analysis of successful resumes in this field, we've compiled a list of key tips to help your resume stand out and land you an interview.
Highlight your lab skills and techniques
Hiring managers want to see that you have hands-on experience with common lab techniques and equipment. Be specific about the skills you've used in your work or academic experience.
- Proficient in operating and maintaining HPLC, GC-MS, and NMR instruments
- Skilled in organic synthesis, purification, and characterization techniques
- Experienced in conducting titrations, spectroscopy, and chromatography experiments
Avoid being too general or vague about your skills:
- Familiar with lab techniques
- Used various instruments
Quantify your research impact and results
Employers like to see measurable outcomes and impact from your research projects or work experience. Use numbers and data to show the significance of your contributions.
- Developed a new catalyst that increased product yield by 25%
- Optimized a synthesis protocol, reducing waste by 30% and saving $50,000 annually
- Published 3 first-author papers in top-tier chemistry journals (impact factor > 5)
Compare this to research descriptions that lack concrete details:
- Worked on developing new catalysts
- Helped optimize a synthesis procedure
- Published papers on my research
Tailor your skills to the job description
Read the job posting carefully and make sure your resume highlights the key skills and qualifications they are looking for. Different chemistry roles may emphasize different areas.
For example, an analytical chemist resume might include:
- Experience with method development and validation for HPLC assays
- Expertise in analyzing and interpreting complex data sets
- Proficiency in statistical analysis software (e.g. Minitab, JMP)
While a materials chemist resume could emphasize:
- Knowledge of structure-property relationships in polymers and composites
- Experience with materials characterization techniques (DSC, TGA, SEM)
- Familiarity with quality control testing and regulatory requirements
Include relevant coursework and certifications
For entry-level candidates or career changers, relevant coursework can help show your knowledge base. Advanced certifications demonstrate your expertise to employers.
Examples of coursework to include:
- Advanced Organic Chemistry
- Instrumental Analysis
- Physical Chemistry Thermodynamics
Certifications that can set you apart:
- Certified GLP (Good Laboratory Practices) Professional
- Six Sigma Green Belt Certification
- EH&S (Environment, Health & Safety) Certification
Showcase your problem-solving approach
Employers value chemists who can think critically and solve complex problems. Use your resume bullet points to illustrate your problem-solving skills in action.
- Identified and resolved a long-standing issue with impurities in a key raw material, improving product quality and consistency
- Designed and implemented a new QC testing protocol that reduced defect rates by 15%
- Led a cross-functional team to troubleshoot and optimize a critical manufacturing process, resulting in a 20% increase in production capacity
Avoid generic statements that don't provide insight into your problem-solving approach:
- Solved problems with raw materials
- Improved QC testing
- Optimized a manufacturing process
Emphasize your communication and collaboration skills
Chemistry roles often involve working with colleagues across R&D, manufacturing, quality, and regulatory functions. Showcase your ability to collaborate and communicate effectively.
Accomplished chemist with strong record of collaboration with cross-functional teams to bring new products to market. Skilled at translating complex technical data into actionable insights for diverse stakeholders.
Compare this to a resume summary that lacks emphasis on communication:
Experienced chemist with expertise in organic synthesis and analysis. Proven track record of developing new products and optimizing processes.
Other ways to highlight your communication skills:
- Presented research findings at the American Chemical Society National Meeting
- Collaborated with manufacturing team to scale up new synthesis process from pilot to full production
- Created and delivered training on new QC methods to lab technicians
Action Verbs For Chemistry Resumes
Chemists are inquisitive, analytical, and scientifically inclined. You can work well with others to solve complicated problems and can patiently chip away at intricate, seemingly impenetrable enigmas. At the same time, you are great at communicating with others and keeping close inventory of all the equipment and processes you are working with. It’s important to not only have these skills, but also be able to communicate them in your application. As you write your chemistry resume, make sure to use powerful action verbs such as “researched” and “proposed” to emphasize your analytical skills. Try your best not to use generic verbs. Instead, use strong action verbs that create an image in the reader’s mind of a confident, well-organized chemist.
- Implemented
- Investigated
- Interpreted
For a full list of effective resume action verbs, visit Resume Action Verbs .
How To Write a Resume Summary for a Chemistry Resume
If you're a senior-level employee, or you're changing careers to become a Chemistry, it's useful to add a paragraph at the top of your resume highlighting your most impressive accomplishments. This is called a resume summary. Here's an example of a summary that can be used on a Chemistry resume.
A resume summary is a totally optional section, and in most cases, it's better to leave it out of your resume than include it. For example, if you're a student or mid-level hire, you should not include a summary, and instead use the space to add to your work experience.

To learn how to write an effective resume summary for your Chemistry resume, or figure out if you need one, please read Chemistry Resume Summary Examples , or Chemistry Resume Objective Examples .
Other Research & Science Resumes

Environmental Scientist
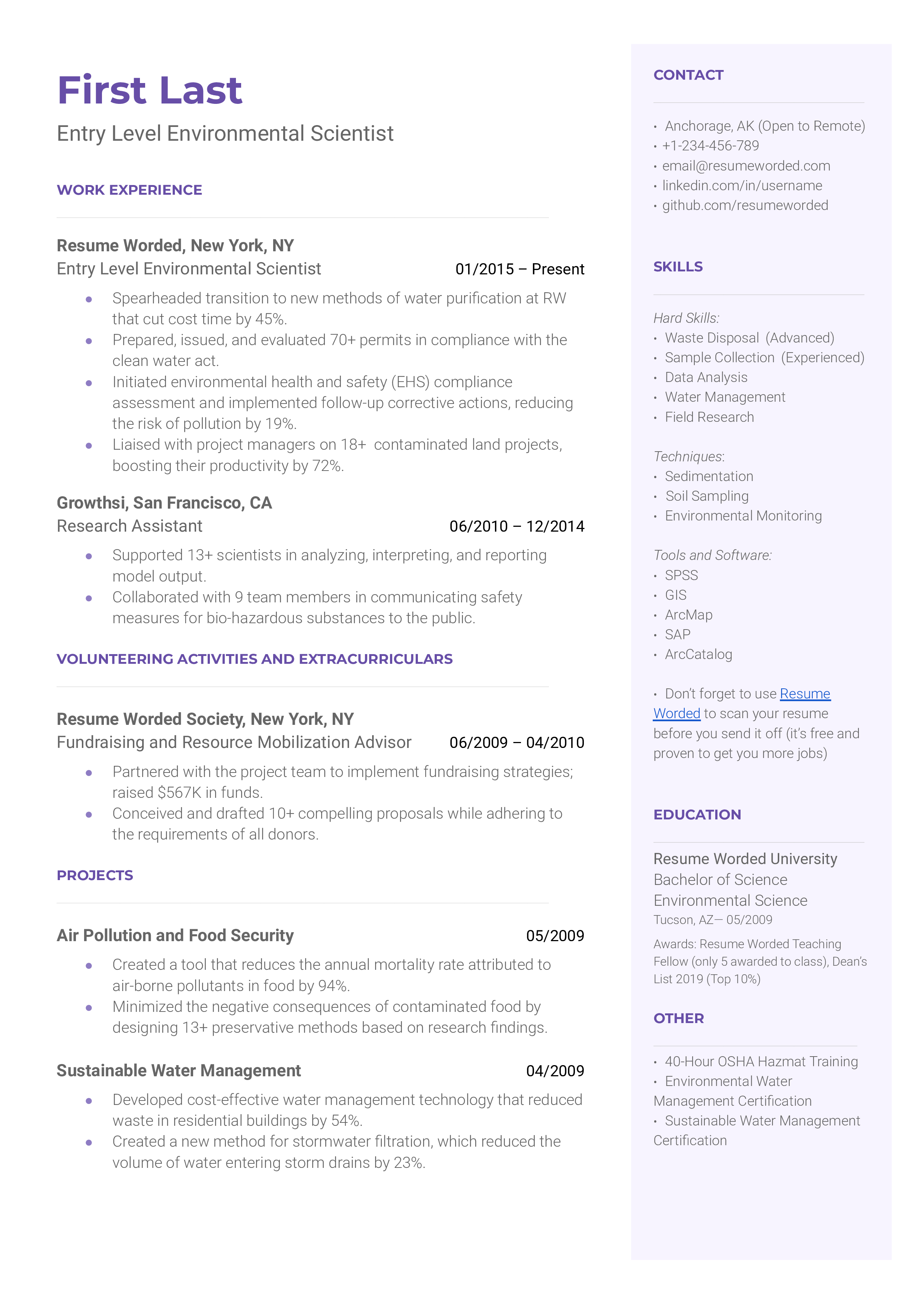
Research Assistant

Chemistry Resume Guide
- Research Assistant Resume Guide
- Quality Control Resume Guide
- Clinical Research Resume Guide
- Environmental Scientist Resume Guide
- Health and Safety Resume Guide
- Chemistry Lab Assistant Resume Example
- Chemistry Lab Technician Resume Example
- Chemistry Research Student Resume Example
- Skills and Keywords to Add
- Tips for Chemistry Resumes
- All Resume Examples
- Chemistry Cover Letter
- Chemistry Interview Guide
- Explore Alternative and Similar Careers
Download this PDF template.
Creating an account is free and takes five seconds. you'll get access to the pdf version of this resume template., choose an option..
- Have an account? Sign in
E-mail Please enter a valid email address This email address hasn't been signed up yet, or it has already been signed up with Facebook or Google login.
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number. It looks like your password is incorrect.
Remember me
Forgot your password?
Sign up to get access to Resume Worded's Career Coaching platform in less than 2 minutes
Name Please enter your name correctly
E-mail Remember to use a real email address that you have access to. You will need to confirm your email address before you get access to our features, so please enter it correctly. Please enter a valid email address, or another email address to sign up. We unfortunately can't accept that email domain right now. This email address has already been taken, or you've already signed up via Google or Facebook login. We currently are experiencing a very high server load so Email signup is currently disabled for the next 24 hours. Please sign up with Google or Facebook to continue! We apologize for the inconvenience!
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number.
Receive resume templates, real resume samples, and updates monthly via email
By continuing, you agree to our Terms and Conditions and Privacy Policy .
Lost your password? Please enter the email address you used when you signed up. We'll send you a link to create a new password.
E-mail This email address either hasn't been signed up yet, or you signed up with Facebook or Google. This email address doesn't look valid.
Back to log-in
These professional templates are optimized to beat resume screeners (i.e. the Applicant Tracking System). You can download the templates in Word, Google Docs, or PDF. For free (limited time).
access samples from top resumes, get inspired by real bullet points that helped candidates get into top companies., get a resume score., find out how effective your resume really is. you'll get access to our confidential resume review tool which will tell you how recruiters see your resume..

Writing an effective resume has never been easier .
Upgrade to resume worded pro to unlock your full resume review., get this resume template (+ 5 others), plus proven bullet points., for a small one-time fee, you'll get everything you need to write a winning resume in your industry., here's what you'll get:.
- 📄 Get the editable resume template in Google Docs + Word . Plus, you'll also get all 5 other templates .
- ✍️ Get sample bullet points that worked for others in your industry . Copy proven lines and tailor them to your resume.
- 🎯 Optimized to pass all resume screeners (i.e. ATS) . All templates have been professionally designed by recruiters and 100% readable by ATS.
Buy now. Instant delivery via email.
instant access. one-time only., what's your email address.

I had a clear uptick in responses after using your template. I got many compliments on it from senior hiring staff, and my resume scored way higher when I ran it through ATS resume scanners because it was more readable. Thank you!

Thank you for the checklist! I realized I was making so many mistakes on my resume that I've now fixed. I'm much more confident in my resume now.

Scientist, Chemistry Resume Sample
The resume builder.
Create a Resume in Minutes with Professional Resume Templates
Work Experience
- 10-12+(BS), 8-10+(MS), or 0-4+(Ph.D) years of experience
- Conceive and execute medicinal chemistry research and development that achieves project and area goals
- Act as a lead scientist in his/her area of expertise on one or more projects
- Critically evaluate relevant scientific advances and integrate this knowledge into research programs
- Demonstrates in-depth knowledge of respective scientific discipline and familiarity with related scientific disciplines
- Develops and implements detailed experimental plans for non-standard situations
- Leads the innovative efforts and advancing the organization’s intellectual property within own projects
- Present and publish research findings in conferences and peer review journals
- Directly interfaces with customers on a regular basis
- Works to understand customer needs and makes recommendation
- Proactively develops and maintains broad knowledge of the technical disciplines in own functional area(s); applies broad knowledge of trends and
- Proactively develops and maintains technical knowledge in specialized area(s), remaining up to date on current trends and best practices
- Experience with surface chemistry is highly desirable
- Develop new and existing material systems and application methods for Illumina’s sequencing and sample preparation platforms
- Perform in-depth analyses using techniques such as ellipsometry, DSC, TGA, FTIR, Raman, SEM, XPS, TOF-SIMS, AFM, high resolution spectroscopy and microscopy to characterize dimensions at the interface between Illumina’s surface chemistry and substrate materials
- Industrial experience
- The role is tasked with solving high complexity analytical problems related to the development of formulation and manufacturing process for the commercialization of Merck’s innovative drug products
- Responsible for method development, testing procedures, and implementation of analytical controls to help the advancement of new molecular entities (NME’s)
- BS or MS in chemistry, chemical engineering, bioengineering or equivalent
- Working knowledge of bioconjugation & particle science
- Proficiency in basic programming (Matlab, JMP, etc.) and scripting
Professional Skills
- Experience or proficiency in LabView or other computer language programming; data manipulation in Excel, DynoChem, KaleidaGraph, or related software; Design of Experiments (DoE); handling air-sensitive materials in a glovebox or using Schlenk techniques; and operating under cGMP
- Proven written and verbal communication skills; the ability to effectively communicate in a team setting
- Demonstrate strong synthesis skills and understanding of organic chemistry
- Strong problem-solving and troubleshooting skills including experimental design and execution
- BS with 10+ years of experience; MS with 8+ years of experience, or PhD with 0+ years of experience
- Experience and skills in LC method development
- Basic organic synthesis skills / experience
How to write Scientist, Chemistry Resume
Scientist, Chemistry role is responsible for design, basic, chemistry, training, compensation, analysis, manufacturing, research, assembly, insurance. To write great resume for scientist, chemistry job, your resume must include:
- Your contact information
- Work experience
- Skill listing
Contact Information For Scientist, Chemistry Resume
The section contact information is important in your scientist, chemistry resume. The recruiter has to be able to contact you ASAP if they like to offer you the job. This is why you need to provide your:
- First and last name
- Telephone number
Work Experience in Your Scientist, Chemistry Resume
The section work experience is an essential part of your scientist, chemistry resume. It’s the one thing the recruiter really cares about and pays the most attention to. This section, however, is not just a list of your previous scientist, chemistry responsibilities. It's meant to present you as a wholesome candidate by showcasing your relevant accomplishments and should be tailored specifically to the particular scientist, chemistry position you're applying to. The work experience section should be the detailed summary of your latest 3 or 4 positions.
Representative Scientist, Chemistry resume experience can include:
- Very good organizational, planning, communication (oral, written), and problem solving skills
- Demonstrate the ability to resolve key scientific hurdles by effectively utilizing available information and technical expertise
- Effective communication and collaboration skills in a team environment
- Demonstrated experience in elemental impurity testing using ICP-MS and ICP-OES
- Effectively communicate both orally and in writing to management and both internal/external customers
- Demonstrated effective planning and execution of regulatory strategies
Education on a Scientist, Chemistry Resume
Make sure to make education a priority on your scientist, chemistry resume. If you’ve been working for a few years and have a few solid positions to show, put your education after your scientist, chemistry experience. For example, if you have a Ph.D in Neuroscience and a Master's in the same sphere, just list your Ph.D. Besides the doctorate, Master’s degrees go next, followed by Bachelor’s and finally, Associate’s degree.
Additional details to include:
- School you graduated from
- Major/ minor
- Year of graduation
- Location of school
These are the four additional pieces of information you should mention when listing your education on your resume.
Professional Skills in Scientist, Chemistry Resume
When listing skills on your scientist, chemistry resume, remember always to be honest about your level of ability. Include the Skills section after experience.
Present the most important skills in your resume, there's a list of typical scientist, chemistry skills:
- Dissect problems with strong analytical and critical thinking skills
- Have extensive analytical development experience and demonstrated effective planning and execution of scientific and regulatory strategies
- Demonstrate the ability to resolve project hurdles and assumptions by effectively utilizing available information and technical expertise
- Strong small molecule purification skills
- Experience with/strong understanding of biocatalysis, modern biotechnology and high-throughput screening techniques
- Be an accomplished scientific leader with outstanding problem solving skills and the ability to work in an interdisciplinary team environment
List of Typical Experience For a Scientist, Chemistry Resume
Experience for senior scientist, chemistry resume.
- Weighing, transfer, mixing of chemicals as required
- Proficiency in fluorescence microscopy & other imaging methods
- Perform review and trending of QC data
- Conduct technical data reviews, and present data interpretation and conclusions to customers for making informed decisions
- Assist with nonconformances and laboratory investigations
- Train and coordinate the activities of other team members
- Support LEAN concepts (5S, Kaizen, etc.)
- Perform other responsibilities to support the needs of the department as assigned
Experience For Associate Scientist, Chemistry Resume
- Performs chemical acquisitions activities including structure data entry and chemical information review for the Repository collection’s chemical inventory database
- Development of analytical methods in product development projects and for later use in the QC department
- Be the technical expert and resource person in R&D covering a broad range of different methods to be used by QC of new products. The methods would cover wet chemistry, physical methods, chromatographic techniques and magnetic characterization
- Be a driver for exploring new analytical techniques for better characterization of our magnetic beads
- In collaboration with our service engineer take responsibility for analytical instruments in R&D
- Design, develop and apply innovative methods and strategies through diverse screening, genomic and proteomic approaches to qualify novel targets and chemoproteomic relationships
- Design, synthesize, and characterize chemical probes and/or drug candidates in target identification and validation, as well as hit-to-lead through lead optimization projects
- Engage and collaborate with the external scientific community to help identify and validate new targets
- Responsible for the routine method development and analysis of chiral and achiral compounds as well as interpreting data and communicating results to medicinal chemistry team members
Experience For Quality Control Scientist, Chemistry Focus Resume
- Maintain the high-throughput purification lab and associated equipment, including open access instrumentation and guiding others on the use of the instrumentations
- Basic lab skills and knowledge of safety precautions
- Maintain, troubleshoot and validate equipment as appropriate
- Develop and validate trace analysis methods for quantitation of potentially mutagenic (genotoxic) impurities in complex sample matrices
- Under supervision, carry out synthesis and purification of project-specific small focused libraries
- Routine use of an electronic laboratory notebook (ELN)
- Presentations in departmental or project meetings, as needed
- Review and approve completed batch records
- Carry out Mass Spectrometry-based method development, method validation, and routine sample analysis with minimal supervision. Where required, perform experiments in accordance with cGLP/cGMP regulations, established business processes and protocols, and applicable SOPs
Experience For Principal Scientist Chemistry Resume
- Document laboratory work that is detailed, timely and in compliance with cGLP/cGMP requirements
- Explore and implementinnovative mass spectrometry methodology and procedures for analytical problem-solving and to improve analytical results and process efficiency
- Mentor and train others in various mass spectrometric techniques
- MS or PhD in Chemistry or related scientific field
- Proficient in wet chemistry sample extraction techniques, modern chromatographic separations (HPLC, GC), and ICP coupled with mass spectrometry
- Work independently, and to present analytical results and conclusions to customers
- Provides direct on-site preformulation support to enable efficacy, PK, and toxicology studies for San Diego based discovery groups
Experience For Associate Scientist / Scientist, Chemistry Resume
- Conducts drug substance physicochemical characterization to evaluate the risk in development
- Conducts solubilization enhancement studies, and supports enabled formulation development (amorphous solid dispersion, nano formulation, lipid based formulation) to improve exposure in the preclinical species
- Conduct solid state characterization and preliminary salt and polymorph screening
- Experience in the pharmaceutical industry in discovery support, or formulation development group
- Highly organized and disciplined record keeping
- Understand relevant scientific literature and apply theoretical framework to solving problems within scientific discipline in a timely manner
Experience For Scientist, Chemistry, Mfg & Cntrl Resume
- Design and synthesis of novel chemical entities with the goal of enabling drug discovery programs to positively impact human disease
- Ensure synthesized compounds progress through decision-making assays
- M.S. or PhD in Chemistry, Pharmaceutical Sciences, or related scientific discipline
- Assist to carry out method development, optimization and validation for the analysis of drug substances, and finished products in accordance with cGMP regulations, established protocols, and applicable SOPs
- Performs reference standard qualification/requalification tests
- Documents laboratory work that is detailed, timely and in compliance with GLP/GMP requirements
- Synthesize libraries in a high throughout manner to advance Structure Activity Relationships
Experience For Scientist Chemistry Bausch Lomb Resume
- Understand the broad objective of the projects and contribute to the generation of project-related documents and presentations
- Learn, understand and master new chemistry technologies
- BS, MS, or PhD in Organic Chemistry or related field. 10+( BS), 8+ (MS), 0+ (Ph.D) years of relevant experience. Postdoctoral or pharmaceutical industry experience desirable
- Exceptional communication skills; ability to develop, implement and clearly communicate chemistry strategies and progress to team
- Synthesize, purify and characterize biologically-active organic small molecules and linkers
- Contribute to development and optimization of analytical characterization methods for linker dugs
- Knowledge of common spectroscopic and analytical techniques (NMR, MS, HPLC)
- Independently design and conduct critical experiments that further project goals
Experience For R&D Scientist, Chemistry Resume
- Understand the goal and maintain a high proficiency in his/her projects as well as the overall program
- Interpret results and draw conclusions from own multistage experiments, note significant deviations, then suggest, design, and pursue relevant experiments
- Perform routine and complex tasks competently and independently and generate reliable and consistent results
- Impact projects mostly through lab and/or pilot plant based activities
- Theoretical and practical knowledge to carry out the job functions
- Work in a multi-functional team environment
- Daily interactions with various allied scientific disciplines in Discovery and Development
- Proficient with analytical techniques such as HPLC, GC, and NMR spectroscopy
- Willingness to work occasional nights and week-ends, when necessary
Experience For Scientist, Chemistry R&D Resume
- Assess synthesized compounds’ profiles to determine whether they are appropriate for further study
- Assisting in method development, conducting method validation, performing laboratory research and/or routine sample analysis under minimal guidance of a supervisor
- Assist senior scientist to carry out methods development, optimization and validation for the analysis of drug substances, and finished products in accordance with cGMP regulations, established protocols, and applicable SOPs
- Knowledge of Dissolution methods developing and testing
- Manages assembly of regulatory information for submission
- Using the client's templates for dossier sections, author CMC sections for routine submissions (e.g., clinical trial amendments, Type I variations, IND/NDA/BLA annual reports, CMC renewals), including adding technical data and descriptive text
- PhD. Advanced academic research (i.e. post-doctoral) and/or industrial experience (1-3 years) desirable
- BS, MS, or PhD in Organic Chemistry or closely related field with 10+ years (BS); 8+ years (MS); or 0+ years (PhD) of industry or postdoc experience
- Using the client’s templates for dossier sections, author CMC sections for routine
Experience For Scientist, Chemistry, Manufacturing & Control Resume
- Great insurance and pension schemes, as well as several additional benefits such as company cabins and training with a personal training during working hours
- The ability to take part of exciting projects to improve human condition
- An inclusive and innovative working environment highly characterized by pride
- A basic understanding of kinetics, thermodynamics
- Search the chemical literature (e.g., using SciFinder)
- Collaborate with Data Verification Specialist to have submission content verified
List of Typical Skills For a Scientist, Chemistry Resume
Skills for senior scientist, chemistry resume.
- Experience in preparative chromatography and sufficient skills and experience in instrumental analysis (HPLC, SFC and HPLC-MS)
- Independently lead own chemistry research and effectively function in a goal-oriented team environment
- Consults literature and subject matter experts for background information and procedures for assistance in effectively accomplish goals
- Works cooperatively and effectively with coworkers
- Effectively communicates data and ideas with colleagues/team
- Independently lead his/her own chemistry research and effectively function in a goal-oriented team environment
- Be able to communicate effectively and follow detailed written and verbal instruction
Skills For Associate Scientist, Chemistry Resume
- Demonstrated industrial experience
- Demonstrate strong interest in synthetic organic chemistry
- Mechanical aptitude. Experienced in the use of basic tools. Able to troubleshoot, maintain, and operate mechanical systems such as Parr pressure reactors
- Independently lead own chemistry research and effectively function in a goal-oriented team nvironment. Key Leadership Competencies
- Extensive hands-on experiencewith MS based quantitative method development and validation
- Method development, method validation, laboratory research and/or routine sample analysis on API and solid oral dosage form under minimal guidance
- Formulation development can include vehicle screening, small scale screening for amorphous solid dispersion or nanosuspensions
- Strong understanding of chromatography instrumentation, principles and troubleshooting ability
Skills For Quality Control Scientist, Chemistry Focus Resume
- Demonstrate independent, scientifically-directed, and innovative thinking
- Effective interaction with other technical, regulatory, research and manufacturing groups is critical
- Experience doing synthetic method development, new reaction discovery, study of reaction mechanisms or total synthesis
- Have experience working with CMO’s and CRO’s
- Experience in synthesis, purification and analytical characterization including HPLC, MS, and NMR
Skills For Principal Scientist Chemistry Resume
- Significant experience authoring relevant CTD sections of regulatory submissions (IND, NDA, MAA, JNDA, etc)
- A proven track record of solving complex synthetic chemistry problems
- Experience with quality control and trending analysis of data is highly desirable
- Experience with programming languages (R, python, etc.)
- One year of manufacturing or industry related experience
- Experience in the pharmaceutical industry in discovery support, or formulation development for small molecules
Skills For Associate Scientist / Scientist, Chemistry Resume
- Process scale-up and process validation support
- Familiarity or experience with techniques of small molecule-protein conjugation, purification, isolation and characterization
- Experience with small molecule API synthesis
- Ph.D. in Analytical Chemistry/Organic Chemistry or other relevant discipline with a 0-3 years of relevant experience
- Work on multiple projects in parallel and be able to adjust to rapidly changing priorities
- Ph.D. in Chemistry with no more than 4 years of industry experience in drug discovery
- Experience with synthetic method development, new reaction discovery, study of reaction mechanisms or total synthesis
Skills For Scientist, Chemistry, Mfg & Cntrl Resume
- Significant experience in analytical development
- Significant experience in analytical technology transfers
- Experience in methodology/catalysis, total synthesis, chemical biology, or physical organic chemistry
- Experience in therapeutic design and interpretation of biological data
- Experience in synthetic organic chemistry methodology/catalysis, total synthesis, or physical organic chemistry
- Write and review validation protocols and reports
- Dissolution experience is desirable
- Functional experience in modern synthetic organic chemistry, compound purification, and organic chemistry mechanisms
Skills For Scientist Chemistry Bausch Lomb Resume
- 10+(BS), 8+(MS), or 0+(Ph.D) years of experience
- Experience with automated preparative HPLC systems
- Proven track record of solving complex problems
- Experience in Methodology/Catalysis, Total Synthesis, Chemical Biology, and/or Physical Organic Chemistry
- Experience with common molecular biology techniques used with DNA, such as PCR
- Experience with analysis of complex data sets
- Experience with statistical software packages is highly desirable
- MSc or PhD with more than 3 years industrial experience in relevant analytical methods
- Knowledge of both physical and wet chemistry methods linked to polymer and solid phase chemistry would be an advantage
Skills For R&D Scientist, Chemistry Resume
- Proven expertisein various mass spectrometric techniques
- Demonstrated expertise in trace analysis of impurities and metabolites in pharmaceutical and biological sample matrices
- Contribute data for preparation of development, validation and technology transfer reports
- Experience with HPLC, GC, FT-IR, KF etc. required
- Build strong relationships with peers and cross functionally with partners outside of teams to enable higher performance
Skills For Scientist, Chemistry R&D Resume
- Contribute to optimization and validation of synthetic routes applicable for synthesis of linker-drugs on multigram scale
- Maintain excellent experimental records for research and development efforts
- BSc or MSc in Synthetic Organic Chemistry 3-4+ years of laboratory experience
- Experience in multistep organic synthesis
- Excellent laboratory practices and safety techniques are essential
- Strong knowledge and technical background in the development and execution of synthetic organic chemistry routes
- Experience with complex chemical synthesis and interpretation of analytical characterization data
- Understanding of function as it relates to achievement of department or project needs and goals
- Understanding of medicinal chemistry principles and practices as it relates to analog / library design
Skills For Scientist, Chemistry, Manufacturing & Control Resume
- Research done following best practices for conducting experiments, acquiring data and interpreting results
- Assist with general laboratory housekeeping including cleaning, maintenance and calibration of analytical laboratory instruments
- Have working knowledge of CMC filing requirements
- Responsible for the routine purification needs for the medicinal chemistry team - Chiral purification using SFC, achiral purifications using HPLC
- Perform elemental impurity testing using ICP-MS and ICP-OESinstrumentations
- Proficiency with analytical techniques including-HPLC, GC, spectroscopic techniques, and mass spectrometry (structure elucidation and characterization)
- Quickly adapt to changes and develop appropriate plans for managing risks
- Ph.D. in chemistry, biochemistry or related field. Anticipated attainment of Ph.D. in 2018 matching noted criteria is also acceptable
- Capable of working with multi-disciplinary team
List of Typical Responsibilities For a Scientist, Chemistry Resume
Responsibilities for senior scientist, chemistry resume.
- Solves chemistry problems with some assistance from colleagues or supervisor
- Contributes technical knowledge and offers to help others with project workload as opportunities arise
- Responsible for timelines and adapts plans as project objectives change
- BS/BA in Chemistry or related discipline with at least 7 years of relevant pharmaceutical development experience OR MS in Chemistry or related discipline with at least 5 years of relevant pharmaceutical development experience OR PhD in Chemistry or related discipline with 0-3 years of experience
- Knowledge of stability chambers and qualification of stability chambers
- Knowledge of Excel, Word, Power Point and LIMS system knowledge
- Opportunity to work with the best teams in the industry, ranging from international top level scientists to cutting edge process- and production teams and highly creative business- and marketing teams
- Highly self-motivated and able to work independently
- Mentor more junior scientists
Responsibilities For Associate Scientist, Chemistry Resume
- A broad range of functional expertise in modern synthetic organic chemistry, compound purification, spectral analysis/interpretation
- An ability to search the chemical literature and evaluate potential solutions to synthetic problems
- A career in a global company certified as a Great Place to Work
- Experience in Chemical Biology, as well as, methodology/catalysis, total synthesis, and/or physical organic chemistry, along with a proven track record of solving complex problems
- Author analytical procedures, methods development and validation protocols and reports, technical reports, experimental designs, regulatory submission documents, etc
- Have working knowledge of developing various analytical techniques (e.g., chromatography, dissolution, spectroscopy) and product specification to support drug substance and drug product development and registration
- Responsible for project science within one's area of expertise on one or more project teams
Responsibilities For Quality Control Scientist, Chemistry Focus Resume
- Plan and organize oneself in a consistent manner
- Be a self-starter with the ability to positively motivate others in a cooperative fashion
- Maintain confidentiality of sensitive laboratory information
- Perform computer operations for data entry
- MS in Organic Chemistry (MS) or BS in Chemistry
- Desire to work with biocatalysts to identify and optimize reactions toward target molecules
- Search the chemical literature to ascertain viable approaches to chromatographic problems
Responsibilities For Principal Scientist Chemistry Resume
- Willingness to work occasional nights and weekends when necessary
- Basic analytical chemistry skills (understanding, routine use, interpretation, and troubleshooting of HPLC, LCMS, GC, and GCMS and the ability to perform basic NMR interpretation)
- Strong basic science and reasoning skills. The ability to plan, execute, critically interpret, and track the results from a logical progression of experiments in order to efficiently obtain definitive results
- Broad range of functional expertise in modern synthetic organic chemistry, compound purification and spectral analysis/interpretation
- Search the chemical literature to find viable solutions to synthetic problems
- Willingness to work occasional nights and weekends, when necessary
- BS & 10+ years of experience; MS & 8+ years of experience; PhD and 0+ years of experience is required to ensure that incumbent has necessary theoretical and practical knowledge to do the job
- Broad range of functional expertise in modern synthetic organic chemistry, compound purification, spectral analysis and interpretation
Responsibilities For Associate Scientist / Scientist, Chemistry Resume
- Search the chemical literature to ascertain viable approaches to synthetic problems
- Work on multiple tasks / projects simultaneously
- Motivation to work in a fast paced, dynamic biotech environment
- Possess strong verbal and written communication skills, and have significant experience in analytical development and technology transfers, and authoring relevant CTD sections of regulatory submissions (IND, NDA, MAA, JNDA, etc) and response to regulatory inquiries
- Experience in synthesis, purification and analytical characterization. Experience in methodology/catalysis, total synthesis, chemical biology, or physical organic chemistry. Experience in synthesis, purification and analytical characterization including HPLC, MS, NMR, and biophysical methods
- Experience in a structured workflow (manufacturing, QC, GXP, etc.) is highly desirable
- Knowledge of next generation sequencing techniques is highly desirable
Responsibilities For Scientist, Chemistry, Mfg & Cntrl Resume
- BS or MS in Organic Chemistry or related field
- BS in materials science, chemistry, biology, or a related field is required
- In depth knowledge of liquid and ion chromatography principles and ion exchange materials
- Hand on experience on using characterization tools such as IC or HPLC chromatography, particle size analysis, surface area measurements, and other related techniques
- Well versed in analytical chemistry principles and analytical method development

Responsibilities For Scientist Chemistry Bausch Lomb Resume
- Accurately plan, define, and manage project tasks
- Product and process characterization
- Preparation of relevant CMC documents for global registration
- Adheres to the client’s defined submission preparation timelines
- Publish project related research in high impact factor peer reviewed journals, R&D reports, patent applications and/or regulatory documents/filings
Responsibilities For R&D Scientist, Chemistry Resume
- Characterization work can include HPLC, XRD, TGA, DSC, and DLS
- Provides direct on-site preformulation support to enable efficacy, PK, and toxicology studies
- Familiarity with common analytical instrumentation
- Train team members in aspects of chemical synthesis
- Anticipate the need for contingencies and develop alternative strategies
- Registration specification development
- Drug product stability studies
- Shelf life establishment
Related to Scientist, Chemistry Resume Samples
Scientist, analytical chemistry resume sample, scientist, immunology resume sample, scientist, research resume sample, science resume sample, research science resume sample, life science resume sample, resume builder.

Chemist CV Example for 2024 (Skills & Templates)
Create a standout chemist cv with our online platform. browse professional templates for all levels and specialties. land your dream role today.

Welcome to our Chemist CV Example article. Here, we'll provide you with a detailed look at the requirements and qualifications for a Chemist position, as well as a sample CV to help you craft your own. With this guide, you'll be able to create a CV that highlights your skills and experience, stands out from the competition, and makes employers take notice.
We will cover:
- How to write a CV , no matter your industry or job title.
- What to put on a CV to stand out.
- The top skills employers from every industry want to see.
- How to build a CV fast with our professional CV Builder .
- What a CV template is, and why you should use it.
What does a Chemist do?
A chemist is a scientist who studies the composition, structure, and properties of matter. They use their knowledge of chemistry to develop new products and processes, diagnose and treat diseases, analyze and interpret data, and design new materials, technologies, and processes. Chemists also work in a variety of other fields, such as environmental science, food science, forensic science, and chemical engineering.
- Chemist CV Sample
- Geologist CV Sample
- Lab Technician CV Sample
- Mathematician CV Sample
- Mining Engineer CV Sample
- Nuclear Engineer CV Sample
- Physicist CV Sample
- Process Engineer CV Sample
- Quality Engineer CV Sample
- Reservoir Engineer CV Sample
- Validation Engineer CV Sample
What are some responsibilities of a Chemist?
- Developing new products and processes
- Conducting laboratory experiments
- Analyzing data and interpreting results
- Maintaining laboratory equipment
- Writing reports and presenting findings
- Researching chemical properties and reactions
- Developing safety procedures and protocols
- Teaching and mentoring students
- Consulting with clients and industry professionals
Sample Chemist CV for Inspiration
Personal Details Name: John Smith Phone: 123-456-7890 Email: [email protected] Address: 1234 Main Street, Anytown, USA
Summary John Smith is an experienced chemist with a proven track record of success in laboratory and research settings. He has a strong background in both organic and inorganic chemistry, and his research has been published in numerous scientific journals. With the ability to work both independently and as part of a team, John brings a unique skillset to any organization.
Work Experience
- Chemist, ABC Laboratories – Anytown, USA (03/2015 – Present)
- Conducted advanced chemical research and development for new products.
- Assisted in the formulation of new products and processes.
- Monitored laboratory safety procedures and maintained accurate records.
- Research Assistant, XYZ University – Anytown, USA (09/2011 – 03/2015)
- Assisted with advanced scientific research and development.
- Conducted laboratory experiments and analyzed results.
- Reviewed and revised existing protocols and procedures.
Education PhD in Chemistry, XYZ University – Anytown, USA (09/2011 – 03/2015)
- Organic and inorganic chemistry
- Laboratory safety
- Research and development
- Data analysis
- Scientific writing
Certifications
- Certified Laboratory Chemist, ABC Chemist Association – Anytown, USA (05/2018)
Languages English (fluent)
CV tips for Chemist
Crafting an impeccable CV that kickstarts your career is a challenging endeavor. While adhering to fundamental writing principles is beneficial, seeking guidance customized for your unique job pursuit is equally prudent. As a newcomer to the professional realm, you require Chemist CV pointers. We've curated top-notch advice from experienced Chemist individuals. Explore their insights to streamline your writing journey and enhance the likelihood of fashioning a CV that captivates potential employers' attention.
- Highlight essential skills and experience in the first section of the CV
- Include relevant research experience and recent publications
- List any awards, honors, or certifications earned
- Include any relevant volunteer experience or extracurricular activities related to chemistry
- Proofread for errors and typos before submitting your CV
Chemist CV Summary Examples
A Chemist CV Summary or CV Objective is an essential tool for job seekers looking to make an impression on potential employers. It helps showcase the candidate's qualifications, experience, and skills to employers in a concise and engaging way. It should be tailored to the specific job position and highlight the unique value the candidate can bring to the role. Additionally, it can demonstrate the candidate's enthusiasm and commitment to the position. For Example:
- Recent Chemistry graduate offering a strong background in laboratory research, chemical synthesis and analysis.
- Highly motivated chemist with experience in advanced laboratory techniques, including chromatography and spectroscopy.
- Organized and detail-oriented chemist with expertise in organic synthesis, data analysis and process optimization.
- Experienced chemist and project leader with a strong track record of success in developing new chemical processes.
- Resourceful chemist with a strong interest in green chemistry and a record of developing safe and efficient laboratory protocols.
Build a Strong Experience Section for Your Chemist CV
Building a strong experience section for a chemist CV is important because it is the most important part of a resume for a chemist position. A chemist’s experience section should highlight their previous positions in the field, their skills and accomplishments, and any relevant research they have conducted. It should also include any internships, co-ops, and volunteer experiences. This section is the employer’s first impression of the candidate and should include enough detail to demonstrate that the candidate is qualified for the position. For Example:
- Designed and executed experiments to study the synthesis of new polymeric materials.
- Conducted research on the effects of various additives on polymer properties.
- Developed protocols for the characterization of polymers materials.
- Prepared technical reports summarizing the results of experiments.
- Performed chemical analysis with a variety of analytical techniques.
- Managed a lab with multiple students and post-docs.
- Implemented safety protocols to protect lab personnel and equipment.
- Authored several peer-reviewed articles about polymer science.
- Presented research findings at national and international conferences.
- Collaborated with colleagues in other departments to optimize research methods.
Chemist CV education example
A Chemist typically needs a Bachelor's degree in Chemistry or a related field, such as biochemistry. Many Chemists also pursue advanced degrees such as a Master's or Doctorate. Additional certifications or licenses may also be required depending on the type of work a Chemist is doing. Here is an example of an experience listing suitable for a Chemist CV:
- BSc in Chemistry, University of Oxford, UK, 2019
- GCSEs in Maths, English, Chemistry, Physics and Biology, Sacred Heart High School, London, UK, 2017
Chemist Skills for a CV
Adding skills to a Chemist CV is important because it allows employers to quickly scan and assess a candidate's qualifications. Skills should be tailored to the position being applied for. By including relevant skills, employers can see that the candidate has the specific knowledge and abilities needed to excel in the role. This can help the employer to make a quick decision about whether or not to move forward with the candidate. Examples of skills that may be relevant for a Chemist CV include knowledge of analytical chemistry, laboratory safety, and instrumentation. Soft Skills:
- Critical Thinking
- Problem Solving
- Organizational
- Communication
- Time Management
- Attention to Detail
- Analytical Skills
- Research Skills
- Analytical Chemistry
- Organic Chemistry
- Inorganic Chemistry
- Laboratory Skills
- Data Analysis
- Quality Control
- Equipment Maintenance
- Data Interpretation
- Safety Practices
Common Mistakes to Avoid When Writing a Chemist CV
In today's competitive job market, an average of 180 applications floods employers' inboxes for each vacant position. To streamline this influx of CVs, companies frequently employ automated applicant tracking systems that weed out less qualified candidates. If your CV manages to surpass these digital gatekeepers, it must still captivate the attention of the recruiter or hiring manager. Given the sheer volume of applications, a mere 5 seconds is typically allocated to each CV before a decision is reached. With this in mind, it's crucial to eliminate any extraneous information that might relegate your application to the discard pile. To ensure your CV shines, consult the list below for elements to avoid including in your job application.
- Skipping the cover letter: A well-crafted cover letter is an opportunity to showcase your suitability for the role and express your enthusiasm for it.
- Excessive jargon: CVs laden with technical terms can alienate hiring managers who lack specialized knowledge.
- Neglecting vital details: Incorporate your contact information, education, work history, and pertinent skills and experiences.
- Relying on generic templates: Tailoring your CV to the specific job exhibits your commitment to the position and company.
- Errors in spelling and grammar: Proofreading is essential to eliminate typos, spelling errors, and grammatical blunders.
- Overemphasizing duties: Highlight accomplishments to underline your candidacy's value.
- Sharing personal information: Steer clear of revealing personal details like age, marital status, or religious affiliations.
Key takeaways for a Chemist CV
- Highlight relevant experience and achievements
- Include a clear and concise summary statement
- Include a list of relevant technical skills
- List academic qualifications and certifications
- Draw attention to any relevant research experience
- Include links to any published work
- Include any professional affiliations or awards


Department of Chemistry
Search form.
- Affiliated Faculty
- Administrators & Staff
- Research Faculty & Staff
- Research Associates
- Graduate Students
- Update/Submit Alumni Information
- Emeritus and Retired Faculty
- In Memoriam
- Research Facilities
- Centers & Programs
- Astrochemistry
- Bioanalytical
- Biophysical Chemistry
- Catalysis and Energy
- Chemical Biology
- Chemical Education Reserach
- Imaging and Sensing
- Inorganic and Organometallic Chemistry
- Nanosciences and Materials
- Organic Chemistry and Synthesis
- Surface Chemistry and Spectroscopy
- Theory and Computation
- Non-Thesis Master's Program (1 Year)
- PhD Program
Applying to the PhD Program
- Information about Charlottesville
- Chemistry and Multidisciplinary Courses
- Graduate Handbook
- Professional and Career Development Opportunities
- Graduate Chemistry Program Clubs & Organizations
- Fellowships and Awards
- Graduate Program Calendar
- Graduate Student Forms
- 2023-24 Bi-weekly Payroll Calendar
- Diversity, Equity, and Inclusion Initiatives
- Disability Accommodation Information
- Prospective and Transfer Students
- General Chemistry Options
- Undergraduate Advisors
- Process for declaring a major, minor, DMP, or ACS Certification
- B.A. in Chemistry
- B.S. Chemistry
- B.S. Specialization in Biochemistry
- B.S.Specialization in Chemical Education
- B.S. Specialization in Chemical Physics
- B.S. Specialization in Environmental Chemistry
- B.S. Specialization in Materials Science
- B.A./M.S. or B.S./M.S. in Chemistry ("3+1" Degree Option)
- American Chemical Society Poster Session
- Undergraduate Publications
- Undergraduate Research in a pandemic
- How to Prepare and Present a Scientific Poster
- How to Prepare and Present a Scientific Talk
- Guidelines for Final Report
- Distinguished Majors
- Study Abroad
- First and Second Years
- Third and Fourth Years
- Graduation Information
- Undergraduate Resources
- Upcoming Seminars
- Seminar Archive
- Request Seminar Date
- Named Lectures
- Spring 2023 Newsletter
Ready to apply!
Check out application requirements below and click on this link: Apply to Graduate School
Application Requirements
The Admissions Committee reviews applications to identify candidates with the broad training in core areas of chemistry necessary to succeed in graduate coursework, teaching, and research. In addition to applicants with bachelor degrees in chemistry or biochemistry, candidates with degrees in allied disciplines (such as biology, chemical engineering, or physics) and who possess significant training in one or more core areas of chemistry will be considered. Generally, students are admitted to the PhD program for the fall term. In some years, applications for the spring term can be considered. Applications and all supporting documents must be submitted through the Graduate School of Arts & Sciences' (GSAS) online application portal , which opens on October 1st . The deadline for PhD Applications is December 1 .
Admissions decisions are made on a rolling basis between January and mid-April; given the size of our applicant pool, we are unable to provide status updates to applicants during this period.
Technical questions related to the application system should be addressed to the Graduate Admissions Office, by email ( [email protected] ) or phone (434-243-0209).
The following items are required for an application to be complete:
- Statement of purpose
- Unofficial transcripts (students who accept an offer of admission must then have official transcripts sent directly from their university to the Graduate Admissions Office)
- 2 confidential letters of recommendation submitted directly by the letter writers
- An application fee of $85
Additional materials for international applicants whose first language is not English
- Self-reported TOEFL/IELTS scores from a test taken within the last 2 years (unless they completed a 4-year undergraduate degree in the U.S. or at an institution with English-only instruction); students who accept an offer of admission must then have official scores sent directly from the testing agency to the Graduate Office of Admissions).
- International students are strongly encouraged to submit GRE Chemistry (or a related subject) test scores.
Admitted students residing in the U.S. will be invited to UVA Grounds for a visitation event in March.
For more information, please visit The Graduate School of Arts & Sciences ’ website, including the page of Frequently Asked Questions about admission.
Application Fee Waivers
Due to the substantial volume of requests and the limited availability of departmental fee waivers, the Chemistry Department has implemented a two-step fee waiver policy:
Step 1: Applicants should first ascertain whether they are eligible for a fee waiver from UVA's Graduate School of Arts & Sciences (GSAS).
• US citizens and permanent residents may qualify for GSAS fee waivers based on participation in specified organizations, training programs, and the United States Armed Forces; graduation from a Minority Serving Institution (MSI); and financial hardship. Click on the following link for eligibility requirements and directions for submitting a fee waiver application, https://graddiversity.virginia.edu/application-fee-waiver .
• International students may qualify for an automatic GSAS fee waiver based on their citizenship. Click on the following link for the GSAS Admission Requirements page, https://graduate.as.virginia.edu/requirements , then click on the “Fees” section for details and directions.
Step 2: Applicants who do not qualify for a GSAS fee waiver may request a fee waiver from the Department of Chemistry—though these are extremely limited and rarely granted—by sending a CV ( and only a CV ) containing the information below to [email protected] .
- Current address
- Citizenship
- Current position
- Educational background (previous institutions, degrees, and GPAs)
- Research experiences
- Research interests
- Publications, awards, honors, and presentations
- UVA faculty members of greatest interest
Applicants will be notified by email when their application fee waiver request has been evaluated. Allow a minimum of two weeks for the review process.
Given the limited number of Departmental fee waivers available, we strongly encourage applicants to apply for our graduate program through the normal process if they are able to do so. This fee waiver application review is separate from the standard application review process, so if an applicant applies for a fee waiver and then later chooses to apply through the normal process, there will be no prejudice against their admission application.
Admission FAQs and Answers to Common Questions
Can I contact faculty directly by phone or email to learn more about research opportunities in their particular laboratories?
Yes, contact information is available on individual faculty pages; additionally, many research groups maintain their own websites and list current research opportunities there.
Do I need to apply directly to a particular laboratory, or do I apply to the Chemistry Department as a whole? Where do I indicate which labs I find interesting?
No, applicants apply to the Graduate Chemistry Program, not to a particular lab; however, you can list up to three faculty you hope to work with in your application and discuss the research group(s) you hope to join in your Statement of Purpose, although you are not obligated to stick with those selections if you are admitted.
Do you accept students for admission in the spring semester?
Most students are accepted for the fall semester; however, under special circumstances we can consider spring admission.
Can I begin research in the summer before my first semester of graduate school?
Students entering the program during the fall semester may begin research in the preceding summer if a research group sponsors them. Admitted students may contact the faculty member(s) they are interested in working with to discuss possible options. Please note that there is no obligation to continue working in the same lab after that summer, and faculty members cannot guarantee summer students a permanent lap position.
If I am an international student and my native language is not English, am I required to submit TOEFL or IELTS scores?
Applicants whose native language is not English but who have an undergraduate degree from a U.S. institution, or from an institution whose instruction is entirely in English, do not have to submit TOEFL/ IELTS scores. All other international applicants self-report test scores on their application. If they accept an admissions offer, they need to have official scores sent from ETS (or IELTS) directly to the Graduate Admissions Office. For more information, see the following website: https://graduate.as.virginia.edu/requirements
What are the minimum TOEFL/IELTS scores?
The minimum internet-based (iBT) TOEFL score for general admission to the Graduate School is 90, with minum scores in each component as follows: 22 for Speaking, 22 for Writing, 23 for Reading, and 23 for Listening. The minimum IELTS score requirement is 7.0 in each section. The date of the reported test must fall within two years of the application deadline.
Is the GRE general test scored required or accepted?
No, the Chemistry Graduate Admissions committee will not consider scores from the General GRE exam.
If admitted to the program, can I visit the Chemistry Department?
Yes, we offer a visitation event (generally in the middle of March) and cover travel expenses up to a specific amount. Upon admission to the program, you will receive information about this event. In addition, although we encourage you to attend the visitation event, we can arrange one-day individual visits to the department if you are unable to attend the larger visitation event.
Does your department have a minimum GPA?
The Graduate School has set 3.0 as the minimum GPA for admission to the graduate program. However, in certain cases warranted by extenuating circumstances, the Chemistry Department can request a waiver of the minimum required GPA. If your GPA is below 3.0, but you believe that there are circumstances that render your application competitive, you are encouraged to apply.
What is the average GPA for students who receive a favorable admission decision?
The average GPA of students who are admitted changes every year. Since evaluation of applications considers many different factors, and the average GPA can change each year, we believe that the average GPA is not, on its own, a definitive metric to gauge an applicant's competitiveness.
Is undergraduate research essential for admission into your program? Will a student's prior research area influence whether or not they are granted admission?
We recognize that students are applying from programs with differing access to undergraduate research opportunities, and undergraduate research is not essential for admission to our program. To the extent possible, we aim to judge each applicant on the basis of their potential for future work in their stated area of research interest. Changes in research topics and areas of interest between undergraduate and graduate level research are to be expected.
Resume Builder
- Resume Experts
- Search Jobs
- Search for Talent
- Employer Branding
- Outplacement
- Resume Samples
- Science and Biotech
Analytical Chemist Resume Samples
The guide to resume tailoring.
Guide the recruiter to the conclusion that you are the best candidate for the analytical chemist job. It’s actually very simple. Tailor your resume by picking relevant responsibilities from the examples below and then add your accomplishments. This way, you can position yourself in the best way to get hired.
Craft your perfect resume by picking job responsibilities written by professional recruiters
Pick from the thousands of curated job responsibilities used by the leading companies, tailor your resume & cover letter with wording that best fits for each job you apply.
Create a Resume in Minutes with Professional Resume Templates
- Develops, implements, and maintains analytical methods for use in lubricant product development, problem solving, and customer assistance
- Provides analytical method development to the process development group
- Assisting Application Development and Technical Service personnel in providing training and demonstrations
- To identify and make recommendations for improvements as part of a team within or outside the department in order to ensure continuous improvement
- Independently perform routine and complex analysis using established analytical methods and development of analytical methods when no SOP is in place
- Data collection and experimentation assistance in support new product development
- Perform routine performance checks on instruments that are being used for project support (e.g. HPLC, XRPD, moisture sorption, DSC, TGA)
- Manage the chromatography and mass spectrometry laboratory and provide timely analytical support to global R&D of the Silicones business
- Support for formulation development
- Technical and analytical support for product and process improvement projects
- Development and validation of both physical and analytical test methods in support of reference standards, metered dose inhaler and transdermal products
- Development and validation of both physical and analytical test methods
- Develop new analytical techniques and methods that will be adapted in other labs within the company
- Writing and routing of technical documents and change management requests
- Leading the development and validation of both physical and analytical test methods in support of metered dose and transdermal products
- Providing technical and analytical support for product and process improvement projects
- Leading studies to support regulatory submissions and improvement projects
- Writing of documents for regulatory submissions
- Other duties as assigned
- Develop, evaluate, troubleshoot, validate, and transfer analytical methods
- Author, review, and execute method validation and transfer protocols and reports
- Good record keeping skills and excellent attention to detail
- Ability to quickly produce accurate results and reports to resolve urgent factory quality issues
- Excellent computer skills particularly with MS Office, ChemStation, Mass Hunter and/or Labware (or a comparable LIM system)
- Demonstrable experience of sample preparation techniques e.g. liquid:liquid extraction, solid phase extraction etc
- Demonstrated ability to run high throughput analytical workflows requiring fast sample turnaround and consistent data quality
- Excellent “teaming” skills. Able to work effectively on cross-functional teams and projects
- Ability to learn from, work with and professionally respect colleagues/peers
- Highly organized and able to work well with customers and employees at all levels in the organization
- Strong attention to detail
- Strong comfortability in working with various instruments and analysis software
15 Analytical Chemist resume templates

Read our complete resume writing guides
How to tailor your resume, how to make a resume, how to mention achievements, work experience in resume, 50+ skills to put on a resume, how and why put hobbies, top 22 fonts for your resume, 50 best resume tips, 200+ action words to use, internship resume, killer resume summary, write a resume objective, what to put on a resume, how long should a resume be, the best resume format, how to list education, cv vs. resume: the difference, include contact information, resume format pdf vs word, how to write a student resume, analytical chemist industrial quality resume examples & samples.
- Independently organize work tasks for the day according to business priorities and backlog
- Effective hand-off of work and priorities, and good communication practices, with colleagues on other shifts
- Coordinate with other chemists to ensure that supplies are stocked and equipment is maintained and calibrated
- Perform quality audits of production areas as needed
- Conscientious, positive in attitude, self-motivated, reliable, and responsible
- Ability to make decisions independently and assess when escalation is appropriate
- Detail-oriented with a demonstrated proactive and analytical approach to problem solving
- Excels at interpersonal communication and able to form effective relationships across departments
- Sense of urgency in daily work and towards resolving issues
Analytical Chemist Intern Resume Examples & Samples
- Sample preparation, including method development, to reveal characteristic features
- Development of proficiency using analytical instrumentation to generate meaningful information in pursuit of materials and chemcial characterization or failure analysis goals
- Collection of results on specified materials using analytical instrumentation
- Analysis of material qualification and performance testing data using statistical methods
- Documentation of analysis results using specified formats and data storage systems
- Communicating effectively with management, coworkers, and suppliers
- Ensuring that all work carried out conforms with safety and housekeeping requirements
- Education: Working toward Master or Ph.D. degree in either Analytical Chemistry or Materials Science
- Experience: No industrial laboratory experience needed, although familiarity with working in an industrial or academic research environment is desired
- Demonstrated ability to apply technical skills and understanding of the fundamentals involved in chemistry or materials science
- Demonstrated ability to work independently to achieve desired results from general objectives
- Recording experimental set-up, data, observations, and results in a lab notebook
- Ability to document work by putting together summary reports and presentations
- Demonstrated ability to work in and around hazardous chemicals without any adverse health effects
Analytical Chemist Resume Examples & Samples
- Ph.D. in Chemistry, Biochemistry or related discipline
- 1+ years of industrial experience in analytical development for proteins and mAbs
- Solid technical experience and understanding of mass spectrometric techniques
- Technical proficiency with HPLC methods (RP-HPLC, IEC, SEC and HIC, etc.)
- Experience in sequence variant analysis for mAbs preferred
- Familiarity with a range of protein analytical techniques including CE, icIEF, UV-Vis spectroscopy, and immunoassay (ELISA) preferred
RD&A Analytical Chemist Resume Examples & Samples
- Supporting flavour development through analysis and interpretation
- Using a range of software, techniques and equipment to carry out research and analysis
- Accurately recording data in accordance to guidelines
- Liaising with customers, suppliers and research/scientific staff
- Writing and presenting reports, reviews and summaries
- Keeping up to date with scientific and technical developments
- Developing new analytical methods
- Validating methods and equipment
- Maintenance and upkeep of equipment
- Ensuring that health and safety issues are adhered to
QC Analytical Chemist Resume Examples & Samples
- Perform Chemical analysis on finished drug substance, drug product, in process materials and stability samples
- Responsible for calibration and use of laboratory instrumentation
- Complete documentation associated with analytical results in a timely manner
- Report and document any non-conformances to the QC Supervisor
- Assist in the preparation and review of area documentation (SOP's, Reports or Protocols)
- Assist in the training of other analysts
- Ensure training compliance within assigned work area and QC
- Perform and assist in additional duties as directed by the QC Supervisor
- Experience in any of the following: High Performance Liquid Chromatography (HPLC) with Empower, Capillary Electrophoresis, Part Mat, Osmolality, pH, appearance testing, UV, Karl Fischer, SDS Coomassie
- Experience with Lab systems an advantage. (LIMs, CDAS, Trackwise, PDOCs)
- Third level qualification preferably with a minimum of 2 years previous laboratory work experience in a cGMP pharmaceutical environment
- Lead conduct work on analytical-based projects supporting all areas of the business involving beauty, cosmetic, and personal care products. Work independently, utilizing a broad range of analytical techniques and scientific knowledge, to arrive at conclusions that guide business decisions
- Understand big picture and in-depth technical details related to projects
- Communicate results clearly and concisely to associates of all levels across R&D in oral and written form
- Develop new and improve existing analytical methods to increase accuracy, efficiency, and robustness
- Explore and evaluate new analytical technology and methodology for potential application to innovative research
- Set specifications for raw ingredients and finished products
- BA/BS or MS degree in chemistry or equivalent scientific discipline with 2-10 years experience
- Self-starter able to see projects through to conclusion
- Broad and in-depth knowledge of analytical chemistry techniques, including chromatography (HPLC, GC), spectroscopy (IR, UV/VIS), DSC, Wet chemistry techniques
- Strong problem solving skills and the ability to develop creative and scientifically sound solutions
- Highly flexible, adaptive to changing business priorities, completing projects in a fast-paced work environment
Process Analytical Chemist Resume Examples & Samples
- Bachelors Degree or higher in Chemistry/ Life Sciences
- Strong background in method development and validation for HPLC, GC-MS and NMR
- 5+ years relevant experience in GMP Manufacturing/Testing environment in FDA Regulated Medical Device/Pharmaceutical Industry, particularly analytical testing associated with combination product development
- To troubleshoot, develop and validate robust, sound analytical methods
- To prepare Submission documentation in support of license applications
- To take part in/ lead laboratory analytical investigations
- To liaise directly with customers and attending conference calls
- To attend internal project review meetings
- To draft Test Methods, Protocols and Reports
- To support all other on-going laboratory functions & requirements
- To ensure that all work carried out is in compliance with the required standards conforming to company, cGxP, SOPs, regulatory regulations and guidelines, safety and environmental guidelines
- To test and analyse raw materials & finished products in a timely and efficient manner
- To perform additional team tasks as agreed to support effective running of the Business
- Qualified to a minimum of degree level
- At least two years experience working in a related technical environment
- Instrumentation experience including any of the following: HPLC, GC, Wet Chemistry
- Bachelor of Science in Chemistry
- 4+ years of experience as an Analytical Chemist
- 2+ years of experience in Gas Chromatography or Gas Chromatography-Mass Spectroscopy or Liquid Chromatography experience
- 2+ years of experience writing technical reports
- Career goal to work as an Analytical Chemist in the Laboratory
- Experience developing methods and macros using Agilent and Perkin Elmer Chromatography software
- Experience in Capillary Electrophoresis
- Demonstrated ability to
Senior Analytical Chemist Resume Examples & Samples
- Minimum of six (6) years of good manufacturing practice (GMP) laboratory experience in an analytical capacity including method development and validation experience
- Bachelor’s degree or higher in Chemistry from an accredited university
- Experience with metered dose inhaler and transdermal products
- Strong working knowledge of High Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC)
- Strong knowledge of FDA and USP requirements for metered dose inhaler and transdermal products
- Self-driven with excellent organizational and leadership skills
- Strong data analysis and statistical skills
- Change management experience
- Empower chromatographic data system experience
Advanced Analytical Chemist Resume Examples & Samples
- Analysis of registration stability samples, collation of data, stability data analysis
- Addressing additional studies to support preparation of regulatory submissions
- Minimum of three (3) years regulated environment laboratory experience
- 3M experience working across multiple disciplines, businesses and/or departments
- Strong working knowledge of Gas Chromatography (GC) and/or High Performance Liquid Chromatography (HPLC)
- Meticulous data collection and ability to maintain a laboratory notebook
- Ability to adhere strictly to SOPs, good technical skills and working familiarity with laboratory procedures and processes
- StarLIMS Lab Information Systems software experience
- Basic understanding of statistical techniques, data analysis and variability
- Conduct raw material, in process and final product testing in GMP Analytical labs
- Analyze and interpret results of technical work and testing
- Crossover and transfer of methods
- Identify and implement improvements to daily lab operations, standard work, testing work flow/cycle time, test methods and lab capability using Lean Six Sigma methodologies
- Bachelor's degree or higher in a Science or Engineering discipline from an accredited institution
- Minimum of one (1) year of experience in a laboratory environment (an Internship or Co-op is considered qualifying)
- Bachelor’s degree or higher in Chemistry, Analytical Chemistry, Engineering or related discipline from an accredited institution
- Experience performing analysis of drug, combination product and medical device products and materials in GMP lab setting [Food and Drug Administration (FDA) Drug GMP, U.S. Pharmacopia (USP), International Council on Harmonisation (ICH) guidelines relevant to drug and combination products industry)
- Functioning knowledge of High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), Fournier Transform Infrared (FTIR) Chromatography, wet chemistry, and analytical laboratory techniques
- Experience in successfully leading projects. Able to coordinate multiple tasks/projects and manage priorities accordingly
- Demonstrated proficiency in the use of statistical and quality data analysis tools such as exploratory data analysis, basic statistics, Gage Repeatability and Reproducibility (R&R), Analysis of Variance, Failure Modes and Effects Analysis (FMEA), Statistical Process Control and Capability, acceptance sampling, correlation and regression, and experimental design
- Demonstrated skills in technically sound written and oral communications
- Knowledge of data collection systems and conformance to 21 Code of Federal Regulations (CF Part Eleven (11), such as Empower, Electronic Laboratory Notebook (ELN), etc
- Producing, analyzing and interpreting data from various analytical techniques
- Use of ICP-OES, surface area, chemisorption, classical analysis and combustion analyzers
- Data collection and instrument maintenance
- Work effectively in a multidisciplinary team environment
- Be integrated in internal operating system and contribute as requested to Dept. targets
- Actively contribute to Honeywell’s world class Safety goals
- Additional tasks per assignment by manager
- Studies: BS degree (3 years) in Chemistry or a closely related science discipline is required
- Specific certifications (CISCO, ORCLE, ITIL, PMI, etc): Proficiency in Microsoft Office (Excel, Word, PowerPoint, Outlook)
- Experience: Minimum 3 years hands-on experience on operating and maintaining analytical instruments for materials analysis related to the petroleum industry
- Additional hands-on experience on surface area, chemisorption and elemental analysis techniques is a plus
- Previous experience in a manufacturing Quality Assurance or Research and Development laboratory environment is preferred
- Familiarity with statistical process control (SPC) and SPC software is desirable
- Good interpersonal skills and ability to interact effectively with other departments
- IT skills HW/SW (OS, Software, Tools, SW development languages, etc): Proficiency in Microsoft Office (Excel, Word, PowerPoint, Outlook)
- Language skills: fluency in Italian and English is mandatory requirement
- Provides leadership in all areas of the Quality System, including but not limited to Risk Management, Corrective & Preventive Actions, Nonconforming Materials, Design Controls, etc
- Prepare analytical method development plans, validation documents and test method documentation
- Review laboratory data for accuracy, consistency and completeness; identify results with potential issues requiring further investigation
- Interpret MSDS and TDS
- Prepare summary reports from laboratory data
- Process improvement
- A minimum BS degree in chemistry, chemical engineering, material science or a related technical discipline - A Master’s degree is a plus
- At 6-8 years of experience is preferred
- Experience with analytical testing (chemical, biological, mechanical) is a definite plus
- Experience working in a regulated industry is preferred, as is a good understanding of Quality Systems processes to be able to effectively lead and support the implementation, improvement and maintenance of department programs
- Good oral and written German and English language skills are requiredR&D
Analytical Chemist, / I-nmr Lab Resume Examples & Samples
- Performing routine NMR analysis including sample preparation, data collection, data processing for a variety of coatings-related materials
- Providing NMR support for structural elucidation, impurity identification, quantification and raw material screening
- Performing maintenance and troubleshooting of NMR instruments, including cryogen refills and instrument calibrations
- Enhancing collaborations with other NMR labs and analytical colleagues to provide technical support and improve productivity
- Providing excellent customer service through timely, clear, and accurate reporting of the analytical data
- Bachelor’s degree in Chemistry or related field
- One year of experience in analytical chemistry, including high pressure liquid chromatography (HPLC)
- Experience with Excel, Word and Power Point
- Knowledge of basic chemical reactions and basic calculations
- Ability to use analytical balances and pH meters
- Ability to perform volumetric analysis
- Generate, organize, interpret, and report data in support of technical projects and service requests, including but not limited to fundamental knowledge development, plant support, customer issue support, new method development, and competitive analysis
- Troubleshoot and maintain test instruments, equipment, and procedures to ensure the highest level of accuracy and reliability in our data
- Apply current knowledge of, and lead research efforts in NMR (and optionally LC and/or GC) analysis, involving and working with the broader analytical team as needed
- Integrate new technology for new applications and product support
- Perform hands-on research as well as direct and prioritize the work of technical staff
- Drive projects from inception through completion
- Communicate and interact with a broad range of internal and external customers through technical reports, presentation, and 1-on-1 conversations
- Meaningfully convey technical information across departments and divisions
- Maintain familiarity with current literature and recent advances in analytical chemistry techniques, including NMR, and optionally LC and/or GC analysis, to keep our labs and methods at the current edge
- Participate in the recruitment, training, and mentoring of technical staff
- Actively participate in Lubrizol safety programs, ensure compliance with safety procedures, and maintain good housekeeping
- Top-tier Analytical Chemist who will push boundaries
- Design and execute forced degradation, excipient compatibility, cleaning validation, and stability experiments
- Evaluate and interpret analytical data and communicate results to management and functional teams
- Recommend specifications and methods for process or product characterization
- Anticipate, recognize, and resolve simple and complex technical issues through knowledge, use of technical resources including technical literature and experts and well formulated, logical experimentation
- Provide scientific guidance and training for junior analytical chemists
- Write and review SOPs
- Manage analytical projects. Establish schedules, define priorities, and direct activities of other scientists and non-scientists as well as provide training and development for others
- Minimum of 5 years of pharmaceutical laboratory experience in analytical R&D
- Extensive knowledge of cGMPs, GLPs, and FDA/ICH guidelines and their application to drug development
- Technical expertise in developing and running analytical methods using common quantitative techniques including UPLC/HPLC-UV/FL/ELSD/MS, UV/Vis, GC, and wet chemistry. Bioanalytical experience is also beneficial
- Experience with in-vitro release testing, such as USP dissolution, dialysis, Franz diffusion cells
- Experience with a broad range of dosage forms such as tables, capsules, device/implants, suspensions, lyophilized products, and novel formulation types is preferred
- The ability to manage multiple projects simultaneously, set priorities, and meet key deadlines
- Exceptional written and verbal communication
- Capable of working independently
Microscopist / Analytical Chemist Resume Examples & Samples
- BS Chemistry/Analytical Chemistry, Chemical Engineering, Material Science and Engineering, or Metallurgical Engineering and 3-5 years of lab experience
- Experience with analytical lab equipment/instrumentation, careful sample preparation, testing, and understanding of analytical techniques for problem solving and characterization of coatings systems
- Must be able to write reports utilizing computers with Microsoft Office
- Experience in creating and following standardized procedures, with close attention to detail
- Previous coatings formulation and application experience
- Experience with optical and/or scanning electron microscopy
- Experience in metallurgical sample preparation (e.g., cross-sectional analyses)
- Experience with conducting maintenance on analytical equipment or other equipment
- Excellent problem solving skills and experience with statistical data analysis
- Must be self-motivated and have independent project management skills
- Good interpersonal skills and ability to communicate detailed information are required to work effectively in a team environment
- The ability to establish and maintain positive productive working relationships with peers, colleagues, internal and external customers is a must
- Ability to use computers for complex scientific applications and apply these skills to their work
- Candidate must be able to distinguish colors in order to perform analysis
- Must have a steady hand to handle small or delicate specimens
- Work safely with paint related materials and chemical reagents
- Must be committed to Safety, Quality, 5S, and TS16949 requirements
- One year analytical chemistry experience in a laboratory environment including traditional wet-chemistry procedures
- Knowledge of basic analytical chemistry procedures such as solvent extractions/digestions and titrimetric, gravimetric, and colorimetric procedures
- Skills with Word, Excel and PowerPoint
- Provide day to day support in the analysis of formulations being developed by the R&D group
- Develop and validate analytical methods to assay the formulations which are nearing commercialization
- Provide analysis support for the formulations being produced in the pilot plant
- Provide analytical and troubleshooting support for product quality issues
- Assist in the transfer of developed analytical methods to the production facilities
- Stay abreast of new developments In Analytical Instrumentation that would meet the needs of the business
- Ph.D. with 1 to 2 years’ experience or B.S. in Chemistry with 10+ years of industrial experience analyzing formulated products
- Hands on experience using HPLC, GC and IC instrumentation
- Strong technical understanding of fundamental analytical chemistry
- Theoretical and operational understanding of chromatographic techniques
- Knowledge of a broad breadth of analytical tools and their relevant applicability
- Strong fundamentals and practical experience in the use of statistics to develop data reliability information
- Excellent interpersonal skills that support a diverse team environment
- Demonstrated creativity and strong problem solving skills
- Manage the chromatography and mass spectrometry laboratory and provide timely analytical support to global R&D of the Silicones business
- Communicate analytical results with other scientists in the organization
- Give technical presentations and provide scientific guidance and training for junior analytical chemists
- Minimum of 3-5 years of industrial or postdoctoral experience
- Technical expertise in mass spectrometry (GC/MS, LC/MS with emphasis on mass spectral interpretation), gas and liquid chromatography, thermal analysis and spectroscopy
- Must be self-motivated, able to work independently, and in the meantime contributing to a team effort to achieve business and technical objectives
EPA Senior Analytical Chemist Resume Examples & Samples
- Participate in all regulatory inspections: FDA, DEA, Board of Pharmacy, and Internal Audits
- Review and approve data generated by other chemist
- Review and update standard operating procedures
- Develop Quality Reports
- Strong knowledge of and troubleshooting HPLC, UPLC, and UV vis
- Knowledge of Aseptic Technique A PLUS!
- Knowledge of Chromatographic analysis, GLP documentation of experiments, and Quantitative Analysis
- Ability to travel up to 5%
- Implementation of bioanalytical methods for the determination of drug and metabolite concentrations in biological fluids and tissues
- Utilize LC-MS/MS instrumentation
- Preparation of samples and processing of data
- Documentation of experimental results in electronic notebooks
- Writing report summaries and collaborating with other scientists in the bioanalytical group. Bioanalysis work includes both preclinical GLP toxicology and clinical studies
- Knowledge of bioanalysis using LC/MS in a regulated environment
- Experience with any of following: Waters Acuity UPLC, Sciex quadrupole MS/MS systems; Watson LIMS and Analyst software; Hamilton robotic system
- Adept in variety of sample preparation techniques: protein precipitation, liquid-liquid and solid phase extraction techniques
- Ability to troubleshoot LC/MS method and instrument problems
- Bachelor's degree or Master’s degree in biology, chemistry, or other related degree concentration, or equivalent directly-related experience (two years of directly related industry experience is equivalent to one full-time year of college in related major)
- At least 1 year experience working as an analytical scientist in a pharmaceutical environment
- Well-organized, great attention to detail, an avid learner, can follow SOPs, and excellent time management skills
- Ability to handle human and animal biological samples
- Perform method development and validation
- Maintain equipment such as HPLC and GC, and oversee preventative maintenance
- Write protocols
- Perform pH detection, moisture testing, pipetting, micro balances and testing using GC
- Perform tests according to GLP/GMP regulations
- Review documentation for accuracy and completeness
- Relevant experience including HPLC to develop and validate methods, and GC in GLP/GMP laboratory environment
- Experience with Agilent chromatography software
- Experience with method development and validation
- Strong initiative and self-direction
- B.S. in Chemistry, Biology, or related science plus at least four years related experience, or M.S. in Chemistry, Biology, or related science plus at least three years related experience
- Responsible for utilizing analytical methodologies, systems and processes to support GMP release and stability testing of clinical supplies for drug substance and drug product, throughout all phases of development. This may include excipient, packaging and post packaging support
- Analytical testing for release would include: compendial testing (e.g., excipients / packaging release testing), disintegration, dissolution, LC, and generic testing (e.g., solvents, water, ash, titrations, heavy metals, ICP/MS)
- Set up and analysis of drug substance and drug product stability samples
- Method development and validation of analytical methods for drug substance and drug product
- Collect and utilize information from a variety of sources to understand the current state of analytical equipment/instrumentation for Data Integrity requirements such as system validation, user access/roles, data/metadata lifecycle/storage, audit trail content, data retention, electronic records/signatures, etc
- Partner with Subject matter Experts (SMEs) to clarify business processes and requirements needed for the generation and management of electronic data for analytical equipment/instruments including observation of equipment/instruments in a lab environment
- Author documents related to Data Integrity assessments for analytical equipment/instruments that are clear, concise and complete
- Ensure alignment of DI assessments and supporting documents across Analytical Chemistry areas
- Effectively communicate status of ongoing activities with project team leadership and department management
- At least one year experience in GMP analytical chemistry
Test Engineer / Analytical Chemist Resume Examples & Samples
- Operate and improve processes for strain analysis in Bioworks1
- Apply DOE approaches to refine and improve sample extraction and assay methods
- Translate assays to be performed by software + automation
- Work hand in hand with software and automation engineers to scale up sample extraction methods and assays 100-1000X
- Develop processes for verification and quality control of assays to ensure low CV and reproducibility
- Collaborate with software engineers to ensure that all generated data is captured, stored and analyzed in Ginkgo's LIMS
- Perform analytical laboratory tests on a daily basis for Dosage Pharmaceuticals (such as purity, related substances, uniformity, dissolution, etc.) in accordance with approved methodology and cGMP, FDA, and DEA regulations when required
- Set-up, adjustment, calibration, operation, and troubleshooting of laboratory equipment and instruments, as well as any associated software
- Follow methods as written and must have an understanding of chromatographic theory to adjust methods as needed to improve resolution, etc
- Preparation of solutions, solvents, and mobile phases
- Accurate preparation and analysis of samples and reference standards
- Accurate and timely documentation of all procedures, calculations, and results
- Must be able to independently perform and adequately document investigations for out-of-specification/out-of-trend results
- Review and approve analyses performed by other laboratory personnel for compliance to analytical methods, as well as cGMP, FDA, and DEA regulations
- Complete assignments in a timely manner in accordance with current SOPs
- Work effectively in a team environment. Good oral and written communication skills
- Comply with company, site, and safety policies / procedures
- Able to perform basic statistical analyses
- Basic computer skills including Word, Excel and database software
- Ability to understand, apply, and audit for compliance to cGMP, SOP, and DEA regulations when relevant
- Accurately and carefully follows established procedure and initiates action to correct quality problems/notify others of quality issues
- Managing Work: Effectively manages time and resources to ensure that work is completed efficiently
- Adaptability: Maintains effectiveness when experiencing major changes in work tasks or environment, can adjust to work within new structures, processes, or requirements
- Four year degree in a science based field, BS in Chemistry or Bio-Chemistry preferred
- Must have two year’s industrial analytical chemistry laboratory experience. Four years is preferred
- The candidate should possess excellent organizational, written, and oral skills and have basic computer skills
- Experience in an analytical chemistry testing laboratory performing wet chemistry testing and/or instrumentation testing including Dissolution, HPLC, AA, IR and UV/Vis
- Working familiarity with word and excel required and operational experience with Water’s Empower data acquisition preferred
- Working knowledge of the US and European pharmacopeias preferred
- Support project teams for analysis of pharmaceuticals, and products / subcomponents within a cGMP lab environment
- Perform analytical testing in support of ongoing R&D and process development projects
- Support product failure analysis activities
- Working knowledge of cGMP requirements/procedures required
- Working knowledge of drug formulation, degradation mechanisms, and processing effects is desired
- Must demonstrate strong communications skills (written and verbal), ability to manage/prioritize high volumes of analysis requests
- Consistent attention to detail
- Aptitude for experimental design and methods development
- Broad knowledge of analytical techniques, including spectroscopy (FTIR, NMR, mass spectrometry), wet chemistry, and chromatography
- Excellent written skills and knowledge of test method validation is required
- Ability to work independently, as well as on teams
- As a generalist, the candidate should be able to provide direction for problem solving by applying internal and external resources (e.g. contract laboratories)
- Well versed in a broad range of standard tests and have hands on experience with a variety of instruments
- Perform chemical analysis and research for projects involving trace analysis of components in environmental samples and complex matrices
- Perform a variety of standard preparation, sample preparation, and analysis using LC-MS and/or GC-MS systems in compliance with applicable methods, test plans, and SOPs
- Perform aspects of equipment operation and maintenance including calibration, operation and maintenance of GC-MS and LC-MS systems
- Perform calculations and conversions, and compile the data generated. Coordinate and perform data analysis and prepare data reports
- Experience with analytical method development
- Previous research experience related to chemical, biological, radiological, nuclear, and/or explosive (CBRNE) threats
- Experience working with toxic chemicals
- Experience operating in GLP/GMP/ISO environments including the preparation and validation of standard operating procedures
- Minimum of a Bachelor’s degree in Chemistry, Analytical Chemistry, or a related field of study
- Experience working in an analytically diverse environment
- Familiarity with method validation procedures, SOPs, and electronic systems
- Hands-on experience with the following: HPLC, GC, FTIR, and NIR
- Self-motivation, good interpersonal and communication skills, and strong customer orientation
- Prior work experience in a manufacturing analytical laboratory
- Hands-on experience with the following: ICP-OES, ICP-MS, LC-MS, GC-MS, and IC
- Working knowledge of other analytical methods (including wet chemical procedures)
- Familiarity/experience with manufacturing sampling planning
- Experience in communicating with manufacturing chemists and/or unit operators
- Minimum of a PhD degree in Analytical Chemistry or a related field of study
- Experience in analytical method development and validation (industrial or academic)
- Hands-on experience with chromatography, mass spectrometry, and NMR
- Self-motivated with good interpersonal and communication skills, and strong customer orientation
- Experience working with process chemists and engineers
- Experience in the field of phosphorus chemistry analytics
- Working knowledge of the following: FTIR, multivariate statistics, thermal analysis, particle size measurements, scanning electron microscopy (SEM)
- Minimum of one or more years of experience in industry
- Hands-on experience with all of the following: HPLC, ICP-OES, ICP-MS
- University level research with thesis
- Hands-on experience with GC, LC-MS, GC-MS, and/or IC
- Prior experience with Atlas software
- Working knowledge of other analytical methods such as wet chemical procedures, UV/Vis, and/or FTIR
- Familiarity/experience with manufacturing sample analysis
- Prior experience working for a global organization
- Prior experience in the agricultural industry
- Minimum of a Bachelor’s degree (or higher) in Analytical Chemistry or a related field of study
- Minimum of three or more years of related industrial experience
- Experience in development and validation of GC-MS/MS methods
- Hands-on experience in analytical method development and validation with emphasis on liquid chromatography - mass spectrometry (LC-MS/MS) based techniques
- Self-motivation and attention to detail
- Demonstrated ability to troubleshoot analytical methods and equipment
- Excellent oral/ written communication and interpersonal skills
- Agility to work on and balance multiple projects, while maintaining the focus to meet business objectives
- Ph.D. degree in analytical chemistry or closely related field
- Experience developing, validating and executing residue analytical methods in two or more matrices (i.e. crop, soil and water) that meet Regulatory guidelines
- Experience and proficiency in the use of Laboratory Information Management Systems (LIMS) and other electronic systems
- Experience with air sampling, monitoring and associated analysis
- 4+ years of related polymer chemistry experience
- ACS Certification
- Hands-on experience with the operation, application, and interpretation of analytical chemistry instrumentation and test methods including FTIR, UV/Vis, AA/AE, NIR, DSC, DMS, TGA, HPLC, GC, GCMS, ESCA, Auger, XPS, SIMS, EDAX, TEM and STEM
- Experience with wet chemical analysis techniques including absorbance spectroscopy and electrochemistry
- Experience with the development of test methods to quantify the effectiveness of chemical process including metal cleaning, etching, plating, and passivation, ceramic and polymer treatments to enhance surface chemistry, texture, and free energy for metallization, coatings or adhesive bonding
- Project management experience including planning, labor estimating, technical direction and reporting for investigation of “root cause” failure analysis, development of new or improved chemical processes and analysis techniques, operational effects of mission specific environments on material physical properties, polymer thermo-mechanical and thermodynamic performance sensitivities
- Experience with mechanical, electrical, optical, and physical properties of organic and inorganic materials
- Experience with and understanding of Polymer Chemistry mandatory
- MS degree in Analytical or Polymer Chemistry
- Authorship of technical papers in society journals and participation as a peer reviewer
- Understanding of mechanical and electrical design tools such as proE, FEA, and SPICE
- Excellent communication skills, attention to detail, follow through and multitasking abilities
- Knowledge of mechanical, electrical, and chemical simulation methods
- BS degree in Analytical or Polymer Chemistry
- Provide scientific, technical, and practical expertise to the Formulations Group to support and take part in the development, validation, and implementation of analytical methods for analysis of pesticide formulations
- It is also expected that this Scientist have basic formulations chemistry skills and the ability to formulate complex formulations (often 2 and 3 way mixes) to help support Formulation team goals when necessary
- B.Sc. in Chemistry or related discipline and 5 years related experience or MS in Chemistry or related discipline and minimum of 3 years related experience or PhD in Chemistry or related discipline and minimum of 2 years related experience; non-degreed with minimum of 15 years practical bench level analytical and formulation experience would also be considered
- Thoroughly conversant with the theory and use of all instrumentation and equipment in the formulations and analytical laboratory, with ability to assist with formulation of samples
- Liaise with similar positions within customer laboratories is expected, together with EPA, State labs, etc
- Good communication, persuasion, and influencing skills are needed, together with strong scientific credibility
- Ability to innovate, principally in the area of analytical method development, possibly on new molecules and formulations
- Working knowledge of industry standard operating procedures and government regulations that will impact the workings of the formulations area including: safety, EPA product chemistry guidelines, and GLP regulations
- Ability to transfer knowledge by providing training and guidance to other chemists within the group
- The job holder is expected to be pro-active and independently provide solutions to problems encountered during projects; it is expected that practical judgment is utilized to extensively evaluate facts before requiring the assistance of the supervisor
Inorganic Analytical Chemist Resume Examples & Samples
- Prepare samples of inorganic polymers for testing by performing sample dilution and acids digestion
- Operate analytical instruments to conduct elemental analysis
- Work with the Senior Scientist to ensure that all analytical results are accurately recorded and delivered safely
- B.S. in Chemistry (analytical)
- Preferably have worked with inorganic polymers
- 1-3 years lab experience, including ICP-OES, ICP-MS and AA
- Experience with preparing samples that include acids (i.e. hydrofluoric) and performing wet chemistry testing
- Become a subject matter expert in analytical test methods, procedures and instrumentation to run ICP-OES, IC, HPLC and auto-titration equipment
- Preform routine analyses on raw materials and finished goods to endure proper QC
- Participate in analytical method development and validation
- Conduct occasional bench top experiments to simulate manufacturing processes
- Ensure all data is generated in a timely and complaint fashion
- Abide by all date input / output specifications
- Perform routine maintenance or troubleshooting on laboratory equipment
- Exercise a high regard for safety and chemical hygiene practice
- Observe and promote exemplary housekeeping within the laboratory
- Train junior chemist and technician to assist with the responsibilities listed above
- Assignment will be a pre-defined rotating schedule to support continuous operations
- BS or MS in chemistry or chemical engineering, with relevant bench-top chemistry experience
- Working knowledge of and experience with analytical equipment including but not limited to ICP-OES, IC, HPLC and auto-titration
- Solid foundation in acid-base chemistry
- Flexibility with work schedule
- Ability to establish priorities, work independently and productively, and deliver results with minimal supervision
- Ability to work in a fast-paced environment that may include rapidly shifting priorities
- Ability to concentrate and perform quality work in a dynamic environment
- Patience and leadership to train and oversee 1-3 chemical technicians per shift
- A strong sense of team cooperation
Research Analytical Chemist Resume Examples & Samples
- 50 %- Utilize analytical knowledge to execute analytical methods that provide support to complex projects providing defined business value. This includes improving and optimizing existing methods and assisting in the development and validation of new analytical methods. Serve on multi-disciplinary project teams as the analytical expert to identify problems, clarify questions and determine needed solutions. Communicate regularly with project team stakeholders. Communicate with other stakeholders as needed, such as business or group management, production, outside vendors, or technology providers
- 25 %-Handle sample requests and conduct routine sample analysis and routine experiments. Organize and communicate results. Write SOPs and reports as needed
- 10 %- Maintain familiarity with a range of analytical instrument options and be able to troubleshoot assigned, key instruments
- 10 %-Effectively communicate and coordinate with other team members to prioritize tasks and execute plans
- 5 %- Ensure corporate EHS and Food Safety compliance including proper waste disposal and perform any other duties as assigned
- Bachelor’s degree in applicable science (chemistry, biochemistry, biology, microbiology, food science ). `
- Minimum 2 years relevant industrial experience
- Experience in chromatography (multiple detection systems) techniques (HPLC and GC)
- Experience with Chromatography Data Systems (CDSs), such as Chromeleon, Empower, OpenLab, MassHunter, etc
- Ability to analyze results and use this analysis in assisting the development or validation of a method
- Demonstrated understanding of the implications of other disciplines on technical problems
- The ability to identify and resolve a problem
- Demonstrated ability to prioritize tasks between projects or assignments
- The ability to rapidly acquire, understand and communicate new information
- Extensive knowledge of HPLC is required and must include practical hands-on experience with the qualitative and quantitative analysis of small molecule pharmaceuticals
- Experience in an R&D environment and knowledge of GMP regulations is required
- Participates in determining objectives of assignment
- Accomplishes assigned tasks using own discretion and judgment as to the specific approach or technique
- Internal/external resource for technical issues
- Evaluates and defines function activities for projects and proposes appropriate timelines; keeps functional activities on schedule
- Represents analytical function as core team member for R&D projects
- Contributes to CMC sections for ANDAs
Principal Analytical Chemist Resume Examples & Samples
- Knowledge of liquid chromatography is required
- Experienced with method development, validation, and transfers is required
- Considerable interpretation is required to develop alternatives and solutions for a broad range of problems
- Solutions require integration of multiple factors and often require collaboration, imagination, and thoroughness to ensure consistency with objectives
- Internal/external resource for technical issues; expert within functional group
- Evaluates and defines function activities for projects and proposes appropriate timeline; coordinates activities in functional area; keeps functional activities on schedule
- Demonstrates knowledge in scientific techniques, theory, troubleshooting, etc
- Familiar with ICH guidelines and applies guidelines as appropriate
- Familiar with requirements of various pharmacopeias (USP, EP, JP) and basic regulatory requirements and applies knowledge appropriately to activities
- Contributes to preparing summaries for IND and/or to preparing sections of the CMC for ANDAs or NDAs
- Familiar with CDFSS/ DFSS (Operational Excellence) tools and applies these tools as appropriate
- Conduct raw material, in process and final product Good Laboratory Practice (GLP)
- Write, review and approve test results and reports
- Conduct Out of Specification investigations
- Generate protocols, summary reports, Standard Operating Procedures (SOPs) and other lab-related documents
- Maintain data integrity, systems, and records
- Ensure safe laboratory conditions and practices through good housekeeping, equipment maintenance, and safety education
- Minimum of one (1) year of experience in a laboratory environment (an internship or Co-op is considered qualifying)
- Bachelor’s degree or higher in Chemistry, Analytical Chemistry, or related discipline from an accredited institution
- Functioning knowledge of Inductively Coupled Mass Spectroscopy (ICP-MS), Ion Chromatography (IC), Fourier Transform Infrared Spectroscopy (FTIR), Gas Chromatography (GC), Flam Atomic Absorption Spectroscopy (FAA), wet chemistry and analytical laboratory techniques
- Demonstrated proficiency in the use of statistical and quality data analysis tools such as exploratory data analysis, basic statistics, Gage Repeatability and Reproducibility (GRR), Analysis of Variance, Failure Modes and Effects Analysis (FMEA), Statistical Process Control and Capability, acceptance sampling, correlation and regression and experimental design
- Knowledge and conformance to 10 Code of Federal Regulations (CFR) 21, 10 CFR 50 Appendix B, ASME Nuclear Quality Assurance (NQA)-1 and International Organization for Standardization (ISO) 17025
- Previous experience in a chemical manufacturing environment
- Excellent communications (oral and written)
- Previous experience working on a cross functional team
- 3M is an equal opportunity employer. 3M will not discriminate against any applicant for employment on the basis of race, color, national origin, religion, sex, sexual orientation, gender identity, age, disability, or veteran status
- Ability to work in a GLP or GMP environment with appropriate documentation and safety practices
- Support formulation development through hands-on generation of accurate and precise analytical chemistry data using routine laboratory techniques such as liquid chromatography (HPLC, UPLC), NMR (liquid), mass spectrometry, vibrational spectroscopy, USP/EP dissolution testing, Karl Fischer titration
- Development of dissolution methodology that will provide some measure of an in vivo / in vitro relationship and serve as a quality control procedure
- Development and execution of studies to ascertain degradation mechanisms in the solution and solid state
- Assess stability risks by evaluating process changes and impact on degradation chemistry or dissolution profile. This would involve DoEs and accelerated stability studies
- Development of assay/purity methods and assessment of method robustness/ruggedness
- Development and execution of method validation protocols
- Transfer of analytical methods to other laboratories and remote sites
- Hands-on generation of data in support of accelerated and registration stability studies
- At least one year experience in product development analytical chemistry
- Perform assays on vaccine products, process intermediates, and related experimental samples using ELISA, HPLC and other biochemical/analytical tests (e.g., spectrophotometric, electrophoretic, immunochemical methods)
- Prepare solutions and reagents
- Laboratory data review
- General laboratory skills, experience with pipettes, plate washers, plate readers, electrophoresis equipment, and automated instrumentation
- Bachelor's degree in Biochemistry, Biology, Chemistry, or other related degree concentration, or equivalent directly-related experience (two years of directly related industry experience is equivalent to one full-time year of college in related major)
- Minimum of 2-5 year related laboratory experience
- Function as a team member with other scientists in, testing, developing and transitioning chemical / biological detection / identification technologies
- Generates test data and contributes to technical report development
- Performs the operation, maintenance, and troubleshooting of various mass spectrometers including a Thermo Fisher Orbitrap
- Performs analytical practices including the generation of a calibration curve with the application of isotopically labelled internal standards. This not only includes the generation of raw data, but also the processing of the data into publication quality figures
- Reports to on-site Leidos team lead and represents Leidos as prime contractor for the Edgewood Chemical and Biological Center
- Requires a Bachelors in Chemistry or related field with 6-8 years’ experience or a, Masters in Chemistry or related field with 3-5 years’ experience. Experience must be related to performing routine and complex mass spectrometry analyses
- Demonstrated experience in the operation and maintenance of mass spectrometers
- Can effectively carry out supervisor’s instructions with minimal oversight
- Applicants must be U.S. citizens and qualify for a security clearance
- Experience in direct-analysis in real time (DART) ionization
- Paper spray experience
- Proficient in problem solving
- Experience in MS proteomics
- Laboratory experience should be well represented within their publication record. Experience with MS analysis of chemical warfare agents (CWAs) or pesticides is desired but not required
- Candidate must have a PhD in Analytical Chemistry degree and a minimum of 3-5 years analytical chemistry working experience
- Demonstrated experience in chemical analysis of small molecules, macromolecules, energetic materials and rubber products
- Must have strong spectroscopic working experience with some chromatographic chemistry experience
- Strong research and problem solving capability in an R&D and/or production support organization
- Experience in NMR, FTIR and MS a plus
- Demonstrated experience and working knowledge of chemical analysis such as GC, GC/MS, HPLC, LC/MS, and Wetlab tests a plus
- Experience with investigation and troubleshooting of Aerospace materials and processing a plus
- PhD in Analytical Chemistry
- Detail oriented but able to understand the big picture
- Development of proficiency using analytical instrumentation to generate meaningful information in pursuit of problem solving solutions or material characterization goals
- Education: Working toward Master or Ph.D. degree in either Analytical Chemistry or Materials Science focusing on Chemical / material Characterizations with various techniques and instrumentations
- Demonstrated ability to apply technical skills and understanding of the fundamentals involved in analytical chemistry or materials science
Associate Analytical Chemist Resume Examples & Samples
- Independently generate precise, reliable and reproducible data in a timely manner as part of the development, validation, and transfer of drug product analytical methods, including excipients, in-process and drug product testing
- Independently or with coaching, design and conduct critical experiments to further project goals. Analyze and critique generated results noting significant deviations and suggest and design further relevant experiments
- May serve as analytical representative to cross functional project teams; actively participate in team efforts and contribute to technical evaluations and solutions
- Manage aspects of analytical projects. Establish schedules, define dependencies and project plans and direct activities of other scientists and non-scientists as well as provide training and development for others
- Proactively and cooperatively communicate with peers and management to ensure awareness of progress and issues; recommend solutions when issues arise
- Capable of working with significant independence
- Maintain and meet the highest standards in quality, customer service and regulatory compliance
Bioscience Center Analytical Chemist Resume Examples & Samples
- Serve as subject matter expert for Analytical Chemistry with a focus on mass spectrometry and chromatographic separations
- Will be expected to develop or maintain a deep scientific understanding of relevant disciplines such as metabolomics, and proteomics
- Possess strong project and program management skills
- Ability to prioritize multiple demands and work successfully in a fast-paced and, at times, ambiguous environment
- Willingness to train others and courage to constructively challenge and discuss analytical results
- Ph.D. (Analytical Chemistry, Chemistry, Biochemistry), or equivalent experience
- Deep experience in industrial research and development. Experience must be proven by a solid track record of publications and/or patents
- Experience developing and optimizing fit-for-purpose analytical methods for analysis of a wide range of analytes (small molecules, proteins, lubricants, fatty acids, fermentation products, anions, cations, and metals) in complex matrices using a wide variety of analytical instrumentation including LC-MS (QTOF and QQQ), UHPLC, GC, GC-MS, IC, ICP, and MIR
- Generating, recording, interpreting, and reporting high quality and accurate analytical chemistry data and information
- Openness to train others on a variety of instrumentation and methods
- Courage to constructively challenge and discuss the analytical results of others
- Ability to communicate effectively with project team leaders to anticipate analytical needs and estimate resource requirements to develop required methodology and execute testing. Writing, optimizing, and validating SOPs and SOMs for a wide variety of analytical instrumentation and methods
- A high level of technical knowledge in a broad range of fields such as metabolomics, proteomics, process analytics, and traditional analytical techniques
- Ability to provide expert advice and innovation to develop solutions to complex technical issues
- Design and execute experiments; interpretation of data from multiple sourcesMust be motivated, possess good interpersonal skills, communicate well within and between teams and be able to work to tight deadlines
- Ability to prioritize responsibilities and work effectively in a multi-tasking and matrix environment
- Participate in project strategy development and implementation, including developing intellectual property
- Demonstrated commitment to safety in the work environment
- Exhibit strong One Team behaviours: “Support those I work with and help to build the effectiveness of my team to achieve the best results”, “Lay the foundations for the future by helping people to develop their capabilities”, “Recognize and acknowledge the contribution of others”, “Enable others to trust me by delivering on my accountabilities and standing by decisions when they are made” and “Put the team first”
- Exhibit strong Respect behaviours including “Respect the views and feelings of others and consider the impact of my words and actions”, “Build strong relationships based on trust and honest discussions”, “Listen carefully and consider different perspectives”, and “Create an inclusive and diverse workplace where everyone is treated with respect and dignity”
- Experience with Agilent mass spectrometry, UHPLC, GC, and GC-MS equipment and software including OpenLab, Masshunter, Bioconfirm, and/or SpectrumMill
- Previous experience of working with academic and 3rd party partners
- Demonstrated experience at executing changes to existing laboratory set-ups through appropriate use of management of change processes
- Ensure analytical methods are properly validated/qualified as appropriate in compliance with local and Group SOPs before routine use in QC
- Ensure analytical instruments are properly qualified and calibrated in compliance with local and Group SOPs
- Ensure all QC technicians are properly trained on all routine methods
- Manage the instrument calibration program for the QC laboratory
- Manage the QC Analytical Reference Standards program
- Manage the QC laboratory supplies procurement process
- Attention to detail and strict adherence to GMPs is a must
- Wet Chemistry, Titration, HPLC, GC, IR, Data Integrity requirements
- Solid technical skills
- Effective problem solving/troubleshooting skills
- Experience with analytical method validation per GMP requirements
Analytical Chemist Scientist Resume Examples & Samples
- Demonstrated ability to perform hands-on testing in a laboratory environment and summarize results
- Problem solving capability in an R&D and/or production support organization
- Experience Preferred
- Experience in thermal analysis (DSC, TGA, thermal and mechanical properties measurement), combustion measurement, wet lab tests, chromatography and/or spectroscopy a plus
- Demonstrated independent work skills
- Demonstrated innovation and/or problem solving skills
- Technical knowledge and expertise in the use and application of specialized analytical instrumentation to support Nutritional testing, pre-mix, pre-blends, mixer studies, raw ingredients, developmental products, and finished product analysis. Specific instrumentation includes, but not limited to, ICP, ICP MS, Leco Protein Analyzers, Fat Extractors, Auto Titrators, TGA, Wet Chemistry tests
- Skilled in sample preparation, clean-up, separation, and assay
- Supervises and develops laboratory specialists
- Frequently interacts with other Regional and Global associates to Implement test protocols, report data, clarify results, validate data, release HRCI data, coordinate new method transfers, and recommend future direction
- Collaborates with the Global Laboratory, Regional Laboratories, Plant Quality Manager, Plant QFS technicians, Regional Laboratory Manager and others in the validation of analytical results and development of new methods
- Directs instrumentation maintenance and calibration
- Directs additional laboratory procedures (nutritional / oxidation / sample preparation) depending on need
- Evaluates regional nutritional laboratory needs on regular basis
- Manages instrumentation including evaluating, recommending, calibrating, operating, maintaining and arranges training for specialists
- Maintains documentation of analytical results in a company issued
- Creates Collaborative Relationships
- Product Quality Custodian
- Masters the key scientific principles associated with Nutritional testing and preparation
- Demonstrates knowledge and understanding of laboratory safety, chemicals
- Bachelor’s degree in chemistry or natural or food sciences with emphasis in Analytical chemistry
- 5-9 years’ experience with chemical and physical analyses in food or related industries (advanced education may partially substitute for required experience)
- Knowledge and accuracy in running and maintaining necessary analytical instrumentation is required
- Master’s preferred
EPA Associate Analytical Chemist Resume Examples & Samples
- Performing sample analysis per cGMP/GLP Guidelines
- Maintains Supply Inventory for Laboratory
- Degree in Chemistry or other applicable science (college laboratory research experience accepted)
- Ability to execute planned projects
- MS or PhD Analytical Chemistry (or equivalent) with minimum of 5 years direct analytical experience using LC/MS, GC/MS and associated sample preparation methods & techniques
- Experience with colloidal systems, reverse and normal phase chromatography, identification of unknowns using TOF and QTOF mass spectrometry, and sample prep of acid/base and solvent systems
- Strong technical, collaborative and organizational skills
- Experience with Agilent and Bruker analytical equipment & software
- SQL expertise (Oracle, MySQL, MS SQL Server, MS Reporting Services)
- Data automation experience
- Programming background (python, Java, C#)
- Demonstrate strong technical capabilities in GC, GC-MS and other analytical techniques
- Analyze data for trends and statistical significance to provide clear experimental conclusions
- General troubleshooting and maintenance of laboratory equipment
- Maintain necessary documentation in accordance with company SOPs and GMP guidelines
- Remain current on industry specific issues and novel analytical techniques that may provide solutions to future problems
- Write reports and present project information to team members as necessary
- Perform general lab duties to assist the team in ensuring smooth daily lab operation
- Maintain safe, clean work area and adhere to L’Oréal’s policies and procedures
- PhD with 1-5 years, Masters with 2-8 with years, Bachelors with 5-11 years’ experience in Analytical Chemistry; other disciplines considered with a strong analytical instrumentation focus
- 1-3 years’ experience in the cosmetic, personal care, consumer goods, raw material supplier, or foods industries
- Strong working knowledge of several analytical techniques including various forms of spectroscopy and chromatography
- Organization and time management skills necessary to lead multiple projects to completion
- Exhibit strong emotional intelligence and interpersonal effectiveness
- Perform and/or review routine and non-routine analytical testing on final APIs and stability samples as needed in a GMP environment: e.g., prepare samples for analysis and record data from analyses or experiments in a log book or laboratory notebook, etc
- Experiments may range from analyses using LC, GC, IR, NMR, and other instrumental methods to non-instrumental methods (wet chemical methods, etc.)
- Set-up and execute analytical method validation experiments per approved protocols
- Maintain and troubleshoot lab instruments/lab problems
- Participate in OOS investigations
- BS degree in chemistry or related discipline and have a minimum of 2-4 years of relevant background
- Strong GMP compliance and documentation skills
- Strong comprehension and facility in quantitative/qualitative analytical chemistry
- Familiarity with Agilent ChemStation (HPLC, GC)
- Familiarity with compendial testing and OOS investigations
- Data review experience in a compliance-oriented laboratory
- Minimum of a Bachelor’s degree in Analytical Chemistry or a related field of study
- Minimum of three or more years of related experience
- Experience in analytical method development and validation
- Hands-on experience with gas chromatography, gas chromatography-mass spectrometry, and sample preparation techniques
- PhD in Analytical Chemistry or a related field of study
- Experience with quantitative, low level analytical chemistry testing
- Perform a range of routine analysis using chromatographic techniques under minimal supervision of a senior member of staff
- Work as part of an efficient analytical team
- Take responsibility as required for elements of specific projects and/or specific areas of general laboratory/sample administration
- Responsibility to lead/participate in assigned RD&I development projects and assignments, and to report progress and results
- Responsibility to order and perform method development, chemical analyses and other laboratory evaluations as part of project and assignments
- Responsibility to initiate and support product development tasks in implementation stages, like production scale up, technical and analytical development and customer evaluation
- Ensure progress in assigned projects through leading and coordinating team members
- Ensure customer satisfaction through knowledge support and trouble shooting in customer related problems
- Cooperate with other laboratories mainly in AkzoNobel
- Master of Science in Chemistry / Chemical Technology
- Experience within the area of Analytical Chemistry, Process Technology and Process Analytical Technology
- Familiar with statistical tools
- Experience from laboratory work
- Strong communication skills (both in writing and verbally), fluent in Swedish and English
- Conduct laboratory testing in an accurate and timely manner on a variety of analytical instruments including particle distribution (by sedimentation, laser diffraction, and/or sieve analysis), BET surface area and porosity, density measurements, color/brightness analysis, and thermal analysis
- Conduct or assist with analyses involving X-ray diffraction, X-ray fluorescence, FTIR spectroscopy, differential scanning calorimetry, optical microscopy, scanning electron microscopy, and transmission electron microscopy with emphasis on problem-solving
- Complete sample preparation, which may include crushing and grinding of ore samples, polishing, working with chemicals, and various other sample preparation techniques.Coordinate submission of samples to external laboratories if needed
- Assist with sample database log-in and organization of projects throughout stages of preparation, analysis, and reporting.Use electronic laboratory notebook to record and manipulate data.Assist with organization of raw data files, calculation files, reports, standard test methods, standard operating procedure, and/or work flow diagrams, according to ISO quality systems/good laboratory practices (GLP)
- Follow written test methods and standard operating procedures accurately during analysis; assist with updating existing or developing new test methods/operating procedures as needed.Organize inter-laboratory or intra-laboratory studies, calibrations, or other quality control related activities to validate results
- Record and organize data in order to complete or assist with accurate interpretation of results, drawing conclusions, and communicating effectively by written standard internal reporting practices
- Actively contribute to analytical method development
- Coordinate with other Analytical scientists as needed to complete projects
- Work within the Imerys Health, Safety and Environmental procedures
- Sharing of laboratory administrative tasks, such as ordering supplies, organizing maintenance schedules, organizing sample inventory, filing, organization of equipment instructions, etc. is necessary
- Aptitude with laboratory equipment repair and maintenance would be beneficial; possible coordination with various building services functions such as electrical contractors, HVAC technicians, gas delivery, etc
- Other tasks and duties as assigned
- B.S. or M.A. in Chemistry, Material Science, Geology or other laboratory based discipline is desired
- 3+ years of direct experience with working in a laboratory environment
- Background in analytical techniques is beneficial
- Passion for electron microscopy techniques with the dexterity and attention to detail required for accurate analysis and sample preparation
- Demonstrates analytical, project management and problem solving skills
- Ability to develop new analytical processes and methodologies
- Meticulous and inquisitive with a passion for continuous improvement
- Capable of working efficiently and accurately when juggling multiple priorities
- Skill in the use of computerized spreadsheet, relational database, word processing, drawing, plotting and process diagram software
- Knowledge of project management principles, practices, techniques, and tools
- Knowledge and understanding of analytical principles, processes, and techniques
- Minimum three (3) years’ of regulated environment laboratory experience
- Bachelor's degree or higher in Chemistry from an accredited university
- Familiar with working in a regulated environment, preferably GMP or GLP
R&D Analytical Chemist, Materials Scientist Resume Examples & Samples
- Operate and maintain the Materials Engineering R&D chemical analysis laboratory equipment used to characterize non-metallic materials for both oxygen and non-oxygen service. This includes Fourier transform infrared (FTIR), accelerated rate calorimetry (ARC), differential thermal/thermal gravimetric analysis (DTA/TGA), bomb calorimetry, etc
- Work independently to complete routine analytical activities for the chemical and thermal property evaluation of non-metallic materials. Determine effective analysis protocols and procedures specific to individual jobs. Provide interpretation of results
- Perform scanning electron microscopy with energy dispersive spectroscopy (SEM/EDS) on both non-metallic and metallic samples for fractography, general sample characterization, and chemistry
- Lead minor failure analysis and analytical technical service jobs. Regularly provide direct support to various global businesses, operations, engineering, and R&D groups within Praxair on technical issues related to non-metals and oxygen safety
- Assist senior Materials Engineering R&D personnel with analysis in support of major failures and issues
- Support commercialization programs led within R&D. Apply technical expertise to identify and evaluate technical solutions to materials issues
- Communicate results and recommendations through oral presentations and formal reports
- Periodic activities will include design and construction of small scale mechanical and electrical systems for material and component testing test programs conducted at the Batavia test site
- A PhD in Analytical Chemistry or Physical Science or a first degree plus significant, demonstrable experience in the application of analytical techniques and procedures to the characterisation of complex materials. Applicants interested in expanding their knowledge into new areas of science would be welcomed
- Knowledge of the analytical technologies required for the development of quantitative and qualitative methods for new agrochemical active ingredients and formulated products
- Knowledge, gained through practical experience, of analytical methodologies available to apply to solving chemical characterisation problems
- Knowledge of colloid and/or polymer science, analysis and characterisation using modern analytical techniques
- Knowledge of extraction procedures and analytical technologies associated with the characterisation of agrochemicals
- Experience of analytical techniques for the qualitative and quantitative analysis of active ingredients or polymers in one or more of the following techniques: GC, LC, CE, GC-MS, LC-MS, preparative chromatography, thermal techniques (inc. DSC) and spectrophotometry techniques
- Good attention to detail, a high proficiency for multi-tasking & project planning, and flexibility to change
- Effective oral and written communication, in a culturally diverse setting, including technical and non-technical audiences
- Able to work effectively in a cross-functional team setting. Customer focused. Experience of working in a team where different methods have been applied to solve common problems
- A good general understanding of chemistry would be highly advantageous
- Excellent communication skills, both written and spoken along with a high level of numeracy
- 1) Assay support for projects in or transferring to Development. This includes running established assays with consistent data quality
- 2) Analytical support for projects in R&D. The scope of this analytical work is project-dependent, variable, and can include small molecule analysis, protein purification and quantitation; assay of enzyme activities in purified samples, fermentation broth, enzyme concentrates and final products; material characterization testing
- 3) Management of analytical lab including maintaining instruments, ordering material supplies and sample prep/shipping to outside labs. You will have opportunity to work in a high performance team environment and deliver against key project goals. The successful candidate must be motivated, creative, have strong problem solving and critical thinking skills, and be capable of working both independently and collaboratively
- BS degree preferred in Biochemistry, Chemistry or related field or an AA with 2 – 5 years of practical laboratory experience in an academic or industrial setting
- Knowledge of modern analytical techniques and instrumentation for the analysis of organic, inorganic, and macromolecules (some examples: LC/MS, FTIR, NMR, HPLC, GC/MS, CE, UV/Vis)
- Demonstrated hands on expertise in GC, HPLC or other analytical separation techniques
- Experience with biochemical assays for enzymatic activity, ideally on clinical chemistry analyzer platforms such as Konelab (Thermo Scientific) or equivalent
- Experience in cultivation of microorganisms, sterile techniques and fermentation
- Experience with High Throughput Screening techniques and material characterization testing is a plus
- Demonstrated hands-on problem solving and critical thinking skills
- Proven ability to work with multidisciplinary teams across multiple projects
- Develop and validate trace-level analytical methods with minimal supervision
- Conduct routine analytical testing
- Collaborate in a fast-paced team environment, working within and across technical teams
- Author validation protocols, validation reports, methods, technical reports, investigations, and documents for regulatory submissions
- Maintain accurate and detailed laboratory records
- Follow standard operating procedures and keep current on all mandatory training
- Minimum of one (1) year of regulated laboratory environment experience
- Bachelor’s degree or higher in Chemistry, Analytical Chemistry, Chemical Engineering or related discipline from an accredited institution
- Minimum of three (3) years of regulated laboratory environment experience, cGMP a plus
- Strong working knowledge and hands-on experience with High Performance Liquid Chromatography (HPLC) and/or Gas Chromatography (GC)
- GC/Mass Spectrometry (MS) and/or LC/MS experience
- Chromatographic method development and validation experience
- Excellent communication skills, including technical writing
- Must be willing to conduct routine testing
- Ability to troubleshoot instrument and method issues
- Flexible and responsive to changing priorities; able to multi-task
- Lead and/or contribute to laboratory investigations
- Computer skills (Excel, Word, PowerPoint, Minitab)
- Experience with Empower, ChemStation, and/or MassHunter chromatographic data systems
- Experience and/or interest in extractables/leachables and inhalation drug delivery
- Attending safety meeting/activities, carrying out safety inspections, complying and promoting the Eastman Safety Culture. Maintaining a clean work area in line with Eastman’s housekeeping standards
- Using general analytical techniques and instrumentation which is not limited to but includes: chromatography, thermal desportion, spectrometry (mass, infrared and ultraviolet), rheology, NMR, microscopy, DSC etc
- Coordinate with the appropriate groups (to develop, improve, and implement analytical test methods
- Organizing and prioritizing experimental work based on timelines and objectives outlined by supervision
- Stay abreast of new analytical capabilities and technologies that may be appropriate in meeting partner analytical needs
- Assure suitability, accuracy, and reliability of analytical test results and methods
- Define maintenance and calibration for instruments and devices
- Ensure analytical development is documented in technical reports, test methods, and/or laboratory notebooks, as appropriate
- Provide coaching and leadership to Technologists, Technicians and Analysts
- Approve certification/recertification of standards and controls, as appropriate
- Investigate customer inquiries/complaints pertaining to testing/results, as appropriate
- Education - A master or bachelor degree in chemistry, applied or analytical chemistry, biochemistry or a related scientific subject
- Experience/Job Knowledge/Expertise - Ideally the applicant will have expertise using a range of analytical techniques with the theoretical knowledge behind those techniques. In particular, expertise of the analytical techniques used to determine volatile organic compounds and volatile emissions related to coating, sealants and adhesives
- Judgment – The ability to prioritise and schedule work to meet demands set by the department, company or external customer. A logical and independent mind, self-confidence and motivation to investigate and solve complex problems
- Initiative – Must be self-motivated, looking for ways to improve laboratory work processes, testing accuracy and safety. Be able to design and conduct analytical experiments. The motivation, patience and persistence to solve complex problems. Have a systematic approach to tasks. Consistently makes sound decisions based on data and anticipates problems
- Technical Skills – Good knowledge of a broad range of analytical chemistry and techniques. A good standard of numeracy and skills in data analysis. Develops plans for moderately sized projects or portions of large projects. Prioritizes work based on available resources and department objectives
- Computer Skills – Microsoft Office and statistical analysis software is preferred. IT and technology skills to work with advanced techniques. Be able to handle the bespoke software used in various analytical techniques
- Language Skills – Fluent in Dutch and English both written and spoken. A knowledge of other European languages although not required is ideal
- Communication Skills – Must be able to communicate experimental results clearly to peers & supervision through email, verbal communication and/or concise report. Required to update research notebook clearly and promptly. Excellent spoken and written communication skills. Communicating with scientists and customers from both within and outside the company
- Teamwork – Must be able to work with people having different personalities and different job functions. Have a flexible and adaptable attitude. Working in multidisciplinary teams is common
Ph.d Analytical Chemist Resume Examples & Samples
- Develop gas chromatography methods for petroleum applications and provide troubleshooting support for manufacturing issues
- Provide technical leadership/support for established methods (e.g. ASTM or company specific) in the Quality Assurance laboratory
- Develop broad analytical skills to solve problems in manufacturing
- Develop technical leadership in chromatography within ExxonMobil
- Participate in cross-functional business teams
- Interact effectively with business groups
- Additional experience with petroleum characterization using advanced analytical techniques
- Strong analytical and communication skills
- Demonstrated work experience in upstream operations, inventory control, oil field accounting, and contract administration
Months Undergraduate Placement Analytical Chemist Resume Examples & Samples
- Expanding and optimising the LC ToF libraries
- Development of GC ToF libraries
- The right to live and work in the UK
- Design and execute limited test method development
- In association with maintenance and analytical vendors as needed install, qualify and maintain the required laboratory equipment within the Technical Services laboratory
- Document and maintain data/test results per GLP and GMP, produce reports and present results as required for management updates
- Ensure that all data comply with cGMP, FDA and/or other appropriate regulatory guidelines
- To perform this job successfully, an individual must be able to perform each essential duty satisfactorily. The requirements listed are representative of the knowledge, skill, and/or ability required. Reasonable accommodations may be made to enable individuals with disabilities to perform the essential functions
- The individual must have demonstrated thorough working knowledge of chemical analysis, including UP/HPLC, GC, prep HPLC, LCMS, tablet/suspension dissolution. The individual must also have a fundamental understanding of algebra and statistics
- The individual must have the ability to comprehend complex instructions and tasks and have strong analytical and logical problem solving abilities
- The individual must have strong project management skills
- The individual must have the ability to excel in a cross-functional working environment and the ability to multi-task, prioritize and handle multiple projects at once
- The individual needs to be able to collaborate with other teams, as well as maintain and strengthen cross departmental relationships
- Ph.D, preferred with 5+ year’s experience or BS in Chemistry with 10+ year’s of experience in the analytical units within the pharmaceutical or related industry including product troubleshooting
- Follows instructions, responds to management direction; takes responsibility for own actions; keeps commitments; commits to long hours of work when necessary to reach goals; completes tasks on time or notifies appropriate person with an alternate plan
- Provide analytical chemical testing/laboratory work in support of the Technical Service department, Operations, and Quality where required for commercial product support including but not limited to product testing, degradation profiles, impurity identification, extractable leachable testing, and material contact studies. Requires analytical method problem solving and trouble-shooting. Material scope includes but is not limited to API, in process testing and finished drug products of the product forms noted in the company summary
- In coordination with other Technical Service personnel design investigation schemes to support commercial product troubleshooting and execute designed lab experiments
- Troubleshoot and remediate analytical test methods for a wide range of API, excipients and finished products
- Perform the analytical laboratory work to serve as the sending lab for test method transfer as needed within Akorn sites, contract manufacturing facilities and contract laboratories
- Operate and maintain the Technical Service Laboratory within GLP/GMP and SOP requirements and within budget
- Perform other duties as deemed necessary by department management
- Participate in Technical Service and Operation’s management staff meetings
- The individual must have strong verbal communication and writing skills in English
- Some synthetic organic experience is desired
- Strong project management and organizational skills, prioritizes and plans work activities; uses time efficiently; plans for additional resources; sets goals and objectives; organizes or schedules efficiently; and develops realistic plans
- Demonstrates accuracy and thoroughness; looks for ways to improve and promote quality; applies feedback to improve performance; monitors own work to ensure high quality
- 5 - 2 years of lab experience
- LC/MS or LC/MS/MS are required
- IC (would be nice but not necessary)
- Strong method development experience
- Strong chromatography and other separations science knowledge
- Strong MS knowledge and hands-on applications method development
- Experience using MS
- Sample preparation for trace analyte determination
- Use of stable-isotope-internal standards for trace determinations
- Maintaining a safe work place, good housekeeping, safe lab practices, and compliance with all site-wide safety regulations
- Analytical Methods development, substantially but not limited to chromatography, and validation of technical and formulated goods
- Analytical equipment/instrument troubleshooting and maintenance skills are a requirement
- Be comfortable working within a lab setting at a minimum of 75% of the time
- Perform the analytical phase of projects, analysis and stability testing, relating to formulations development
- Execute analytical tasks in support of product development projects within agreed upon timelines
- Provide analytical support relevant to the investigation of product performance issues
- Provide technical support to implement appropriate analytical methodologies
- The interpretation of analytical results to formulation chemists/engineers, biology staff, and others as appropriate
- Report writing will include formal documentation of study results, test methods, validation studies, etc
- When appropriate perform and report upon analytical work requiring compliance with GLP regulations. This individual will serve either as study personnel or as a study director
- Recommend and facilitate the improvement of applied technologies which enhance the level of cost effectiveness and services provided
- May be required to supervise a technician or junior level scientist
- Experience working with Agilent OpenLABS and Waters Empower software are pluses
- Coordinate contract lab activities serving as either a study sponsor and/or study monitor
- Possible domestic travel to provide analytical chemistry support at manufacturing sites
- Successful candidate will have demonstrated analytical knowledge and experience which contains a significant amount of applied analytical chemistry, methodological research, most notably including experience with chromatographic separations and chromatographic separation mechanisms. A working spectral interpretive knowledge is a worthwhile secondary skill enhancing an applicant’s candidacy
- Experience working in a multidisciplinary environment that met strict regulatory requirements is a strong plus
- Familiarity with Agilent OpenLAB and Waters Empower software are pluses
R&D Analytical Chemist Resume Examples & Samples
- Assure accident and incident-free conduct of all aspects of R&D ongoing operations
- Internal publication and/or presentation of proprietary research and development projects
- External publication and/or presentation of non-proprietary research and development projects
- Work with instrument manufacturers and/or develop internal capabilities for new instruments applicable to semiconductor process chemicals and gas analysis
- Participation in semiconductor and analysis-related standards organizations
- Development of new analytical methods for the Production Laboratories
- Development of new analytical methods for new or advanced products
- Provide training for new analytical methods to Production Laboratory personnel
- Develop draft SOPs for Production Lab personnel
- Maintain contact with Production Lab personnel during method lifetime to ensure continued applicability of analytical methods
- Develop the analytical capabilities needed for semiconductor process chemicals and gases
- Perform established tests as documented, either from internally generated methods or externally issued specifications
- Serve as technical resource for laboratory staff including troubleshooting of laboratory equipment/instrumentation
- Assist with completion of laboratory workload as needed
- Serve as primary contact for analytical method transfer from Research and Development (R&D) and train analytical lab personnel on such methods
- Implement a preventive maintenance and calibration program for all laboratory equipment and instrumentation
- Evaluate current laboratory methods in use, including the execution of measurement systems analysis, and recognize areas for improvements
- Assist laboratory manager with the evaluation and selection of laboratory equipment and instrumentation
- Assist laboratory manager with resolution and closure of internal audit findings
- Maintain accurate and complete laboratory records
- Comply with all site and laboratory safety practices
- Additional tasks to include supply inventory, statistical process control, chemical hygiene, safety inspections and training
- Inspections, analytical testing and other scientific analyses to address chemistry-related issues related to the use of materials
- Root cause analysis of a wide variety of failures related to issues arising in development, manufacturing or use of products in a variety of industries including consumer products, medical device, pharmaceuticals, and other markets
- Gathering information from and conveying technical conclusions to individuals in engineering, business, law and industry, as well as communicating testing rationale in the context of product development, product defect claims, and intellectual property disputes
- Oversee management of chromatography instrumentation, related projects, and support personnel
- Oversee ISO 9001 and ISO 17025 Quality System as it applies to laboratory instrumentation
- Actively participates or leads laboratory safety programs
- Ph.D. in Chemistry, Analytical Chemistry, or a related field
- A strong theoretical knowledge of analytical characterization techniques as well as practical, hands-on experience, as applied to material science, is required
- Experience with sampling techniques and statistical data analysis for GC-MS, LC-MS, ICP-MS, ICP-OES, GPC, FTIR Spectroscopy, NMR Spectroscopy, and other techniques
- Familiarity with FDA, ICH, USP, EP lab quality regulations and procedures
- Industrial experience in GMP/GLP or ISO laboratory, preferably with method development/validation experience is desirable
Analytical Chemist, Specialty Gases Resume Examples & Samples
- Safely perform gas / vapor phase analysis of hazardous materials to support new product development, manufacturing development, and customer requests
- Generate new analytical methods for gases and volatile compounds
- Establish detection limits, accuracy, precision and stability for analytical methods
- Identify new analytical needs and implement these capabilities per business unit priorities
- Design, set up, and execute experiments; analyze data and communicate results to the team (or customers) via reports and presentations
- Work as a member of cross-functional new product development teams in order to bring new products from concept and feasibility all the way to commercialization
- Provide technical support to Quality, Marketing, Sales, and Operations
- Manage projects, including planning, schedule, and resources
- Write SOPs, WIs, analysis reports to clearly communicate work to team members and other stakeholders
- Train other team members on use of analytical instruments, methods, SOP’s, etc
- Maintain and troubleshoot gas / vapor phase analytical instruments
- Ability to travel up to 10-20% domestically and internationally
- Bachelors in Chemistry or Chemical Engineering plus experience in analytical instrumentation operation, data analysis, and method development
- Experience with gas-phase analysis, including FTIR, GC, MS, particle detection, including methods development, instrument maintenance / trouble-shooting, data analysis, as well as detection limit, accuracy, and stability analysis
- Experience handling hazardous gases and materials (e.g. high pressure systems, vacuum systems, pressure / flow measurement components, etc.)
- Strong statistics background
- Certifications or training in Six Sigma, Semi, ISO, etc, are a plus
- Experience with programming, controls, and automation a plus
- Experience working in product development a plus
- Experience interfacing with customers on technical issue resolution a plus
- Perform complex testing related to compendial monographs and general chapters; IR/UV analysis, Assays GC/HPLC analysis, ICP-MS analysis, Atomic Absorption spectrometry, etc
- Perform measurements, computations, tabulation, and analyze results related to the testing conducted
- Perform non-routine instrumental analysis and wet chemistry analysis in response to customer inquiries and projects as required. This may include but not limited to developing methods and performing method validation and verifications
- Operate and maintain/calibrate laboratory equipment as per manufacturer’s recommendations and company’s Preventive Maintenance program
- Maintain laboratory records related to all testing activities assuring that they are accurate and up to date
- Perform all analysis and lab related duties as per cGMP
- B.S. in Chemical Engineering, Chemistry or related science
- A minimum of 3 years work experience
- Wide knowledge of wet chemistry analysis using appropriate instrumentation such as UV-Vis, pH meter, Karl Fisher, FT-IR, AAS, Viscometer
- Ability to write Method Validation Protocols, Method Validation Reports, and Test Methods
- Experience in the operation, calibration, and maintenance of GC-MS, HPLC, ICP-MS is a plus
- Experience in a contract testing laboratory setting preferred
- Analytical method development, optimization, and troubleshooting
- Developing and maintaining technical understanding and competence in operations of DAK laboratory testing equipment at all sites with Resins production
- Interface with Technical Marketing, customers (as requested by Technical Marketing), and other areas for special projects where analytical testing expertise and/or new method developments are required
- Oversee DAK working relationships with all existing external PET testing laboratories and also lead the process as new laboratories are added
- Training and mentoring (as needed) laboratory technicians on new or changed methods
- Drive quality assurance best practices / procedural implementation and lab testing alignment across 6 production sites
- Ensure alignment of all DAK Resin’s critical analytical testing results across all sites
- Participate in Technical projects (Cost, Quality, Energy, Capacity, etc.) as needed
- Bachelor’s Degree Required, Masters and/or PhD Degree Preferred
- Minimum of 5 years experience with R&D or related product lines
- Must have extensive knowledge in one or more of the following GC/MS, FTIR, EDXRF, TGA, DSC, DMA
- Prefer extensive knowledge in GC/MS and pyrolysis GC/MS
- Prefer extensive knowledge of polymers or rubber
- Must be willing to learn, understand and operate all instrumentation in the lab
- Ability to provide a broad knowledge about the theory and practice of technologies for which responsible along with other relevant analytical technologies
- Operation, maintenance and modification of equipment as needed and the development of new methods to meet customer needs
- Ability to interpret data generated by advanced technology and reporting results as needed
- Strong written, verbal and interpersonal communication skills in order to communicate complex technical analysis to a wide variety of associates and customers
- Participates in the planning and completion of activities in a team environment
QC Associate Analytical Chemist Resume Examples & Samples
- Must have a four year college degree in Chemistry, Biology or a related scientific or technical field
- Prefer 1-3 years of working experience in cGMP-regulated environments in compliance with FDA legislation and ISO specifications. Prefer industrial experience with LC Mass Spectrometry (LCMS), HPLC, and UV-Vis
- Must have computer skills including Microsoft Office (MS Excel, MS Word, and MS PowerPoint) and Microsoft Outlook
- Knowledge of quality systems and laboratory safety. Previous experience in such environments is desired. Good laboratory technique and safe operation of lab equipment are essential skills
- In addition, the candidate should be comfortable initiating and executing CAPA investigations, nonconformities, and compliance regulations. Experience using TrackWise for deviation investigations would be beneficial
- As often is the case, the analyst will be excellent working in groups. Often, other team members both inside and outside of the department will be needed to conduct investigations for root causes of problems. The analyst will need to work in many capacities to assuage all departments involved
- Support R&D team efforts including but are not limited to: sustaining activities in ion mobility spectrometry, new trace detection products characterization, and support sales team in the field
- Lead efforts related to consumables for IMS products
- Support product development by conducting tests as necessary for critical decision points
- Write test protocols and conduct validation testing
- Conduct and coordinate in-house test and evaluation of IMS and MS instruments
- This job description does not cover or contain a comprehensive listing of activities, duties or responsibilities, other tasks will be assigned as necessary
- B.S. in Chemical Engineering, Chemistry, or related discipline
- Minimum one year experience directly related to the position
- Must have experience in Ion Mobility Spectrometry (IMS)
- Must have superior laboratory skills and demonstrated experience in data analysis and interpretation
- Experience in Analytical Chemistry
- Exceptional verbal, written, presentation skills
- Strong interpersonal skills and superior organizational abilities
- Ability to take initiative, to maintain confidentiality, and to meet deadlines
- Organized, flexible and adaptable; a self-starter
- Must be able to multitask on multiple development projects
- Must always promote teamwork and accountability
- Master’s degree (thesis-based, with laboratory research experience) with a concentration in Chemistry or Environmental Chemistry; Ph.D. strongly preferred
- Post-doctoral research experience or industry experience helpful but not essential
- Experience in regulatory matters is helpful but not essential
- Demonstrated ability to conduct rigorous reviews of scientific literature and communicate results effectively
- Ability to analyze chemistry data and provide oral and written interpretation
- Publication and presentation record based on research in a relevant discipline
- Strategic thinker with strong quantitative skills
- Intellectual ability and comfort to work with and communicate effectively with non-scientists
- Strong computer skills, specifically with Microsoft Word, Excel, Power Point
- Ability to collaborate with diverse colleagues and participate effectively in team projects is essential
- Demonstrates depth of knowledge in analytical chemistry, organic chemistry and polymer chemistry
- Experience with polymer characterization including thermal analysis, mechanical properties, rheology, solution properties, polymer crystallinity
- Broad skillset in polymer characterization, thermal analysis (DSC, TGA, DMA, TMA), rheological and mechanical analysis (rheology, tensile)
- Knowledge of statistical tools and design of experiments [DOE] is desired
- Expertise in the analysis of data, from the qualitative to the rigorously statistical treatments
- Able to draw and defend conclusions based upon analytical data
- Applies expertise to complex materials interaction and issues, providing in-depth evaluation of multiple factors
- Exercises significant independent judgment, working within broadly defined policies and practices. Determines best method for accomplishing work and achieving objectives
- Participates in technical decisions for the project. Evaluates technology risk in partnership with management and stakeholders
- Participates in cross-functional initiatives. Working knowledge in cross-technical disciplines
- Works across organizational boundaries
- May represent the team to external customers/suppliers. Identifies emerging industry standards and adopts them where applicable and beneficial
- Ability to broadly contribute in a fast-paced, industrial R&D and Manufacturing environment
- Strong teamwork and networking skills
- Clear & effective written and verbal communication skills that inform & persuade peers and managers
- Excellent organization/multitasking, communication and analytical skills
- Complex problem-solving using internal and external resources
- Strong problem-solving and project management abilities
- Advanced degree in chemistry, preferably in Analytical Chemistry
- Ability to work in multicultural, multi-site teams
- 10% travel required
- BSc in Chemistry or closely related subject, or equivalent industrial experience
- Demonstrable experience working within an analytical testing laboratory, operating GC-MS and/or GC-MS/MS instrumentation, including performing routine maintenance and troubleshooting
- Ability to manage multiple competing priorities in a fast paced environment
EPA Lead Analytical Chemist Resume Examples & Samples
- Create, review and approve data generated by other chemist
- Maintenance of Laboratory Notebooks
- Lead OOS/Investigative reports
- Train QC Chemist
- B.S. in Chemistry with a minimum of 5 years of pharmaceutical analytical laboratory experience and 1 years of supervisory experience of own projects or staff members
Polymer Analytical Chemist Resume Examples & Samples
- 10+ years of experience in the polymer, refining and/or medical industry
- Knowledge and application of analytical test methods including GC-MS and LC-MS
- PhD in Chemistry from an accredited institution
- Experience with GPC, NMR, Rheology and/or FTIR
- Experience .NET programming languages for lab equipment data collection and processing
- The intern candidate generally will work with a project team to support a project involving the development and characterization of applications for targeted compounds on a new mass spectrometer
- Perform method development including establishing the best methods and protocols in order to achieve the Limits of detection (LOD) and limits of quantitation (LOQ) requirements for the analysis of immunosuppressant drugs in whole blood
- Provide a thorough assessment and characterization of the sensitivity and quantitative performance of the system and determine the useful analytical range compared to the clinical therapeutic range
- Generate a technical report on results and present the results to the project team
- Undergraduate or graduate student in Chemistry, Biochemistry, Analytical or Physical chemistry
- Good experimental design and implementation skills
- Wet lab experience including dealing with solvents, pipetteting, preparing dilutions of samples, and preparing standards
- Strong communication (written and verbal) and presentation skills
- Capable of working with multi-disciplinary teams
- Analytical instrumentation and hands on experience a plus
- Practical knowledge of Mass Spectrometry
QC / Analytical Chemist Resume Examples & Samples
- Conduct analytical testing of high-purity quartz samples in accordance with ISO 9001 procedures
- Responsible for instrument calibration/qualification and maintenance
- Establishing laboratory schedules and procedures
- Communicating results to Manufacturing, Quality and Technology functions
- Commitment to maintaining a safe work environment and following all company EHS procedures
- Familiarity with chemical laboratory equipment and operations
- Demonstrated emphasis on laboratory environment safety
- Ability to work as a solid contributor within a team environment
- Familiarity with ICP-OES, ICP-MS and XRF a plus
- Knowledge of fused quartz and semiconductor industry desirable
- Understanding of Continuous Improvement/5S/LEAN 6-Sigma a plus
Analytical Chemist / Mass Spectroscopy Resume Examples & Samples
- The ideal candidate will have an advanced degree in chemistry with advanced academic training in mass spectroscopy and laboratory experience in chromatographic separations. BS/BA in Chemistry with demonstrated subject matter expertise in mass spectroscopy method development will also be considered
- Able to work independently, strong interpersonal, communication and organizational skills
- Must be eligible to work in the US
EPA Analytical Chemist Resume Examples & Samples
- Understand OOS/Investigative reports
- B.S. in Chemistry with a minimum of 5 years of pharmaceutical analytical laboratory experience
- Experience in FDA regulated industry
- Operate and perform analytical testing and materials characterization using thermal analytical equipment
- Maintain critical analytical equipment and spare parts inventory
- Perform routine analytical testing in support of new product development projects
- Assist with developing and implementing analytical testing of new materials
- Develop daily check sheets, work instructions, safety procedures and safety reviews for analytical equipment and methods
- Make recommendations and implement improvements in efficiency, when possible
- Bachelor degree in chemistry, applied science, materials science or chemical engineering preferred
- Experience in analytical chemistry is preferred
- Experience in semiconductor, optical, or pharmaceutical industries is ideal
- Handling of air-sensitive materials and methods is strongly preferred
- Experience working safety with safe handling experience is preferred
- Analytical training or experience preferred
- Ability to interpret data with Senior team members
- Strong communication skills and experience working in a team environment
- Experience performing analytical methods a plus
- Shown ability to interpret data by performing trend analysis
- Shown ability in technical writing skills
- Shown problem solving and troubleshooting abilities
Hplc Analytical Chemist Curriculum Manager Resume Examples & Samples
- Designs and develops education solutions for instructor-led and virtual training using instructional design principles. Training material may include laboratories, lectures, eLearning modules, eManuals, assessments, videos, webinars, and animations
- Collaborates with a cross-functional team of experts to determine appropriate curriculum and course content including product support, product managers, regional specialists, customers, and industry leaders
- Adheres to training development standards, guidelines and quality requirements as indicated by the ECS product life cycle
- Maintains a high level of technical expertise
- Project manages education product development with vendors and consultants
- Supports the deployment of courses to the local delivery organizations to ensure seamless transition from R&D to delivery
- Delivers select training through a variety of mediums including video and virtual modalities
- Leads and participates in various projects to support improvements in the development and delivery function of the customer education business, i.e. testing new forms of virtual delivery
- BS degree with a minimum of 1 year of relevant experience OR a MS degree with no more than 3 years of experience in biotech or pharmaceutical industry
- Experience with analytical and protein chemistry
- Working experience with other routine analytical and biophysical techniques such as capillary electrophoresis and spectroscopy
- BS degree focused in chemistry, biochemistry or biology is preferred
- Some experience with HPLC and/or LC/MS and LC/MS/MS analysis of protein therapeutics would be desirable
- A good understanding of protein structure and function relationships
- Knowledge and hands-on experience in various protein and oligosaccharide separation and characterization techniques would be a plus
- A highly motivated individual with excellent communication skills, both written and verbal
- An instinctive team player with excellent people skills
- Looking for a highly motivated individual who will support the analytical testing for vaccine development
- This involves the chemical labeling of biological samples, HPLC separation, and data analysis
- This individual needs to ensure that the analytical results are of high quality and all work is documented in a clear, concise and timely manner
- A BS or MS degree or equivalent in Biochemistry, Analytical Chemistry or a related field is required
- The successful candidate should show ability to work independently, follow procedures precisely, and generate analytical data consistently
- Experience in HPLC separation and using Empower would be an advantage. Ability to work on different projects in parallel is required
- Supervise activities in the analytical laboratory in order to assure acceptability of raw materials, active ingredients and preservative levels in cosmetic drug products, assures test methods, specifications and standards are available
- Provides training in analytical techniques such as wet chemical and instrumental analysis
- Perform analytical/instrumentation analysis of raw materials, bulks and finished goods
- Review new and innovative instrumentation/test methods and operating procedures to improve the efficiency and accuracy of the lab
- Supervise activities in the Analytical Laboratory for timely evaluation of raw ingredients, cosmetic drug bulk batches and finished goods. Assures that appropriate testing procedures are followed and that necessary controls are maintained. Plan, organize and supervise activities of the laboratory staff
- Coordinate with Research Center to obtain necessary analytical specifications, test methods and raw ingredient standards. Review test method and specification problems and discrepancies. Recommend revisions to standards and/or specifications as necessary
- Review and establish procedures to maintain all analytical instruments in proper calibration. Initiate preventive maintenance to insure a minimum of down time on equipment. Maintain calibration program for Product laboratory
- On-site expert in analytical/instrumentation methodology, validate issued test methods and resolves issues associated with those methods
- Performs varied analyses on raw materials, bulks and finished products including drug stability testing
- Prepare and maintain appropriate records to assure compliance with Corporate and Regulatory requirements
- Investigate raw material, bulk batches and finished goods out of specification test results. This includes interaction with vendors, Revlon operations and other Revlon sites recommending corrective action, tracking issues to closure
- Requisition of chemicals and other laboratory supplies as needed
- Conduct regular safety training and take action to prevent unsafe acts in the Analytical Lab
- Maintain and update physical standards for raw materials. This includes identifying raw materials for which there is no standard. This information is compiled from purchasing reports, MIF update reports, and R&D new formula reports requesting standards from the R&D analytical department in a timely manner as to allow time for the standard to be on site two weeks prior to receipt and processing requirement
- Provide certificate of analysis for raw materials shipped from the Oxford facility to Revlon international sites. Coordinate activities to assure that information is provided on a timely basis to service international production requirements
- Compile data and report information to support the raw material vendor certification program
- Perform special projects as requested by management, reporting results and recommending solution/corrective actions
- Science degree in applicable scientific discipline, or equivalent on-the-job experience
- Working laboratory experience, preferably with FMCG experience
- Working knowledge of GSK Quality standards, GLP and GMP requirements
- Working knowledge of validations, calibration systems, and instrumentation
- Working knowledge of applicable GSK policies and procedures
- Problem solving/continuous improvement skills
- Developed skills in coaching, interpersonal communications, team building and training facilitation
- High level of computer literacy
- Ability to interact with all levels of the organization
- Experience in writing, reviewing and revising SOPs
- Working knowledge of Microsoft Word, Microsoft Excel and Laboratory Information Management System (LIMS)
- Ability to work and succeed in a team based environment
- Flexibility to work extended hours (including weekends) to achieve results as needed
- 1) Support Operations by analyzing raw materials, intermediate batches, packaging and finished products as required
- 2) Responsible for trouble shooting and investigating out of specification results. Perform analytical testing on all incoming Raw Materials as per our reduced testing program as well as an annual full testing using USP/NF, FCC, Pharmacopoeia and GSK methodology
- 3) Responsible for the disposition of raw materials, intermediate, batches, and finished products in a timely manner
- 4) Data entry of test results
- 5) Investigate out of specification results
- 6) Support method and/or product transfer and NPI activity as necessary
- 7) Preparation, documentation and maintenance of test solutions and standards. Maintain, calibrate, and validate laboratory equipment
- 8) Ensures that GMP, Quality, Health & Safety are considered in all aspects of role
- 9) Perform other duties as directed by QC Specialist or QC manager
- Applies a range of sophisticated analytical techniques to accurately and effectively support research, troubleshooting and technical support activities
- Conducts specialty analytical testing for product failure, customer complaints and process related issue analyses and provides accurate and expert interpretation of analytical data to solve product and process issues
- Provides creativity and innovation in strategic aspects of analytical technology, in proposing technology developments which could provide the company a competitive edge
- Displays good bench techniques and initiatives in carrying out tasks
- Adheres to all the safety, quality and environmental procedures in the laboratory
- Demonstrated knowledge and capability in troubleshooting and analytical method development
- Excellent analytical skills in a wide range of analytical instrumentations and areas of method development
- Creativity and innovation with a technical learning ability
- Customer-focused with sound decision-making skills
- Ability to work well within a team and independently
- Good laboratory practices and techniques
- Stops and/or reports any unsafe work or conditions
- Follows safety & environmental policies and procedures
- Supports safety & environmental goals and initiatives
Analytical Chemist / Lab Manager Resume Examples & Samples
- Operate and maintain the analytical laboratory equipment used to identify chemical tracers
- Work independently to complete routine analytical activities. Provide interpretation of results
- Regularly provide direct support to operations within Praxair on technical issues related to chromatography and sample analysis
- Assist technicians with troubleshooting field equipment including software and hardware
- Apply expertise to identify and evaluate technical solutions to customer issues
- Some travel will be required on short notice for field analytical support
- Complete full report drafts with minimal guidance from senior staff and manager
- Training of new technicians on analytical equipment
- Provides analytical method validation and method transfer to the QC group
- Designs and perform experiments that contribute to project goals
- Participates in evaluation of technical feasibility of new projects
- Clearly documents experimental research in laboratory notebooks
- Maintains up-to-date laboratory records in accordance with company guidelines
- Conducts literature research to identify and select relevant method development protocols
- Ability to maintain a positive attendance record
- Know and adhere to Standard Operating Procedures (SOP’s)
- Maintenance of an orderly and safe working environment
- Responsible for ensuring that information security is carried out while performing day-to-day functions, duties and tasks
- Seeks out, accepts, and applies feedback and coaching
- Responsible for launching, overseeing and carrying out projects for the process development group
- Exercises independent judgment within defined practices and policies in selecting methods and techniques for creating solutions
- This position requires a BS/BA degree with minimum of eight (8) years of experience or MS/Ph.D. degree with a minimum of four (4) years of experience in analytical chemistry
- Demonstrated ability to impact multiple project goals by utilizing technical knowledge and techniques in projects regarding organization, research, production and operation
- Demonstrated independent judgment in selecting methods and techniques for creating solutions to a wide range of challenging chemical processes
- Demonstrated ability for launching, overseeing, and carrying out projects in Process Development
- Lead lab configuration for new project testing requirements
- Aid in the determination of specific lab equipment needed for project testing and work with vendors to obtain quotes
- Responsible for working with site personnel to order equipment necessary
- Must be willing to study and understand the new chemicals coming into the lab and train current lab technicians on proper handling and PPE
- Support installation and set-up of new instrumentation in lab for project testing
- Demonstrated work with Gas Chromatography instrumentation
- Experience with a variety of laboratory instrumentation and equipment including, but not limited to
- Provide chromatography support to R&D and manufacturing
- Develop chromatography methods and solve chemical and process problems
- Analyze samples, report results and document methods
- Train technical support staff in QC
- Maintain and operate chromatography instruments
- Maintain excellence in safety, health and environment practices
- B.S. in Chemistry graduating May 2016- May 2017 preferred or at least 5 years of relevant experience
- Experience in chromatography and spectroscopy techniques desired
- Strong strategic thinking and analytical problem solving skills
- Highly organized and excellent verbal and written communication skills
- Strong interest in challenges of industrial analytical chemistry for a wide variety of products
- Responsible to work on SCIEX range of LC and MS instrumentations in the manufacturing plant. He/she will be performing QC, troubleshooting and stability test of MS systems before shipment
- Development of new testing method in manufacturing on MS systems before shipment
- Performed specialized testing on MS systems before shipment per customer’s special requirements
- Performed troubleshooting and improvement of existing test protocols and implement changes if required in manufacturing
- Support MFG engineering team to troubleshoot failures and provide technical guidance during manufacturing
- Provide expert knowledge on various customer applications of LCMS which includes high flow and nano flow LC operations
- Provide support in chemical validation and preparation process for chemicals used in manufacturing per GLP requirements
- Other duties/ ad hoc tasks as assigned
- Perform analytical lab work including LC-MS and GC-MS
- Analyze data from the lab
- Document labwork in laboratory journal
- MSc or BSc or equal experience in analytical chemistry
- Analytical skills
- Fluent in English and Swedish both verbally and in writing
- Experience from working in a regulated environment, e.g. GxP or ISO 17025
- Method development and validation of compounded drug products and API
- Ability to perform testing with high precision and accuracy
- Method validation of compounded drug using chromatographic techniques
- Responsible for documenting all lab work per cGMP and company standards
- Participates in the planning and execution of raw material, components, and vendor qualification or alternate supplier qualification studies
- Writes, edits, and revises test methods and procedures into company SOPs; reviews, writes, or assists in writing SOPs for analytical development
- Conduct analysis using wet chemistry, HPLC, LC/MS, KF, IR, and UV as per cGMP standards
- Provides analytical testing support for manufacturing process validation
- Initiate Change Controls related to the analytical documentation and testing
- Maintain the analytical laboratory in accordance with cGMP
- Creating and performing stability studies for compounded drug product
- Author specifications and test methods, as needed
- Review of Analytical raw data and documents
- Testing performed in a high quality and timely manner and meets all procedure and regulatory requirements
- Provide troubleshooting support for analytical method or drug product issues, as needed
- Superior communication skills. Written and Verbal
- Minimum 7 years of pharmaceutical cGMP related experience in a Quality Control laboratory or development lab
- Intermediate proficiency in MS Office products (Word, Excel, PowerPoint)
- Ability to multitask and organize
- Comprehensive understanding of cGMP, DEA regulations, ICH Quality guidance, FDA guidance, safety standards and hazards associated with a pharmaceutical laboratory environment
- Extensive knowledge of analytical instrumentation including LC, LC/MS and all forms of spectroscopy
- Maintain a safe working environment for all employees, ensuring compliance to all local, state and federal codes and regulations
- Self-motivated individual with the ability to learn, lead and strengthen and Pentec Health team
- Extensive knowledge and experience in analytical separation techniques and method development/validation for pharmaceutical products
- Ability to perform or direct development of new analytical test procedures based on emerging technologies
- Previous supervisory experience in a laboratory environment
- Maintained analytical lab equipment and involvement in validation of new equipment
- BS in Chemistry and/or related science discipline
- Perform a variety of chemistry laboratory support activities as assigned such as routine cleaning and waste removal
- Properly maintain work areas and supplies necessary to perform testing
- Operate, install, maintain and perform change control activities as it relates to equipment
- Write protocols, reports and execute method transfers and/or validations
- Ensures testing meets the necessary compendial requirements
- Performs complex analyses using unfamiliar or new methods, asking for scientific guidance as needed. Learn to design and independently conduct tests for chemistry projects and provide initial analysis
- Participates in new equipment and instrument assessment and qualification, as appropriate
- Recognizes deviations from normal results and inform Study Director, Principal Investigator, and/or management of any problems and/or deviations that may affect the integrity of the data and participates in corrective action of problems. Identifies problems and makes suggestions for modifications in test methods or procedures Able to adapt to new procedures or particular needs as directed Able to identify critical steps in an assay. Initiates, drives and implements process improvement initiatives
- Setting up and performing sample analysis per cGMP/GLP Guidelines
- Peer Review of Data via other chemist/lab personnel
- Follow Standard Operating Procedures
Assistant Analytical Chemist Resume Examples & Samples
- Prepare samples ready for analysis using established techniques according to standard operating procedures
- Extract and clean-up samples in preparation for analysis
- Be aware of and follow the appropriate quality assurance and safety procedures
- Support sample prep during busy periods - log samples onto the Laboratory Information Management System (LIMS) and prepare samples when required
- Practical IT experience, in particular Microsoft Packages such as Word and Excel
- Previous laboratory experience, preferably within an environment of analytical chemistry
- Ability to keep clear written records
- Ability to follow written and verbal instructions efficiently
- Ability to recognise when things have potential to go wrong
- Flexible and adaptable approach to work
- Self-motivated and have an ability to develop good working relationships with colleagues and other team members
- A methodical approach to work and attention to detail
- Experience of operating within a quality system (e.g. UKAS)
Analytical Chemist Physical Characterizations Resume Examples & Samples
- Develop and provide specialized safety training to technicians working in their labs on unique hazards present
- Responsible for the quality and reliability of the data from each method and instrument in their lab. Ensure proper SPC and calibration are done. Ensure maintenance is done in collaboration with the Analytical Reliability Coordinator
- Troubleshoot odd results and answer methods and results questions from the customers of the lab results (plant, RT Sales, R&D)
- Train technicians on the use of lab equipment. Continually improve and develop skills of technicians working in lab area
- Responsible for ensuring the correct disposal of hazardous waste generated in their lab
- Ability to lead and influence in a matrix environment
- Communication- ability to clearly communicate results and expectations
- Coaching and mentoring- ability to train others on analytical methods and equipment and continuously develop their skills and level of responsibility
- Strong focus on both internal and external customers to deliver the right results at the right time
- B.S. or higher in chemistry or analytical chemistry
- Demonstrated hands on experience in an analytical lab with responsibility for data quality and equipment operation
Analytical Chemist ICP Analysis Resume Examples & Samples
- Subject matter expert on equipment and methods for their respective areas
- Serve as the liaison between ASC and designated Operations in Curtis Bay. Attend weekly plant production meetings. Communicate testing status to the plant and pro-actively identify opportunities for the plants and ASC to work better together by optimizing sample types and frequency
- Troubleshoot equipment when problems arise, providing on-call 24/7 support if question arise
- Responsible for updating existing test methods and writing new test methods as needed to support changes in product chemistry or plant operations
- Responsible for validation of new methods and new equipment installed in the lab
- Serve as an internal company expert on the tests in their area, understanding the scientific fundamentals of the tests and the limitations of the tests. Provide expertise on test methods as required to other Grace locations
- Manage the correlation program between plants for the methods in their area and organize meetings and opportunities for lab staff to share best practices between locations
- Manage ISO compliance for their lab area
- Lead specialized testing in their area of expertise as needed to support plant operations and troubleshooting
- Keep up with equipment and method advances in their area of responsibility through vendor interaction, external training courses, and self-study
- Provide thought leadership and drive continuous improvement in their lab looking for opportunities to improve workflow, equipment, automation and data handling
- Strong safety focus
- Demonstrated hands on experience in lab with responsibility for data quality and equipment operation
- Bias for action and desire to delivery timely, correct analytical results
- Prior experience with ICP equipment and methods
- Good communication skills, conveying information both professionally and concisely
- Actively champion and promote all safety related policies and programs to ensure colleague engagement and commitment to the benefits of working safe
- Understands their role in food safety and quality systems, actively participates in food safety efforts and ensures all activities conform to good manufacturing practices
- Perform analytical methods development, validation, and routine testing using a variety of analytical techniques (e.g. ICP/MS, GC/MS, LC/MS, FTIR, TOC, UV/Vis, and DSC)
- Document the results of all work in accordance with cGMP/cGLP guidance
- Participate in the formulation of strategies to solve complex problems
- Maintain instrumentation in good order and perform simple troubleshooting
- Participate in the analysis of new materials and assist in evaluating material changes for product equivalence
- Remaining current on all applicable SOP, safety, security, and annual quality related training
- Strict adherence to all safety/emergency procedures
- Professional and technical interaction with customers
- Proficiency with Microsoft Office products and other job specific software applications is assumed
- Must be comfortable working in a high throughput environment with minimal supervision
- Must possess the aptitude and desire to learn and adapt to changes in a dynamic technical environment
- Performs other duties related to the position when required by laboratory management
- Experience in any of the following disciplines is desirable, however not mandatory for the right candidate
- Minimum of a good honors degree in chemistry, with relevant analytical experience and/or higher degree
- Good working knowledge of cGMP as applied to an Analytical Laboratory
- Good organizational, planning and communication skills. Able to communicate across a range of scientific disciplines
- Positive, can do approach and enthusiasm
- Good knowledge of Physical Property techniques (e.g. Malvern, XPRD, DSC)
- Demonstrated competency in Analytical Chemistry, familiar with a range of analytical/techniques/new analytical technologies
- Bachelor's degree or higher in a science or engineering discipline from an accredited institution
- Minimum of three (3) years’ of regulated environment laboratory experience
- Experience performing analysis of drugs, combination products, medical devices and materials in a GMP lab setting [Food and Drug Administration (FDA) Drug GMP, U.S. Pharmacopeia (USP), International Council on Harmonization (ICH) guidelines relevant to drug and combination products industry)
- Knowledge of data collection systems and conformance to 21 Code of Federal Regulations Part 11 (21CFR Part 11), such as Empower, Electronic Laboratory Notebook (ELN), Quality Management Systems (QMS), etc
- Functioning knowledge of High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), Fournier Transform Infrared (FTIR) and near infrared (NIR) spectrometry, wet chemistry, United States Pharmacopeia (USP) methods and analytical laboratory techniques
- Experience working in a manufacturing setting and pace
- Demonstrated proficiency in the use of statistical and quality data analysis tools such as exploratory data analysis, basic statistics, Gage Repeatability and Reproducibility (R&R), Analysis of Variance, Failure Modes and Effects Analysis (FMEA), Statistical Process Control and Capability, acceptance sampling, correlation and regression, and experimental design and their applications to process improvement
- Leading projects that require investigation of polyester films, polymers, raw materials or process samples using available tools or coordination with other scientists for required testing
- Operating a variety of analytical instruments including but not limited to GC, mass spectrometer, autotitrator, particle sizer, x-ray fluorescence, optical microscopes, micro profiler, FTIR, Raman spectrometer, SEM, DSC, DMA, TGA, MFI, rheometer, capillary viscometer, and pyrolysis
- Preparing samples and standards using good laboratory technique
- Developing methods when needed to solve a specific product defect or production need
- Utilizing a variety of instrumentation software
- Minimum of one (1) year (academic or work) laboratory environment experience
- Bachelor’s degree or higher in Analytical Chemistry or Materials Engineering from an accredited university
- Minimum of three (3) years of chemical laboratory experience
- Minimum of three (3) years Excel (including performing calculations), PowerPoint, Word and Email experience
- Gas chromatography and gas chromatography –mass spectrometry(GC-MS)experience
- Method development and data evaluation experience
- Sample preparation techniques and basic laboratory skills
- Chemical instrumentation experience
- Commercial instrumentation control software
- Statistical data analysis
- Proven experience in solving problems with analytical measurements
Protein Analytical Chemist Resume Examples & Samples
- Perform analytical (UV/Vis, FTIR, Raman, TOC, conductivity) and compendial testing (pH, osmo, turbidity, appearance), as well as wet chemistry practices in a GMP environment
- Proficient computer skills and knowledge of Microsoft applications
- BS with 2-4 years or MS with 1-2 years of experience in QC within the Pharmaceutical or Biotech industry, in a GMP or GLP regulated laboratory environment
- Prepare laboratory samples, collect and report data within a pharmaceutical development laboratory
- Primary responsibility will be to operate Raman spectroscopy instrumentation for data collection to support active R&D development programs
- Conduct performance, quality checks, and routine maintenance on instrumentation regularly
- Engage operations group and instrument vendors as appropriate to maintain instrumentation
- Troubleshoot computer software and hardware used with instrumentation
- Aptitude with automated systems
- Attention to detail, excellent communication (oral and written), ability to work independently and as part of a team, self-motivation, adaptability, and a positive attitude
- At least one year experience in analytical chemistry
Quality Control Analytical Chemist Resume Examples & Samples
- Perform testing and data review for Analytical Chemistry methods, and routine laboratory procedures, may include HPLC/UPLC, KF Titration, CE, CEX/AEX, GC, TGA, UV/IR
- Perform general laboratory housekeeping activities
- Complete training on assigned tasks
- Comply with safety guidelines and cGMPS/CFRs which include but is not limited to, the maintenance of training records, laboratory documentation, written procedures, building monitoring systems and laboratory log books
- Technical expertise for executing analytical test methods in one or more of the following areas: Chromatographic techniques (HPLC, UPLC, GC), TGA. Karl Fisher, UV, and IR
- Bachelor's degree or Master’s in Analytical Chemistry, Physical, Life Sciences, or other related degree concentration, or equivalent directly-related experience (two years of directly related industry experience is equivalent to one full-time year of college in related major)
- 0-2 years of relevant experience within an academic, pharmaceutical, or biotechnology organization
- Develop, qualify and validate analytical test methods for drug product, intermediates and excipients
- Design and execute analytical development studies
- Write analytical method validation protocols and reports
- Write and maintain applicable SOPs
- Provide analytical support to QC, Process Engineering and Manufacturing as applicable
- Perform externally generated data review
- Assist in development stability study design and protocol writing
- Assist management during regulatory inspections and internal audits
- Experience with variety of analytical techniques, method development and validation
- Hands-on experience in HPLC, GC, LCMS, and GCMSis a plus
- Bachelor's degree in Analytical Chemistry, Biochemistry, Pharmaceutical Science or related discipline with at least 5 - 8 years of contract laboratory/research or relevant industry experience
- Must have hands-on GXP experience
- Support spray dry formulations including completion of necessary release and paperwork to support batch
- Preparation of common vehicles for formulation preparation to support in vivo studies
- Weighing and labelling of samples prior to shipping for studies at other sites
- Solubility measurement in buffers, bio-relevant medium & organic solvents (HPLC, UV-Vis)
- Vehicle Screening (e.g. solubility / stability) for SubQ, IV and PO formulation development
- (HPLC, UV-Vis, XRPD)
- Preparation of (previously developed) formulations for animal studies including processing support for spray drying of amorphous dispersions or other preparation techniques as required
- Preliminary physicochemical characterization of compounds / formulations including (HPLC,
- XRPD, TGA, DSC)
- Formulation analytical testing e.g. release and chemical / physical stability studies (HPLC, XRPD)
- Powder blending and filter analysis for inhaled programs (HPLC)
- Particle size measurements of drug substance and formulations
- At least 1 year related laboratory experience with biochemical, immunological, or related
- General laboratory skills, experience with pipettes, plate washers, plate readers, electrophoresis
- Equipment, automated instrumentation
- Minimum of 1 year laboratory experience
- Bachelor’s degree or higher from an accredited institution
- Minimum of 2 years of regulated laboratory environment experience
- Good Manufacturing Practice (GMP) analytical laboratory experience with any combination of medical device, combination products, and/or drug products
- Bachelor’s degree or higher in an Analytical chemistry, or Science related discipline from an accredited university
- Experience working with HPLC (High Pressure Liquid Chromatography)
- Experience working with GC (Gas Chromatography)
- Good organizational skills, attention to detail, compliance behaviors
- Excellent computer skills (Excel, Word, PowerPoint, Minitab)
- Flexible and responsive to changing priorities and able to multi-task
- Experience working with wet chemical test methods
- Prior experience testing pressure sensitive adhesive
- Working knowledge of data collection systems, such as Empower
- Understanding of FDA Guidance and ICH relevant to Drug/Medical Devices for analytical testing
- Experience with internal and external audits
- Bachelor's degree in Chemistry, Biology or related field or equivalent and relevant formal academic / vocational qualification
- Previous experience that provides the knowledge, skills, and abilities to perform the job (comparable to 4+ years’) or equivalent combination of education, training, & experience
- 1 plus years of experience with HPLC in a regulated cGMP lab is required
- Experience with Particulate matter is preferred
- Experience with Suspensions are preferred
- Regular and consistent attendance
Analytical Chemist / QC Analyst Resume Examples & Samples
- Perform analytical activities including method development, transfer and validation as well as, IPC’s, cleaning verification, release testing of incoming materials, intermediates, API’s/DP’s and reference standards
- Perform QC analytical work to address chemical stability and structure elucidation including forced degradation, stability studies and dissolution testing
- Adhere to cGMP compliant procedures and data management systems to archive and track analytical activities
- Authors/co-authors SOP’s, technical reports and sections for regulatory filings
- Actively communicates, consults and collaborates with colleagues to ensure optimal execution and record of analytical activities
- Significant experience in analytical method development, validation and QC testing for small molecules
- Experience in supporting all phases of API/DP development, ideally spanning early development to registration
- MS/BS in analytical chemistry or related field with 5+ years of relevant experience
- The successful individual MUST be willing and able to work in a highly structured work environment with regard to safe and consistent conformance to operating procedures and work practices
- Ensure timely throughput of product through work queues through a trained and flexible team
- Developing, control, and maintain the Lab Scope and all associated lab documentation and procedures
- Develop and update existing preventive maintenance programs to maximize equipment uptime
- Lead planning analytical activities in an effort to meet customer service supply goals
- Operate required analytical instrumentation (GC’s, FTIR’s, process analyzers, standard blenders, etc)
- Recognize problems with analytical instrumentation and troubleshoot/assist in subsequent repair(s)
- Accurately input and document analytical data into all necessary analytical and plant process logs
- Conforms to ISO 9001:2008 directives included but not limited to policy and procedures. Strictly adhere to all necessary calibration requirements within all lab operations
- Independently preparing and analyzing samples for analysis of e-vapor stability studies using UPLC-UV and QToF
- Analyzing samples using the Waters AutoPurification System for collection and isolation of compounds
- Tracking samples for stability projects, organizing work and documenting results in an electronic laboratory notebook
- Organizing and reporting results from LC-Profiling in a clear and concise manner to meet the needs of our customers
- Delivering high quality data to our customers
- Collaborating with other scientists to provide additional insights to stability
- Maintaining and managing the appropriate documentation for Analytical Sciences to be compliant with ISO 17025 Accreditation, Altria Quality System and Records Management policies
- Participating in internal and external scientific meetings to maintain and expand scientific expertise
- A Bachelor’s Degree in Chemistry, Forensic Science or closely related field with demonstrated experience in an analytical testing laboratory
- Demonstrated experience with UPLC-UV, UPLC-MS and with prep LC collections
- Demonstrated experience working with Waters AutoPurification System and FractionLynx for collection and isolation of compounds
- A minimum of 3 years of experience working with liquid chromatography-mass spectrometry for the analysis of compounds in complex matrices
- Experience working in a regulated environment under ISO 17025 Accredited laboratory or other regulated laboratory environment is preferred
- Experience with sample preparation and extraction techniques (LL, SPE, etc.) for the quantitative analysis of target compounds in complex matrices
- Proficiency with chromatography and mass spectrometry data analysis software such as MassLynx, Empower, ChemStation and MassHunter
- Ability to work independently, to multi-task, and to be a strong team player
- Excellent attention to detail, verbal and written communication skills
- A Bachelor's degree in Chemistry/Analytical Chemistry
- A minimum of 5 years' experience in the use of GC-MS and GC-QTOF-MS
- Demonstrated experience in mass spectral interpretation
- Demonstrated competence in chromatographic data analysis using Mass Hunter and/or MS Data Review software
- Experience in troubleshooting instrument problems and performing routine maintenance of equipment
- Detail orientation to undertake experiments and develop protocols to meet regulatory needs
- Significant demonstrable experience (minimum 3 years) working within an accredited analytical testing laboratory, working with HPLC, LC-MS and LC-MS/MS systems
- Able to demonstrate the capability as well as experience of developing LCMS methods/applications from concept through to validation to final delivery
- Demonstrable experience of a range of sample preparation techniques, including liquid:liquid extraction and solid phase extraction A high degree of flexibility will also be required to deal with the varied matrices and compounds required for testing by RPS’ clients
- Plans, conducts, and may lead one or more projects including determining objectives and organizing efforts
- Works with minimal supervision and has wide latitude and discretion over methods used to achieve technical objectives
- Decisions or recommendations result in achieving significant project objectives
- Participates in setting technical and program goals
- Scouts analytical market and proposes capital budgets in line with business blueprint
- Implements procedures ensuring high quality standards (ISO9001)
- Implements data driven quality (Lean - 6Sigma) improvement plans
- Provide analytical support and problem solving for GE Water and Process Technologies’ internal and external customers
- In-depth analytical characterization of water, deposit and oil samples though in-depth analytical expertise
- Determination of root cause of issue/failure, formulate solutions for though-to-treat systems
- Responsible for the operation, maintenance and troubleshooting of analytical instruments including gas chromatography (GC/MS, GC/FID, GC/NPD, Thermal desorption and pyrolysis), High Performance Liquid Chromatography (HPLC) and Ion chromatography (IC/CD, IC/MS)
- Preparation, analysis and reporting of routine and non-routine samples
- Continuous improvement of analytical methods and associated standard operating procedures
- Develop and validate new methods in line with business requirements
- Recognized as an authority in area of expertise within division
- Responsible for recommending, developing and implementing technical projects to support business goals and objectives
- Compile analytical customer reports
- Assist in setting up investments plans in anticipation of future market changes
- Master or PhD in Analytical Chemistry, Chemical/Environmental Engineering, Materials/Polymer Science or equivalent knowledge or experience with relevant experience in a similar role
- Extended expertise in chromatographic analyses and mass spectrometry as well as in other analytical techniques
- Expertise in one of the following areas is an advantage: Hydrocarbon Process Industry, cooling, boiler and/or waste water treatment, Membrane separations technology
- Affinity for Environment, Health and Safety framework
- Excellent conceptual problem solving skills-ability to consider overall problem
- Strong interpersonal skills and excellent communication skills
- Fluent in English. Other languages are an advantage
- PC literate
- Provide analytical support for nonclinical studies under FDA GLP’s (21CFR58), including dose formulation analysis (concentration and homogeneity), dose formulation stability, analytical method development and validation, as well as data/notebook review and verification
- Develop, validate, and carry out analytical assays that are compliant with GLP regulations
- Proactively identify problems and resolve them using industry knowledge and practice and in consultation with other scientists
- Consistently deliver high quality methods and data with high productivity in an independent and timely manner
- Experience in HPLC, UPLC, SEC, and in-vitro binding assays (i.e. Elisa, MSD)
- Experience in Good Laboratory Practice (GLP)
- Experience with Capillary Electrophoresis and particle analysis (e.g. microflow digital imaging, DLS/SLS) preferred
- GMP experience is preferred but not required
- Minimum qualifications of B.S. or B.A. in Chemistry, with a minimum of 5 years’ industry experience working in a GMP/GLP environment
- Testing of Raw Materials/Excipients/Components/Intermediates/Drug Substance/Drug Product in accordance with site schedules and company policies, procedures and guidelines
- Support the introduction of new products to the site
- Aid in the resolution of issues that may arise during testing
- Participate in assigned projects
- B.S. or M.S. degree in chemistry, biology or related field with 5- 7 years of experience in the Pharmaceutical industry
- Understanding of cGMPs and Good Documentation Practices
- Understanding of various laboratory instrumentation
- Knowledge of Microsoft programs such as word and excel
- B.S. or M.S. degree in chemistry, biology or related field with a minimum of 8 years of experience in the Pharmaceutical industry or a Ph.D. with 5+ years of experience
- Understanding of cGMPs and cGDPs
- Understanding of Regulatory requirements
- Understanding of Safety requirements
- Flexible and able to work in a team environment
- Proficient with Microsoft programs such as word and excel
- Good written skills
- Proficient with a variety of analytical instrumentation. Some examples are HPLC, GC, TLC, Dissolution Baths and Titrators
- Design and coordinate chromatography experiments as a member of a corporate engineering services analytical laboratory
- Participate in development of analytical test methodology and strategies for R&D medical device products
- Perform hands-on testing in the chromatography and analytical chemistry lab
- Prepare test data summary reports/documentation to communicate results to lab customers and the technical community. Reviews and approves results and reports from Chromatography team members
- Completes tasks on analytical lab documentation and quality records
- Creates and maintains test instrument and test method documentation. Validates equipment and test methods
- Identifies areas for improvement, suggests solutions, and submits idea records
- May provide leadership, work direction, and technical oversight to Chromatography team members
- May represent, coordinate work for, and report back from the analytical department to R&D project teams
- Demonstrates a primary commitment to patient safety and product quality by maintaining compliance to the Quality Policy and all other documented quality processes and procedures
- Applies scientific principles and company policies and procedures, analyzes data or situations, and exercises judgment to recommend solutions to solve problems
- Review of all laboratory Quality Systems to ensure compliance to GMP
- Participate in Regulatory, Internal and Vendor audit programs as required
- Provide technical guidance / trouble shooting where applicable
- Review and approval of Laboratory results and documentation
- Bachelor Degree in Chemistry
- 3-5 years industry experience in analytical chemistry in particular with chromatographic and pharmaceutical cGMP analysis; medical device or pharmaceutical experience preferred
- Liquid chromatography experience with both routine testing and method development
- Compliance experience in a regulated environment
- Strong technical report writing skills
- Combination product analytical chemistry testing experience
- Waters chromatographic equipment (e.g., Waters 2695 Separations Module with 2996/8 Diode Array Detector) and Empower software experience
- Drug dissolution, content, or degradants analysis by chromatography experience
- HPLC, GC and GPC experience
- Experience with mass spectrometry detectors
- Receive, log, analyze field samples and send out daily residue reports to customers
- Prepare daily field sample analysis report and discuss the results with technical service personnel
- QC of in-house and field samples
- Maintain day to day laboratory tasks
- Maintenance and upkeep analytical instruments and laboratory
- Manage samples and waste generated from lab
- Provide timely residue analysis reports to customers and filed personnel
- Management of the analytical lab (instruments and seasonal temporary workers)
- Upkeep of Food safety certification (SQF) and logs
- Provide analysis summary reports
- Design and execute experiments for analytical support of biotherapeutic proteins using HPLC, LCMS, and capillary electrophoresis
- Troubleshoot and maintain analytical equipment in good working order
- Develop and optimize analytical methods
- Experience analyzing proteins using HPLC, LCMS, and electrophoretic methods
- Experience developing, qualifying, and testing analytical methods to characterize proteins
- Strong analytical lab skills and technical knowledge of proteins and peptides
- Excellent documentation skills, including an understanding of GxP requirements
- Excellent communication (oral and written) skills and attention to detail
- Strong computer and organizational skills
- Ability to work independently and as part of a larger analytical team
- Biotech/pharmaceutical industry experience preferred
- B.S. with 1-4 years of experience, with degree in Chemistry, Biochemistry, Chemical Engineering, Molecular Biology or related field
Analytical Chemist Tech Resume Examples & Samples
- 1) Independently performing a variety of analytical techniques in support of R&D, customer and regulatory projects
- 2) Formulating and preparing samples and reagents
- 3) Execute stability studies in support of product development
- 4) Providing technical help for routine lab experiments
- 5) Record lab data and compile & analyze results for review with project leaders. Maintain electronic databases, documenting results in laboratory notebooks and reports
- 6) Troubleshooting existing laboratory processes and instrumentation
- 7) Maintaining a safe and clean laboratory as well as all other assigned areas of responsibility
- Operate novel dissolution test equipment
- Run analyses as needed such as drug assay
- Run and operate pharmaceutical formulation and bio-processing lab equipment
- Wet chemistry bench work
- Run HPLC analyses
- Document results
- Assist in evaluation of the results
- Assist in preparation of reports
- Plans and conducts chemical analysis of medical device manufacturing and design processes, materials, and products
- Implements, refines and validates test methods for QC testing
- Test method development and implementation using HPLC and GC through reports and protocols
- Process control charting within manufacturing and quality processes
- Complete internal audits of manufacturing processes
Scientist Analytical Chemist Resume Examples & Samples
- Perform testing and data review for HPLC/UPLC (high and ultra-high liquid chromatography), titre, ATOL purification using TECAN and plate reader, CE (capillary electrophoresis), CEX (cation exchange), HILIC (hydrophilic interaction), TOC (total organic carbon), LAL (endotoxin testing), ELISA, and DNA testing
- Testing and purification of proteins and peptides
- Utilize high-throughput, robotics sample handling and/or auto-sampler systems to manage samples, purify, and test
- Demonstrate independence and ability to produce quality results under minimal direction
- High energy individual who can multi-task and handle a fast-pace, dynamic work environment
- Comply with safety guidelines and site specific procedures which include but is not limited to, the maintenance of training records, laboratory documentation, written procedures, building monitoring systems and laboratory log books
- Interact with multiple functional areas in a highly matrixed-team environment, which may include Manufacturing, Process Development, IS, Facilities, Engineering, and Quality Control
- Technical expertise for executing analytical test methods in one or more of the following areas
- B.S., or M.S. degree in Analytical Chemistry, Biology, Molecular Biology, Immunology, or related field
- Position is full-time, Monday-Friday, 8 a.m.-5 p.m., with overtime as needed. Candidates currently living within a commutable distance of West Greenwich, RI are encouraged to apply
- Masters or PhD in analytical or organic chemistry. Demonstrated track record of technical problem solving in structure elucidation preferred with 3-7 years of experience post degree
- Hands-on experience on Bruker NMR instruments preferred
- Proficient with NMR software such as TopSpin, IconNMR, MNova/ACD
- Skillful knowledge in data acquisition and interpretation of NMR and LC/MS
- Experience with LC/MS software including MassLynx, ChemStation, Analyst, and PeakView would be useful
- Knowledge of Microsoft Office package is an asset
- Problem solving skills in small molecule purification
- Motivated team player with good communication skills, able to work closely with other scientific disciplines involved in drug discovery to solve complex problems
- Laboratory operations: Safely and efficiently providing quantitative measurements using commercially available instruments
- Consultation to and collaboration with DuPont scientists to provide solutions to problems in DuPont businesses through analytical work done in-house and through Contract Analytical Lab partners
- MS or Ph.D. in Physical/Analytical Chemistry, Materials Science, or Physics
- Prior experience with material characterization using ICP and/or ICP/Mass Spec
- Deep understanding of common Elemental Analysis sample preparation methods such as acid digestion and open/closed vessel microwave-assisted digestions
- Experience in the development and validation of analytical methods and effective, technology transfer. Excellent verbal and written communication skills
- Results oriented, with ability to solve complex problems with analytical science tools
- Ability to manage and prioritize multiple tasks
- The ability to relate chemical analysis results to chemical and materials processes. Ability to contribute or collaborate effectively in a cross-disciplinary team environment and lead a project/task team. Prior supervisory experience of lab operations is desirable
- Oversees the testing of incoming base stocks, and additives to determine if material meets the company's specification
- Oversees the testing of products during manufacturing process, final blend products, and during the filling of containers to ensure product quality
- Maintains all laboratory equipment in proper working order/and request repairs or replacement when deemed necessary
- Keeps current on all test methods and procedures and instructs the technician on existing and new test
- Oversees maintenance of MSDS library of products stored and produced at the Portland Terminal
- Ensures the ongoing calibration of laboratory test equipment
- Responsible for following the standard operating procedures defined by the environmental and quality management systems for the department
- Makes detailed observations, analyzes data, and interprets results
- Writes technical reports to summarize research experiments
- This position requires a BS/BA degree with minimum of one (1) year of experience
- Execute 1 to 3 worklists on average per day
- Attend Prioritization meetings as required to support assay transfer, co-validation, qualification and batch production release/stability testing for demonstration, engineering, clinical trial, process validation and/or commercial manufacturing in a GMP environment as needed
- Responsible for becoming proficient in a breadth of methodologies including but not limited to compendial test methods, raw material test methods, HPLC, electrophoresis, spectrophotometric and residual component determination methods
- Executes test methods, tabulate data and provide data interpretation
- Assist in revision of test methods, LIMS builds and creation of training materials as necessary
- Ability to work in a fast paced team-oriented setting and possess good communication skills
- Experience running HPLC methods
- Bachelor's degree in life sciences, biology, chemistry, or other related degree concentration, or equivalent directly-related experience (two years of directly related industry experience is equivalent to one full-time year of college in related major)
- Minimum of 4 years of experience working in the biotech or pharmaceutical industry
- Ensure all raw materials and product shipments conform to the established specifications
- Demonstrate and establish the use of qualified, well maintained analytical equipment
- Cultivate the usage and implementation of our integrated LIMS program
- Improve analytical skills of technicians
- Lead the development in standards preparation needed to calibrate instrumentation
- Possess a Bachelor’s or Master’s degree in Chemistry or closely related field (i.e. Biochemistry, Biology) from an accredited university
- Minimum of 2-5 years of experience in a laboratory setting
- Working knowledge of Laboratory Information Management System (LIMS)
- Experience in both operations and maintenance of analytical instrumentation
- Skilled in Method Development and knowledge of Standards Preparation
- Competent in basic PC skills including MS Office Suite
- Performing analysis on a wide range of sample types utilizing multiple techniques and analytical instrumentation
- Delivering prompt service, quality data and relevant information to our customers
- Providing support to ALCS Quality Compliance and Operating Company Factory Quality Assurance to assist in identifying and resolving product and other quality issues
- Providing support to Research & Development and Regulatory Affairs customers for the analysis of materials in all types of products
- Participating in the development, validation and transfer of analytical methods to Operating Company Factory Quality Assurance
- Participating on cross-functional teams representing Analytical Sciences
- A Bachelor’s degree in Chemistry is preferred. A degree in Biology or closely related field with analytical laboratory experience is acceptable
- At least one year of experience in an analytical testing laboratory is preferred
- Experience with sample analysis using GC-MS is required. Experience with the identification of unknown compounds by GC-MS is preferred
- Experience with sample analysis using FT-IR and/or EDXRF is preferred
- Experience working in an ISO 17025 Accredited laboratory or other regulated laboratory environment is preferred
- A Bachelor’s Degree in Chemistry or Polymer Science
- 10 years minimum experience in a laboratory environment, or; a Master’s Degree in Chemistry or Polymer Science with 5 years minimum experience in a laboratory environment
- The qualified candidate for this position will need to have the ability to function in both a team and an individual environment. In addition, this candidate must possess the flexibility to address changing priorities as they arise
- Understands MRI is a contract research business. Understands MRI administrative and technical operations, including the divisions, sections, and groups
- Immediately notifies supervisor of lack of billable project work. Provides supervisor with summary of time availability and project progress, alerting supervisor to potential overload on project work and impact on project budgets
- Understands that all activities conducted on a project influence client satisfaction and future project
- Conducts experimental work on projects; sets up the procedure and performs the laboratory operation based on established protocols, developed procedures, or experimental designs; keeps records of experiments performed and all data collected. Reviews data for accuracy and completeness
- Follows MRI safety and security policies and procedures and maintain labs and facilities in organized manner
- Maintains equipment and ensures it is in working order. Schedules needed repairs and/or calibrations and documents maintenance in appropriate use logs
- Networks through the Institute to find out how other staff can help solve a problem
- May train technicians in technical procedures and instrumentation and assist with their professional development
- A B.S. or M.S. degree in Analytical Chemistry
- Minimum of 5 years industry experience in the field of analytical chemistry
- The individual must have good interpersonal and communication skills, and be customer-focused
- Individual must be self-motivated, and have ability to independently achieve results
- Must have ability to work in teams and work in a collaborative environment
- Must have flexibility to quickly respond to changing customer conditions and needs
- Must have good technical writing skills and good verbal communications skills
- Must have good problem solving skills, leveraging technical background
- Experience in Gas Chromatography/Mass Spec analytical testing
- Analytical method development
- Experience in working with external customers and industry standards organizations
- Experience in working with manufacturing sites
Senior R&D Analytical Chemist Resume Examples & Samples
- Play a key role in method development and delivery of analytical technologies, including HPLC, GC/MS, NMR, FTIR
- Support the product development teams with technical expertise in the area of analytical chemistry with particular focus on polymer product development i.e. Intrinsic Viscosity, degradant product analysis, polymerization characterization by NMR, etc.,
- Support new product introductions, working with internal and external teams to develop and transfer analytical methodologies. This includes method development, optimization and validation
- Identify and support introduction of systems & testing improvements
- Support the implementation of quality systems
- Management of the Biomaterials Laboratory including testing, documentation and laboratory maintenance
- Maintain laboratory test/validation records in compliance with regulatory requirements
- Lead laboratory chemical risk assessment and introduction of appropriate engineering controls
- Participate in the planning and running of DOE’s and test plans
- Perform trending and analysis of data, and compiling of associated technical reports
- Participate in brainstorming and problem solving sessions
- Maintain appropriate laboratory supplies and equipment to ensure efficient operation of the Biomaterials Test Laboratories
- Independently determines and develops approach to resolving end customers product related issues, through problem solving and troubleshooting test issues, product training and relationship building
Temp Analytical Chemist Resume Examples & Samples
- Prepares and accurately determines metal content and other properties of matrix materials, applying approved analytical and/or instrumentation test methods to support refining, catalyst and chemical production, development or other JM department
- Prepares and maintains proper analysis documentation, including summarized test data and procedures
- Develops a basic understanding of production processes and an ongoing proficiency with all analytical test methods related to customer specifications
- Operates in accordance with all JM safety procedures and SOPs at all times
- Post-secondary major in chemistry or related science required
- Lab experience including commercial analytical/quality control laboratory experience needed
- Instrumentation techniques, for inorganic, elemental analysis, and physical characteristics preferred
- Solid working knowledge of Microsoft Office and strong attention to detail required
- Requires sound communication skills both verbal and written and ability to work successfully in a team environment
- Define and establish the target of analytical team, review and assess its progress and fulfillment
- Define and follow quality procedure, test method development, and coordination with global analytical team
- Based on effective communication, coordinate the suitable analytical resource to support global business technology (GBT) team, technical service team and colleagues from manufacturing/process technology team, establish good work relationships with kinds of partners
- Review the work-flow of analytical lab, record test procedure and lab activity, summarize general report on time
- Lead and organize the analytical support to new projects
- Keep connection with collaborated universities and institutes to support internal unsatisfied analytical needs
- Management of overall analytical lab and showcase display
- Master or above in analytical chemistry, organic chemistry, polymer chemistry or other related majors with minimum 5 years working experiences and 1-3 years automotive coating R&D experience is plus
- Very familiar with modern analytical techniques, including but not limited to Spectroscopy (IR, Microscopy IR, UV-VIS, XRD), Chromatography (GPC, GCs, Pyrolysis-GC/MS), Thermal analysis (TGA, DSC) and Physical properties technology (Surface tension, Contact angle, Optical microscopy, Water content by Karl-Fisher, etc.)
- Knowledge with other separation/analytical technologies: Column separation, Particle size distribution, Rheometer, Elemental analysis
- Strong ability in instrument operation and maintenance
- Fluent in both English and Mandarin
- Strong ownership, work under pressure and independently
- Proactive and self-motivated team leader with good interpersonal communication capability
Analytical Chemist West Lafayette Energy Center Resume Examples & Samples
- PhD in Analytical Chemistry or closely related chemical discipline (e.g., environmental chemistry.)
- One year experience with industrial/government experience in analytical chemistry or experience working in analytical chemistry in an academic lab as a technician (analyst that was not part of working towards a degree.)
- An equivalent combination of education and experience may be accepted
- Experience in conducting sorption and desorption experience under controlled conditions, familiarity
- Experience with extraction methods, Dean Stark analysis, purification of solvents, preparation of analytical standards and buffers, titration of samples, perform colorimetric assays
- Ability to independently use modern chemical, optical, thermal and mineralogical instrumentation (FTIR, Raman, UV-visible-NIR, HPLC/UPLC, powder X-ray diffraction, thermogravimetric analysis, evolved gas analysis, and differential scanning calorimetry
- Aptitude to search for existing methods of analysis from the literature and/or develop new methods for chemical analysis,
- Capability to write, implement, and follow standard operating procedures
- Knowledge of statistics as it relates to accuracy and precision of measurement; knowledge of experimental design; computer literacy
QS Analytical Chemist Resume Examples & Samples
- To perform Development Department, Out of Specification, Process Validation, Method Validation and other miscellaneous analysis, as requested and according to the written procedures of the company, pharmacopoeias and regulatory requirements
- To report any out of specification or anomalous result to a supervisor and to document any deviations from written analytical procedures
- To assist in the investigation of out of specification or anomalous results and assist in qualification activities relating to new and existing equipment
- To operate documented systems to ensure that duties are performed to, and maintain GMP standards
- To perform and document routine calibration and general laboratory duties
- To assist in the preparation of method validation protocols and reports, Development reports, SOP's, specifications
- You should have a Degree in Chemistry, or closely related subject
- You will have previous experience in a cGMP analytical lab
- You will have the ability to work as part of a busy team, while maintaining high levels of accuracy and flexibility
- You will have good communication skills and a responsible attitude to health and safety issues
Senior Analytical Chemist GC Resume Examples & Samples
- Significant demonstrable experience (minimum 3 years) working with GC-MS systems, ideally within an accredited/regulated analytical testing laboratory
- Comprehensive understanding of the theory and practical applications of GC-MS-based systems
- Demonstrable experience of troubleshooting and improving analytical methods
- Demonstrable experience of a range of sample preparation techniques, including liquid:liquid extraction and solid phase extraction
- Experience working within an environmental testing laboratory analysing for trace levels of a range of environmental contaminants in difficult matrices would be highly advantageous
- Learns to conduct routine analyses in compliance with applicable methods, protocols, SOPs, and regulatory agency guidelines
- Learns how to document work and maintain study documentation and laboratory records
- Attends and participates in project meetings
- Learns to perform peer review of data
- Learns to plan assigned workload on a daily basis and effectively schedule multiple assignments
- Contributes to a cohesive team environment. Plans individual workload in coordination with team members
- Serve as subject matter expert for Analytical Chemistry with a focus on chromatographic separations to provide information on chemical and biological reactions
- Ability to prioritize multiple demands and work successfully in a fast-paced environment
- Willingness to train others and courage to challenge and discuss analytical methodologies and results
- Accountable for the maintenance and upkeep of various analytical instrumentation in the lab
- Broad experience in industrial research and development and the analysis of reagents and products
- Courage to constructively challenge and discuss the analytical methodology and results of others
- Design and execute experiments; interpretation of data from multiple sources
- Must be motivated, possess good interpersonal skills, communicate well within and between teams and be able to work to tight deadlines
- Performs routine physical and chemical analytical evaluation/characterization of raw materials, chemical intermediates and finished product while adhering to ISO 13485 Quality system
- Primary Responsibilities: Testing of R&D materials- polyurethanes, collagen, ECM, resorbabale polymers
- May work on method development for materials
- A degree in chemistry or related scientific discipline that would support analytical lab activities
- 2+ years of experience in an analytical or industrial chemistry/physical test environment
- Familiarity with following Test methods and Standard operating procedures
- Experience in chromatography (GC, GPC & HPLC), FTIR analysis, Thermal testing (Melt Flow, Rheology, DSC), Autotitrator using potentiometric analysis
- Experience with polyurethane a plus
- Develop new and/or improved analytical methods for new and existing products and processes
- Validate analytical methods for cleaning verification, raw material assay, in-process controls, stability testing and final product release. Prepare validation protocols, conduct validation work and prepare validation reports
- Execute reference standard qualifications and prepare reference standard reports
- Provide analytical support for QC projects as necessary. Work with multidisciplinary product and technology teams
- Perform appropriate testing and data evaluation for the development and justification of specifications
- Coordinate instrument qualification, maintenance and repair activities for AD equipment. Maintain instrument logs and records
- Maintain supplies and materials for the GAATS area
- Assist in the evaluation and cost estimates for new projects
- Document and record work observation and results according to written SOP’s
- Implement analytical methods to elucidate the types and quantities of impurities present in reaction mixtures, process streams, waste streams, intermediates and finished products
- Work with CRD staff to develop impurity profiles for Active Pharmaceutical Ingredients and regulated intermediates
- Ensure that work in the analytical development laboratories is done safely and in accordance with the site chemical hygiene plan, following any additional regulatory requirements that may apply
- Conduct all job responsibilities in a manner consistent with all applicable safety and environmental laws and regulations, company policies and procedures, regulations and commit to continuously improve safety and environmental performance
- Develop and validate chromatographic (HPLC, GC, and IC) methods for the analysis of pharmaceutical active ingredients and their precursors (intermediates)
- Develop and validate as needed other analytical methods required by regulatory agencies for a pharmaceutical active ingredient and its precursors
- Develop and qualify in-process controls (HPLC, GC, etc) for processes
- Validate analytical methods for cleaning verification, raw material assay, stability testing, waste streams, and final product release
- Modernize analytical methods for existing products and processes
- Develop validation protocols, conduct validation work and write validation reports
- Perform appropriate testing for specification development
- Ensure that all IPC’s are performed on laboratory experiments before they are used to monitor a kilo lab, pilot plant or manufacturing run
- Provide analytical support for CRD and Pilot Plant projects. Work with multidisciplinary product and technology teams
- Operate in compliance with current Good Manufacturing Practices, Good Laboratory Practices and applicable ICH and FDA requirements
- Assist in the preparation of responses to request for proposal / quote, project scope assessments and project timelines for new projects, as needed
- Facilitate detailed process understanding by developing analytical methods in a timely fashion to elucidate the structures and quantities of impurities / side products present in reaction mixtures, process streams as well as intermediates and finished products
- Work with CRD staff to develop an understanding of impurity profiles for Active Pharmaceutical Ingredients and regulated intermediates
- Ensure that work in GAATS is done safely and in accordance with the site chemical hygiene plan, following any additional regulatory requirements that may apply
- Participate in Continuous Improvement Efforts by identifying work-practices to reduce costs and/or improve throughput and quality and bringing them to the attention of laboratory management
- Keep up to date with relevant developments in chemistry and laboratory practices and bring them to the attention of the laboratory management
- Approve reports, methods, and other documents as needed in the absence of supervisor
- Design and execute experiments to achieve development of validated analytical methods for release and stability of protein substances and products
- Manage and monitor progress of methods development, qualification and transfer to contract research laboratories including data review
- Document experimental work and write analytical development reports
- Contribute to the writing of analytical sections for regulatory filing
- Performs routine analytical testing using GMP/GLP practices
- Performs data analysis and results interpretations comparing to specifications, validity criteria, alert limits
- Responsible for maintaining product and QC material inventories
- Responsible for the maintenance and cleanliness of the laboratory, stocking shelves/drawers with testing materials
- Responsible for the maintenance and cleanliness of equipment, calibrating or submitting items for calibration as needed
- Responsible for completing training in a timely manner and maintaining the records
- Responsible for revising QC documents (SOPs, QSs)
- Participates in LI/OOS investigations
- Responsible for training QC analysts on test methods
- Participates or leads a QC department team
- Working knowledge of Enterprise Resource Planning (ERP), QSR, GMP/GLP requirements
- Working knowledge of industry/regulatory standards
- Requires a BS or higher in Chemistry or related field with at least 1 year of hands-on HPLC/analytical chemistry experience in GMP or GLP environment
- Must possess chemistry knowledge and technical skills in operating and troubleshooting analytical equipment including HPLC, FTIR, Mass Spectrometry and UV/VIS Spectrophotometer
- Experience with different modes of chromatography such as: ion exchange, reverse phase
- Experience with Waters Empower software is a plus
- Design and execute test strategies to develop and evaluate improved characterization capabilities for ionic and ionizable analytes
- Design and execution of strategies for ionic and non-volatile analytes
- Collaborate with project team members
- Document, report, and present results at internal meetings
- Ph.D. degree in Analytical Chemistry, Physical Organic Chemistry, or relevant discipline
- Expertise in chromatography, particularly of ionic materials
- Strong interest in solution chemistry
- Familiarity with ion/liquid chromatography instrumentation and software
- Operational experience with IC (or LC) and EC, notably ion/small molecule separations
- Excellent interpersonal skills with ability to work in a team environment
- The ability to work and think both in a team environment and as an independent researcher
- Conduct testing to support regulatory submissions and improvement projects
- Bachelor's degree or higher from an accredited university
- Minimum of three (3) years of experience in a Good Manufacturing Practice (GMP) laboratory environment
- Bachelor's degree or higher in Chemistry or a related discipline from an accredited university
- Strong working knowledge of Gas Chromatography (GC) and High Performance Liquid Chromatography (HPLC)
- Working knowledge of Food and Drug Administration (FDA) and United States Pharmacopeia (USP) requirements for reference standards, metered dose inhalers and transdermal products
- Ability to lead and contribute to laboratory investigations
- Ability to multi-task and respond to changing priorities
- STARLIMS and/or another Lab Information Management Systems software experience a plus
- Develops efficient and selective analytical procedures (with emphasis on HPLC and GC) for assigned projects
- Performs sample analysis and reviews data packages
- Documents all analyses per SOPs
- Volunteers to assist with task not directly related to a specific project
- Demonstrates initiative in handling responsibilities
- Attends project meetings and prepares project updates
- Responsible for trouble shooting and investigating out of specification results. Perform analytical testing on all incoming Raw Materials as per our reduced testing program as well as an annual full testing using USP/NF, FCC, Pharmacopoeia and GSK methodology
- Responsible for the disposition of raw materials, intermediate, batches, and finished products in a timely manner
- Data entry of test results
- Investigate out of specification results
- Support method and/or product transfer and NPI activity as necessary
- Preparation, documentation and maintenance of test solutions and standards. Maintain, calibrate, and validate laboratory equipment
- Ensures that GMP, Quality, Health & Safety are considered in all aspects of role
- Perform other duties as directed by QC Specialist or QC manager
- Grow and develop into roles of increasing responsibility based on your interests and performance
- Provide technical support to the analytical laboratory using instruments such as GCMS, HPLC, FTIR
- Deliver analysis of contamination of coatings and foreign body identification
- Run routine analysis of resin and paint samples using wet chemistry techniques, among others
- Develop best-practice methodology for analytical equipment and analysis, as required
- Maintain all lab instrumentation to ensure optimal performance
- B.S. Chemistry, Material Science or related field with 3-5 years' experience
- Experience with analytical equipment and testing methods. (2+ years preferred)
- MS or PhD in Chemistry, Material Science or other related discipline
- Demonstrated ability to lead multiple projects simultaneously in a fast paced environment
- Ability to work collaboratively and build relationships with cross-functional teams
- Strong technical skills with development/formulation of DoE’s, customer trials, testing, troubleshooting and report writing
- Passion for safe working practices in handling chemicals and working with laboratory equipment
Senior Scientist Analytical Chemist Resume Examples & Samples
- Develop, validate, transfer and troubleshoot analytical methods, including API, raw materials, in-process and finished drug product; author, review and verify validation and transfer protocols and reports (Must have in-depth knowledge and extensive hands-on experience with method development for HPLC, GC and LC-MS)
- Anticipate, recognize and resolve complex technical issues through job knowledge, use of technical resources including technical literature and experts and well formulated, logical experimentation
- Advance the technical capabilities of the department by evaluating, recommending and implementing new technologies to meet business needs
- Serve as the analytical representative and/or lead cross functional project teams; actively participate in team efforts and contribute to technical evaluations and solutions
- Write/co-author Regulatory Submissions sections of the CMC for IDEs and PMAs),memos and scientific reports in support of Regulatory Submissions and support quality control and manufacturing documents
- Manage analytical projects
- Establish schedules, define dependencies and project plans, perform technical and quality risk assessments and direct activities of other scientists as well as provide training and development for others
- Present project results and recommendations to senior management
- Provide guidance (scientific and administrative) for junior analytical scientists
- Maintain and meet the highest standards in quality and regulatory compliance
- Follow, understand and comply with AV SOP’s and policies on cGMP’s and safety
- Must have in-depth knowledge and extensive hands-on experience with method development for HPLC, GC and LC-MS
- Must have extensive experience with method validation and transfers for analytical methods according to current regulatory guidelines
- Internal/external authority for technical issues
- Evaluates and defines function activities for projects and determines appropriate timeline; coordinates activities with functional resources; keeps functional activities on schedule
- Demonstrates expertise in different analytical techniques, theory, application, troubleshooting, etc. Advanced knowledge of area of analytical chemistry is required. Applies advanced principles, theories, and concepts in area of specialty. May contribute to the development of new concepts, practices and standards
- Directly or indirectly supervises/mentors others; delegates activities appropriately
- Develops alternatives and solutions for a range of complex problems. Solutions require creativity, critical thinking, and collaboration, considering a broad range of potential impacts, including those that are downstream
- .Extensive experience in preparing regulatory submissions
- Develop and validate new analytical methods and implement improvements to existing methods to support both new and existing products. This includes conducting designed experiments and applying statistical techniques to determine test method capabilities and limitations, creating laboratory standards, developing calibration techniques, establishing test method parameter and analytical procedures, and conducting training for analysts on new methods
- Evaluate and introduce new analytical technologies to expand the analytical capabilities
- Function as an information source of analytical expertise at the company
- Transfer new analytical methods and improvements to QC group
- Manage contracts and interactions with instrument vendors and outside analytical services
- Conduct Safety Reviews and maintain high standard in lab and safety practices
- Act as analytical expert to support safety, operations and QC functions
- Successfully completes all assigned trainings
- Expected to work extended hours as business needs dictate
- MS or preferably, Ph.D. in Analytical Chemistry or related discipline
- 5+ years of industrial experience
- Extensive hands-on experience with analytical instrumentation such as gas chromatography (GC), mass spectroscopy, FTIR, thermal stability testing (e.g. DSC/TGA), liquid chromatography and NMR
- Experience with handling and analysing air/moisture sensitive materials including both gases and liquids
- Proven track record of developing new analytical methods for novel products and delivering results within deadline
- Possess fundamental Six Sigma knowledge (e.g. applying statistical techniques to determine test method capabilities) combined with demonstrated ability to efficiently design, execute and implement analytical projects
- Knowledge and experience in semiconductor applications are a plus
- Knowledge and experience with polymer materials are a plus
- Excellent team player with good project management and problem solving skills
- Excellent communications skills. Ability to generate high quality reports for both internal and external purposes is highly preferred
- Maintenance and troubleshooting of Waters analytical uPLC- MS and autoprep LC- -MS systems using MassLynx software in an open-access small molecule drug discovery environment working with medicinal chemists
- LCMS purity assessments
- Preparative LCMS separations
- Software and hardware modifications and improvements to existing open-access analytical and chiral method development and small and large scale purification and/or NMR for structure confirmation
- Desirable, but not critical skill set and experience in the area of SFC instrumentation for achiral and chiral method development and small and large scale purification and/or NMR for structure confirmation
- This position requires proficiency with lab computers for data entry, analysis, interpretation and presentation for lab meetings, scientific posters and manuscripts
- Collaborative orientation
- Positive outlook
- Industry experience performing qualitative and quantitative material characterization in a GLP and/or GMP laboratory
- Ability to design, perform, and interpret analytical testing to characterize a wide range of materials
- Demonstrated knowledge of modern analytical techniques including HPLC, LC-MS and wet chemistry techniques
- Familiar with material chemistry on a broad range of metals, plastics, leathers, adhesives, paints, and coatings
- Excellent communication and presentation skills to technical and non-technical audiences
- Demonstrated ability to excel in a fast-paced, schedule-driven environment
Analytical Chemist & Lab Manager Resume Examples & Samples
- Manage team of analytical chemists, setting work direction and priorities to support R&D programs as needed
- Partner with the Quality Organization to develop and implement laboratory procedures needed to support product development under proper regulatory compliance
- Partner with R&D leadership to define and prioritize test methods needed to support R&D activities. Drive development and validation of test method to support program schedule
- Develop, characterize and validated test methods as needed
- Support transfers of test methods to internal and external analytical labs as needed
- Collaborate with other Renal Care Solutions analytical labs to ensure uniform application of lab procedures and best practices
- Collaborate with company-wide analytical labs to develop laboratory metrics and best practices to measure continuous improvement in quality and productivity
- Partner with manager to develop strategic hiring and equipment plan for analytical chemistry lab
- BS in Chemistry, Analytical Chemistry or equivalent
- 10+ years of analytical laboratory experience, including 5+ years in technical leadership and/or management role
- 5+ years analytical chemistry experience in the medical device industry or other regulated industry (ISO or defense)
- Proficiency in laboratory software including Empower, ELN, LIMS
- Expertise is developing methods for analytes in biologic fluids including blood, serum and urine
- Self-starter with strong work ethic and initiative in accomplishing objectives
- Design and conduct experiments using HPLC/UPLC/GC including HPLC-MS and GC-MS in support of method development and validation
- Provide expertise in maintenance and usage of all analytical instrumentation
- Act as a technical team member with cross functional project responsibilities on assigned projects
- Provide expertise in the areas of analytical best practice, structure elucidation of impurities, state-of-the-art methodology and installation and qualification of equipment within AS&T
- Support the network and lead activities related to extractable/leachable methods and instrumentation, as well as troubleshooting and resolving investigations to facilitate solutions to complex challenges
- Collaborate with sites, R&D and Regulatory Affairs in the preparation, management and follow up of Health Authority documentation for extractable/leachable testing, analytical methodology, and equipment
- Utilize proactive thinking to generate creative solutions to complex technical problems
- Completes sample preparations
- Performs sample preparation and analysis of samples following prescribed procedures
- Calibrates and utilizes instrumentation
- Completes all paperwork associated with the analyses (ex., calibration logs, run logs, etc.)
- Manages waste generated as a result of analytical procedures and ensures proper disposal methods
- Tests samples of bulk and drum wastes, as well as various types of environmental matrices
- Performs other duties and tasks as assigned by management as required by the needs of the Clean Harbors business
- Bachelor’s Degree in Chemistry or physical science is preferred
- 1 to 5 years’ experience with GCMS instrumentation
- Familiarity with RCRA SW-846 methodology
- GC/MS experience in Environmental Chemistry
- Competent in GLP
- Superior organizational skills coupled with a strong attention to detail
- Master degree or above, preferably majoring in Analytical chemistry
- Relevant working experience in the field of the analytical chemistry or instrument analysis such as HPLC, GC, MSD, IR will be preferred
- Good verbal and written capability in both English and Mandarin
- Good computer application skill is a must
PhD Analytical Chemist Resume Examples & Samples
- Partner with existing PhD chemists to assess and demonstrate feasibility of new methods/capabilities through experimental design, vendor communication, bench-scale hands-on assessment, partnership with existing technician resources, and development of technical documentation (knowledge capture and procedure development)
- Assess and document safety aspects of new experimentation and their impact on later implementation in terms of safety, quality, productivity and workflow integration
- Pursuing PhD or post-doc in Chemistry, Inorganic Chemistry, Physics, or related field to lead selected, initially-defined projects from ideation to implementation stage, including design, lab hands-on experimentation, partnership with current senior and technician team members
- Independently performing a variety of analytical techniques in support of R&D, customer and regulatory projects
- Execute stability studies in support of product development
- Providing technical help for routine lab experiments
- Troubleshooting existing laboratory processes and instrumentation
- B.S. degree in Chemistry or similar science required
- Experience in the chemical or petrochemical industry is preferred but not required
- Previous experience with liquid chromatography (LC or HPLC), gas chromatography (GC), and gas chromatography mass spectrometry (GC-MS) is highly desirable
Analytical Chemist / Lab Supervisor Resume Examples & Samples
- Strong interpersonal and teamwork skills with the ability to influence leadership, peers, and Quality Control Technicians; able to take a "hands-on" approach to drive change and get results
- Prioritize and manage multiple, complex, time-sensitive projects
- Manage and lead both strategic long-term programs/projects as well as address short-term operational issues
- Drive the qualification of new analytical equipment
- Leads improvements quality control analytical processes, either through technology, process, and equipment upgrades
- Maintains laboratory standards and equipment in accordance with quality certifications
- Supervises a staff of 6 laboratory employees providing testing support across three operational shifts
- BS degree in Analytical Chemistry
- 3+ years Analytical experience
- Chromatography experience including HPLC, GC, as well as GPC is required
- Strong technical, analytical and functional skills
- Demonstrated computer skills including proficiency in Microsoft Office
- Previous supervisory experience a plus
- Executing analytical tests in compliance with GMP and in-house procedures. Tests performed include but are not limited to physiochemical assessments, assay, related substances and dissolution. Analyses may be performed on raw materials and finished pharmaceutical dosage forms
- Documenting in laboratory notebooks and in-house reporting formats without error in a highly regulated cGMP environment
- Evaluating/interpreting analytical data and communicating results to management and functional teams
- Anticipating, recognizing, and resolving or escalating simple and complex technical issues. This is accomplished through knowledge, use of technical resources including technical literature, technical experts and well formulated, logical experimentation
- Participating in the establishment and revision of standard operating procedures and other technical documents
- Maintaining and troubleshooting equipment as an instrument owner where appropriate
- BS/BA degree or higher in a scientific field
- At least 2 years of related experience in the pharmaceutical industry, or equivalent combination of education and experience with knowledge of current Good Manufacturing Practices (cGMP)
- Practical knowledge of cGMPs, GLP, and FDA/ICH guidelines and their application to drug development and release
- Technical expertise in the operation and application of analytical methods using common quantitative techniques including UPLC/HPLC, UV/Vis, GC, and wet chemistry
- Analytical method development experience is preferred
- Experience with a broad range of dosage forms such as tablets, capsules, device/implants, suspensions, lyophilized products, and novel formulation types is preferred
- Proficiency with data acquisition software (ideally Empower) is preferred
- Proficiency with common PC based applications and specifically in the use of MS Office
- General computer proficiency, an understanding of corporate IT networks, or familiarity with electronic data system compliance (Part 11, data integrity, etc) would be desirable skills
- Knowledge of laboratory safety precautions and protocols for safe handling and the disposal of hazardous agents/reagents, chemicals and materials
- Organization, communication and analytical skills
- Intermediate mathematical skills, including: algebraic functions, statistics (mean, median, standard deviation, variance, probability), basic geometry (area, volume calculations), and scientific calculators
- Ability to work varied and extended hours/days, as business dictates
- Perform all functions and duties of Q.C. Chemists and Technicians as needed
- Lead and train Associate Chemists and Technicians
- Perform all analyses associated with HPLC for both companies
- Monitor instrument performance and troubleshoot all functional problems
- Perform all preventative maintenance on HPLC instruments; ensure proper calibration of HPLC and all lab instruments
- Ensure accuracy of all qualitative analysis
- Maintain adequate stocks of necessary reagents and materials for operating instruments
- Develop and validate new analytical and isolation/extraction methods
- Make decisions regarding pass/reject on ingredient and product lots
- Trouble-shoot problem areas within the QC functions and recommend solutions
- Work with production employees to consistently improve sanitation practices
- Participate in the investigation of non-conformances in products
- Must demonstrate skill in establishing and implementing QC procedures for the continuous improvement of the QC Lab Department functions. These include but are not limited to record keeping, trend analysis, specification development, and reporting of data in a timely manner
- Responsible for organizing, storing, clean-up and disposal of HPLC waste and samples tested
- Provide training for new employees as needed
- Adhere to all safety procedures and policies
- Must have regular, punctual attendance
- Perform all other duties as assigned
- Experience with ICP-MS is highly desirable
- Complete sample preparation, which may include crushing and grinding of ore samples, polishing, working with chemicals, and various other sample preparation techniques. Coordinate submission of samples to external laboratories if needed
- Assist with sample database log-in and organization of projects throughout stages of preparation, analysis, and reporting. Use electronic laboratory notebook to record and manipulate data. Assist with organization of raw data files, calculation files, reports, standard test methods, standard operating procedure, and/or work flow diagrams, according to ISO quality systems/good laboratory practices (GLP)
- Follow written test methods and standard operating procedures accurately during analysis; assist with updating existing or developing new test methods/operating procedures as needed. Organize inter-laboratory or intra-laboratory studies, calibrations, or other quality control related activities to validate results
- Required 3+ years of direct experience with working in a laboratory environment
- Required experience X-ray diffraction, X-ray fluorescence, FTIR spectroscopy, differential scanning calorimetry, optical microscopy, scanning electron microscopy, and transmission electron microscopy
- Preferred background from mineral or mining company
- Organizes and conducts routine analyses independently and in compliance with applicable methods, protocols, SOPs and regulatory agency guidelines
- Independently able to use and perform basic troubleshooting of laboratory equipment and software appropriate for assigned tasks, while learning to perform routine and non-routine maintenance
- Provides input and participates in project meetings
- Performs assigned workload on a daily basis and effectively completes multiple assignments
- Contributes to a cohesive team environment and maintains a positive attitude. Plans individual workload in coordination with team members
- Identifies problems, determines appropriate actions, and implements solutions with support or independently as appropriate
- Able to adapt to new procedures or particular needs as directed
- Leads process improvement initiatives
- Completes all required training courses
- Trains and assists less experienced staff
- May assist in optimizing and validating methods for a variety of matrices
- Participates in client visits or calls as needed
- Learns to write text for reports, methods and, or protocols
- Viewed as a resource within their team
- 2-3 years of relevant experience
- Prepare, conduct and document all phases of Residue Analytical Studies according to protocol, guidelines, GLP, SOP´s and Sponsor requirements
- Protocol review, sample preparation, wet chemistry and instrumental analytics, raw data documentation, data evaluation and aggregation, report preparation
- Efficiently carry out procedures necessary to complete each project with minimal supervision
- Accurately prepare all solutions needed for analysis
- Assure accurate and complete collection of study data
- Maintain study data and facility records in organized manner
- Application of IT systems for data acquisition, evaluation, and reporting
- Writing, reviewing and editing SOP's
- Maintain a clean working environment in the laboratory including washing of glassware
- Training and supervision of laboratory staff members in the use of equipment, machinery and/or vehicles
- Ensure awareness and compliance with health and safety precautions
- Maximum of 15% Travel will be required
- Ability to conduct all experimental phases of Residue Analytical studies, including sample homogenisation, preparation, extraction, purification and instrumental analysis (LC-MS/MS)
- Good understanding of the underlying principles of Instrumental Analytics
- Ability to acquire and interpret relevant information for analytical methods
- Excellent weighing and pipetting skills
- Familiarity with MS office (Word, Excel), Analytical Instrument Software, Databases
- Willingness to learn new skills, well organized, capable of adapting to a broad spectrum of responsibilities and able to maintain meticulous and accurate records
- Ability to work with little or no supervision
- Ability to work outside standard business hours, according to team/business workloads
- Bachelor's degree in analytical chemistry, (food) chemistry, or other related degree concentration, or equivalent directly-related experience (two years of directly related industry experience is equivalent to one full-time year of college in related major)
- Ability to travel 15% both nationally and internationally
- Valid US Passport & Driver’s License
- Raw material monograph testing, as needed
- QC analytical chemistry assays, HPLC, GC, IR, UV, etc
- Wet chemistry assays
- Weekly water sample analysis for support of manufacturing
- Routine cleaning sample analysis for support of manufacturing
- Back up for QC analytical instrument maintenance, calibration, and validation
- QC general laboratory equipment maintenance
- QC method qualifications, transfers and validations
- QC method development
- Cleaning validation studies
- Backup for review of QC data
- Assist QC Management, as needed, in the completion of OOS/departure investigations for QC
- Backup for QC analytical sample receipt for internal testing, outside laboratory testing, login, processing, shipment, tracking, distribution of test results, and closure
- Proficient use of computer software, including Microsoft Office Suite (excel, word)
- Strong organizational skills with the ability to multi-task
- Knowledgeable in FDA GMPs (21 CFR, 211, 820, and/or 600); ISO 9001 and 13485 a plus
- Safe storage, testing and disposal of materials which may include acrylates, solvents, acids/bases, reactive monomers, irritants, corrosives, compressed gasses, heavy metals, phthalates and powders
- Laboratory operations; including chemical inventory, storage and disposal of chemicals, accurate measurements, wet chemistry, extractions, standards preparation, titrations, analytical data interpretation, data documentation, graphics and reporting
- Maintain and calibrate instruments, as needed
- Developing new laboratory techniques and methods for testing and analysis, as needed
- Evaluating and troubleshooting instrumentation, as needed
- Participating, leading and documenting meetings as required
- Ordering chemicals and lab supplies according to Armstrong purchasing policy, and other applicable policies or regulations
- Traveling as required for training to support testing, R&D, plant operations or other business needs
- Executing and analyzing data from testing protocols and external laboratories as required
- Perform basic testing in the testing laboratory as applicable
- Perform any other duties and/or tasks that may be assigned to him/her on an as needed basis
- Bachelor's Degree in Chemistry, Forensic Chemistry, or equivalent
- Strong academic, co-op or internship experience
- Demonstrated proficiency with a variety of computer applications, including Lotus Notes, Microsoft Office, ChemStation, Mass Hunter, Labware and/or Minitab
- Familiarity and experience with the following laboratory techniques and equipment: GPC, GC/MS, LC/MS TOF, FTIR, elemental analysis including AA and/or MP-AES, TGA, DSC, DMA, TMA, total volatile emissions testing, standards preparation, solvent extractions, acid digestions, polarized light microscope, stereoscope, digital microscope, scanning electron microscope and EDX
- Self-confident, outspoken, client-focused and creative personality
- Problem solving and detail oriented
- Ability to travel 0-10%
- Experience in research based environment
- Experience in scientific report writing
- Excellent organizational and teamwork skills
- Strong comfortability in a lab environment
- Strong communication skills with varying levels of management
- Ability to multi-task and prioritize work
- Ability to follow established policies and procedures
QC Technician, Analytical Chemist Resume Examples & Samples
- Reponsible for analysis using modern analytical instrumentation such as GC/MS, LC/MS, HPLC, FT-IR in accordance with accepted laboratory procedures
- Media & sample preparation and data analysis
- Immediately notify the Quality Management Team of any deviations from acceptable standards and start the investigation of the issue
- Responsible for entering & maintaining all result data in the JDE database / excel spreadsheets
- Responsible for keeping the assigned areas in a clean and orderly condition
- Responsible for keeping all equipment functioning properly and reporting all malfunctions to the Senior Analytical Chemist and Quality Control Manager
- Responsible for performing other duties as required by the Quality Control Manager
- May need to provide coverage on other shifts, on occasion, when vacation coverage is needed. This will require product sampling training and taste testing
- Weekday and weekend overtime will be required, on occasion
- Minimum 4-year Science Degree or equivalent in Chemistry or Biochemistry is required
- Experience with Analytical testing equipment is preferred
- Good personal hygiene
- Strong mathematical aptitude
- Working knowledge of Microsoft Office including Excel and Word
- Ability to develop and maintain effective working relationships with laboratory and plant personnel
- Ability to assist in all aspects of support for the Quality Control laboratory functions as requested by the Quality Control Manager
- Analyze organic and inorganic materials to determine chemical and physical properties, composition, structure, relationships, and reactions utilizing wet chemistry, chromatography, spectroscopy, and spectrophotometry techniques
- Prepare test solutions, compounds, and reagents for laboratory methods to conduct tests
- Compile and analyze test information to determine process or equipment operating efficiency and to diagnose malfunctions
- Troubleshoot / validate analytical methods in support of operations and regulatory needs
- Interface with technical staff, e.g., Project Leads, ICD, QA, and others on special projects, validations, and method transfers
- Assist QA and Operations on investigations for complaints and product deviations
- Participate in maintenance and creation of laboratory training documentation and records, keep SOPs current, and provide support for proper training of laboratory personnel
- BS in chemistry
- Three – five years’ experience in a fast-paced laboratory environment performing validations and method development
- CGMPs and quality systems in a regulated environment
- SOP writing
- Familiarity with SAP a plus
- Theory, operation, and troubleshooting of HPLC, GC, IC, ICP, FTIR
- Proficiency in Microsoft products: Word, Excel, PowerPoint
- Strong written and verbal communication skills necessary
Related Job Titles
Synthetic Organic Chemistry CV example
To land a top Synthetic Organic Chemistry job, you need a winning CV that will wow recruiters and hiring managers.
Learn how to create your own winning CV with our example Synthetic Organic Chemist CV along with a step-by-step CV writing guide.
Guide contents
Synthetic Organic Chemistry CV example
- CV layout and format
- Your CV profile
- Work experience
Education section
CV templates

This is a good example of a Synthetic Organic Chemist CV which contains all of the information that a hiring manager will need to know, and presents it in a well- structured, easy-to-read manner.
Take some time to study and understand this CV, and refer to it throughout the writing of your own CV for best results.
Synthetic Organic Chemistry CV layout and format
Recruiters and employers are busy, and if they can’t find the information they’re looking for in a few seconds, it could be game over for your application.
You need to format and structure your CV in a way which allows the reader to pick out your key information with ease, even if they’re strapped for time.
It should be clear, easily legible, well-organised and scannable – check out some simple tips and tricks below:
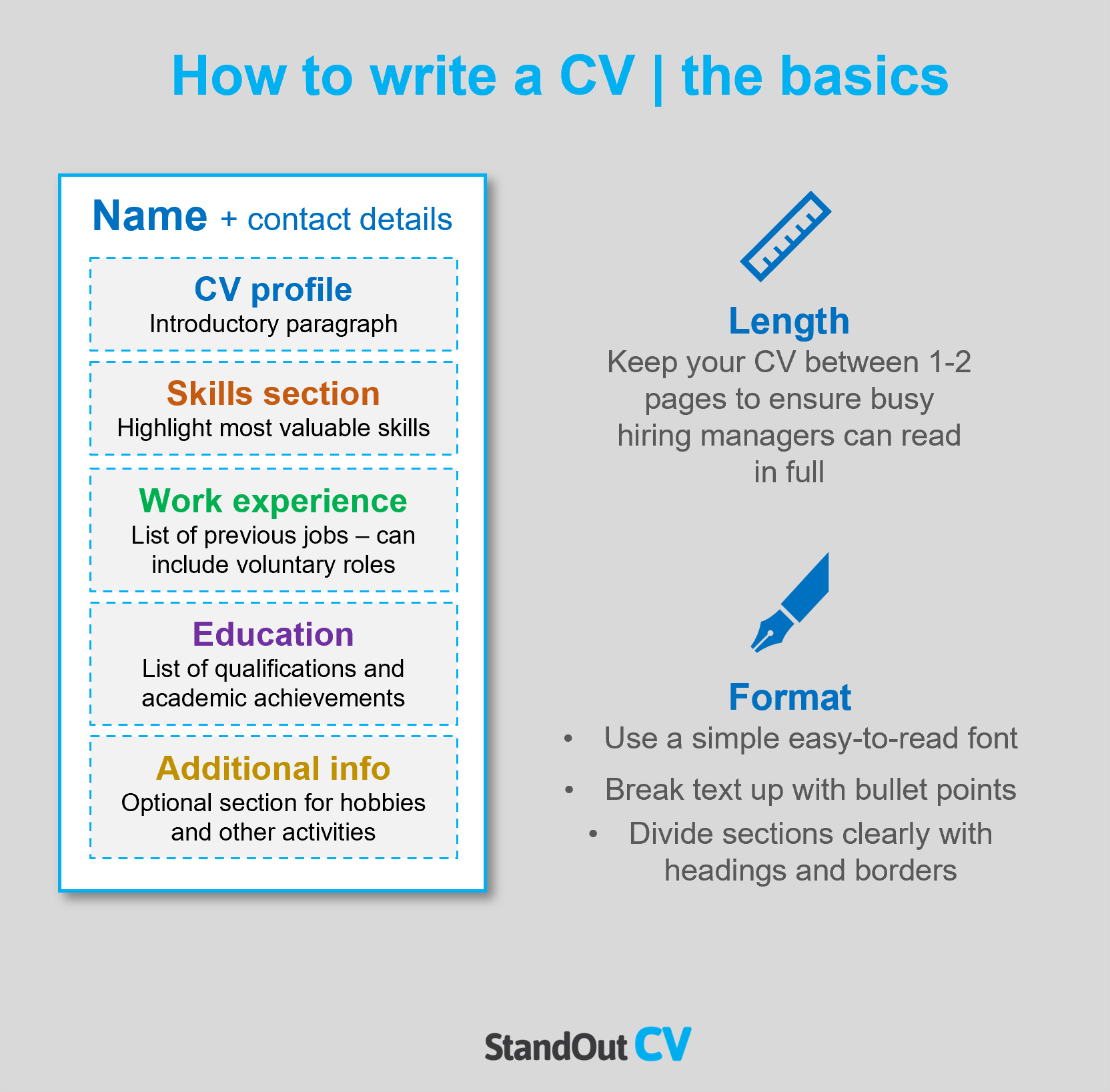
Formatting advice
- Length: Recruiters will be immediately put off by lengthy CVs – with hundreds of applications to read through, they simply don’t have the time! Grabbing their attention with a short, snappy and highly relevant CV is far more likely to lead to success. Aim for two sides of A4 or less.
- Readability : By clearly formatting your section headings (bold, or a different colour font, do the trick) and breaking up big chunks of text into snappy bullet points, time-strapped recruiters will be able to skim through your CV with ease.
- Design: The saying ‘less is more’ couldn’t be more applicable to CVs. Readability is key, so avoid overly complicated designs and graphics. A subtle colour palette and easy-to-read font is all you need!
- Avoid photos: If your CV has photos, images or profile pictures, hit the delete button. They’re not needed and won’t add any value to your applications.

CV structure
As you write your CV , divide and sub-head into the following sections:
- Name and contact details – Always start with these, so employers know exactly how to get in touch with you.
- CV profile – Add a short summary of your relevant experience, skills and achievements, which highlights your suitability.
- Core skills section – A 2-3 columned list of your key skills.
- Work experience – A detailed list of any relevant work experience, whether paid or voluntary.
- Education – An overview of your academic background and any training you may have completed.
- Hobbies and interests – A brief overview of your hobbies and interests, if they’re relevant (optional).
Now I’ll tell you exactly what you should include in each CV section.
CV Contact Details

Kick-start your CV with your contact details, so recruiters can get in touch easily. Here’s what you should include:
- Mobile number
- Email address – Make sure it’s professional, with no silly nicknames.
- Location – Your town or city is sufficient, rather than a full address.
- LinkedIn profile or portfolio URL – Ensure they’ve been updated and are looking slick and professional.
Quick tip: Avoid listing your date of birth, marital status or other irrelevant details – they’re unnecessary at this stage.
Synthetic Organic Chemistry CV Profile
Recruiters and hiring managers are busy, so it’s essential to catch their attention from the get-go.
A strong introductory profile (or personal statement , for junior candidates) at the top of the CV is the first thing they’ll read, so it’s a great chance to make an impression.
It should be a short but punchy summary of your key skills, relevant experience and accomplishments.
Ultimately, it should explain why you’re a great fit for the role you’re applying for and inspire recruiters to read the rest of your CV.

Tips for creating an strong CV profile:
- Keep it concise: It might be tempting to submit a page-long CV profile, but recruiters won’t have the time to read it. To ensure every word gets read, it’s best to include high-level information only; sticking to a length of 3-5 lines.
- Tailor it: Recruiters can spot a generic, mass-produced CV at a glance – and they certainly won’t be impressed! Before you write your profile (and CV as a whole), read through the job advert and make a list of any skills, knowledge and experience required. You should then incorporate your findings throughout your profile and the rest of your CV.
- Don’t add an objective: If you want to discuss your career objectives, save them for your cover letter , rather than wasting valuable CV profile space.
- Avoid cliches: Cheesy clichès and generic phrases won’t impress recruiters, who read the same statements several times per day. Impress them with your skill-set, experience and accomplishments instead!
What to include in your Synthetic Organic Chemistry CV profile?
- Summary of experience: Start with a brief summary of your relevant experience so far. How many years experience do you have? What type of companies have you worked for? What industries/sectors have you worked in? What are your specialisms?
- Relevant skills: Employers need to know what skills you can bring to their organisation, and ideally they want to see skills that match their job vacancy. So, research your target roles thoroughly and add the most important Synthetic Organic Chemistry skills to your profile.
- Essential qualifications: Be sure to outline your relevant Synthetic Organic Chemistry qualifications, so that anyone reading the CV can instantly see you are qualified for the jobs you are applying to.
Quick tip: Even the best of writers can overlook typos and spelling mistakes. Use our quick-and-easy CV Builder to add pre-written content that has been created by recruitment experts, and proofread by our team.
Core skills section
In addition to your CV profile, your core skills section provides an easily digestible snapshot of your skills – perfect for grabbing the attention of busy hiring managers.
As Synthetic Organic Chemistry jobs might receive a huge pile of applications, this is a great way to stand out and show off your suitability for the role.
It should be made up of 2-3 columns of bullet points and be made up of skills that are highly relevant to the jobs you are targeting.

Work experience/Career history
Now that recruiters have a good overview of your skills and abilities, you need to jump into the detail of your career history.
Give them a more thorough insight into what you can do by creating a detailed list of your relevant experience.
Start with your current role, and work backwards through all the relevant positions you’ve held. This could be freelance, contract or voluntary work too; as long as it’s related to the role you’re applying for.

Structuring your roles
Whilst writing your CV, it’s essential to look at it from the eyes of a recruiter.
If they’re met with giant blocks of text which are impossible to navigate, they might get frustrated and skip onto the next CV.
Instead, make use of the 3-step structure shown below, to give them a pleasant reading experience.

Provide a brief overview of the job as a whole, such as what the overriding purpose of your job was and what type of company you worked for.
Key responsibilities
Follow with a snappy list of bullet points, detailing your daily duties and responsibilities.
Tailor it to the role you’re applying for by mentioning how you put the target employer’s desired hard skills and knowledge to use in this role.
Key achievements
Lastly, add impact by highlight 1-3 key achievements that you made within the role.
Struggling to think of an achievement? If it had a positive impact on your company, it counts.
For example, you might increased company profits, improved processes, or something simpler, such as going above and beyond to solve a customer’s problem.
At the bottom of your CV is your full education section. You can list your formal academic qualifications, such as:
- GCSE’s
As well as any specific Synthetic Organic Chemistry qualifications that are essential to the jobs you are applying for. Note down the name of the qualification, the organisation at which you studied, and the date of completion.
Interests and hobbies
The hobbies and interests CV section isn’t mandatory, so don’t worry if you’re out of room by this point.
However, if you have an interesting hobby , or an interest that could make you seem more suitable for the role, then certainly think about adding.
Be careful what you include though… Only consider hobbies that exhibit skills that are required for roles as a Synthetic Organic Chemistry, or transferable workplace skills. There is never any need to tell employers that you like to watch TV and eat out.
Writing your Synthetic Organic Chemistry CV
A strong, compelling CV is essential to get noticed and land interviews with the best employers.
To ensure your CV stands out from the competition, make sure to tailor it to your target role and pack it with sector-specific skills and results.
Remember to triple-check for spelling and grammar errors before hitting send.
Good luck with the job search!

COMMENTS
CV Guide. IMA EXAMPLE (Chemistry) 21 College Street, City, State, Zip. 123-456-7890 | [email protected]. EDUCATION AND TRAINING. Michigan State University, Department of Chemistry. PhD Candidate in Chemistry, September 20XX - present. Michigan State University, Department of Chemistry.
CV templates. Use this CV example as a guide to formatting and structuring your Chemistry Graduate CV, so that busy recruiters can easily digest your information and determine your suitability for the role. It also provides some insight into the key skills, experience and qualifications you need to highlight.
Here is an example of a chemist CV to help you craft your own:Margery BarnesSeattle, Washington012-345-6789margery.barnes@email.comObjectiveEnthusiastic chemist with over five years of experience. Eager to work in the field of drug development.Laboratory experienceChemistChemCorp, Seattle, WashingtonMay 2018-Present.
In 2023-2024, we've seen a transition towards more interdisciplinary roles, blending traditional Chemistry with fields as wide as biotech and environmental science. Hence, a CV that clearly represents your versatility and adaptability can catalyze your career progression. Now let's dissolve a common myth.
Here are the main sections of a resume to add: Header: add the right contact information. Summary: provide a snapshot of your resume in miniature. Experience: your chemistry job accomplishments to date. Education: your schooling, plus skills-based achievements. Skills: your most relevant few skills for the job in question.
View this document to see a sample analytical chemist Ph.D. resume and CV example with qualifications. View Document. Report An Issue.
Resumes and CVs for Graduate Students and Postdocs 2 (314) 935-5930 careerswustl.edu careercenter.wustl.edu The Career Center - Washington University in St. Louis Curriculum Vitae vs Resume C.V. Resume Comprehensive list (unlimited length) of your educational, academic, professional, and work experience Shorter more focused document (1-2 pages)
Applications Advice. Writing a CV for your PhD application is an important part of the process. A CV for a PhD application needs to be an academic CV. These differ from traditional CVs in several key ways. They provide a great opportunity for you to display your education background and any relevant research experience in a short and concise way.
The key to creating a high-impact resume is to look at the document from the employer's point of view. From this point of view, the purpose of the resume review is to screen out applicants who don't fit the job requirements. Your objective is not to include anything that will get your resume stac ked on the "rejects" pile.
2017 - 2020 BSc in Chemistry (2:1), University of York. Comprehensive introduction to the core aspects of chemistry, whilst also allowing for advanced study of specialist areas. Main subjects studied: Research project: The synthesis of 2-alkyl-3-hydroxy long-chain acids and their 6-0-glucose esters. Technical skills: Range of analytical ...
Use measurable achievements to describe your chemist skills and experience. Use action words to make an impact on your chemist CV. Tailor your CV to your target chemist job. Use keywords from the job description throughout your chemist CV. Format your chemist CV so that it is easy to read by ATS software and human eyes.
Template 2 of 6: Chemistry Lab Assistant Resume Example. A chemistry lab assistant is usually the next level up from a chemistry research student assistant. You will likely support more complex chemical procedures, while also performing administrative and clerical duties like cleaning and sterilizing equipment.
Initiate and develop new research projects for the optimization of industrially relevant heterogeneous polymerization catalysts. Manage and drive research projects and schedules. Manage and organize a team of 2 - 3 laboratory technicians within the Catalyst R&D Group. PhD in Chemistry or Chemical Engineering. Phoenix, AZ.
Structuring your CV. Organise your content into the following sections for ease-of-reading: Contact details - These should always be at the very top of your CV. Personal statement - A brief introductory summary of your qualifications, skills and experience in relation to the PhD. Core skills - A short and snappy list of your most relevant ...
Senior Scientist, Chemistry. 02/2018 - PRESENT. Los Angeles, CA. 10-12+ (BS), 8-10+ (MS), or 0-4+ (Ph.D) years of experience. Conceive and execute medicinal chemistry research and development that achieves project and area goals. Act as a lead scientist in his/her area of expertise on one or more projects.
Janet Brown. 123 Fake Street, City, State, Zip Code. E: [email protected] P: 000-000-0000. Professional Summary. Knowledgeable organic chemist with over a decade of experience performing common analytical techniques. Expert in the application of gas chromatography and mass spectrometry for the analysis of organic samples.
4. Add your name. Begin writing your resume by typing your first and last name at the top of the document. It's important for your name to be prominent from the rest of your resume. Consider using a boldfaced font, larger font size or another design element to separate it from the rest of your content. 5.
Create a standout Chemist CV with our online platform. Browse professional templates for all levels and specialties. Land your dream role today! ... PhD in Chemistry, XYZ University - Anytown, USA (09/2011 - 03/2015) Skills. Organic and inorganic chemistry; Laboratory safety; Research and development;
In addition to applicants with bachelor degrees in chemistry or biochemistry, candidates with degrees in allied disciplines (such as biology, chemical engineering, or physics) and who possess significant training in one or more core areas of chemistry will be considered. Generally, students are admitted to the PhD program for the fall term.
QC Analytical Chemist Resume Examples & Samples. Perform Chemical analysis on finished drug substance, drug product, in process materials and stability samples. Responsible for calibration and use of laboratory instrumentation. Complete documentation associated with analytical results in a timely manner.
When considering how to write a chemistry CV that gets you noticed by employers, follow the steps below: 1. Outline the sections of your CV. The sections you include in a chemistry-specific CV usually depend on your experience level. If you're primarily applying for roles in academia or don't yet have much industry experience, you may choose to ...
EXAMPLE 4: Academic Curriculum Vitae Eugene Timmons 1400 North County Road Zurich Switzerland A94724M [email protected] Swiss Federal Institute of Technology (ETH) +41 1 632-4430 (lab) ETH Honggerberg-HCI F330 +41 1 362-7933 (home) EDUCATION 7, Cornell University, Ithaca NYPhD, Chemistry, August 201 GPA: 4.0/4.0
CV templates. This is a good example of a Synthetic Organic Chemist CV which contains all of the information that a hiring manager will need to know, and presents it in a well- structured, easy-to-read manner. Take some time to study and understand this CV, and refer to it throughout the writing of your own CV for best results.